Endocrine Therapy-Based Strategies for Metastatic Breast Cancer with Different Endocrine Sensitivity Statuses: A Systematic Review and Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Study Objectives and Endpoints
2.3. Statistical Analysis
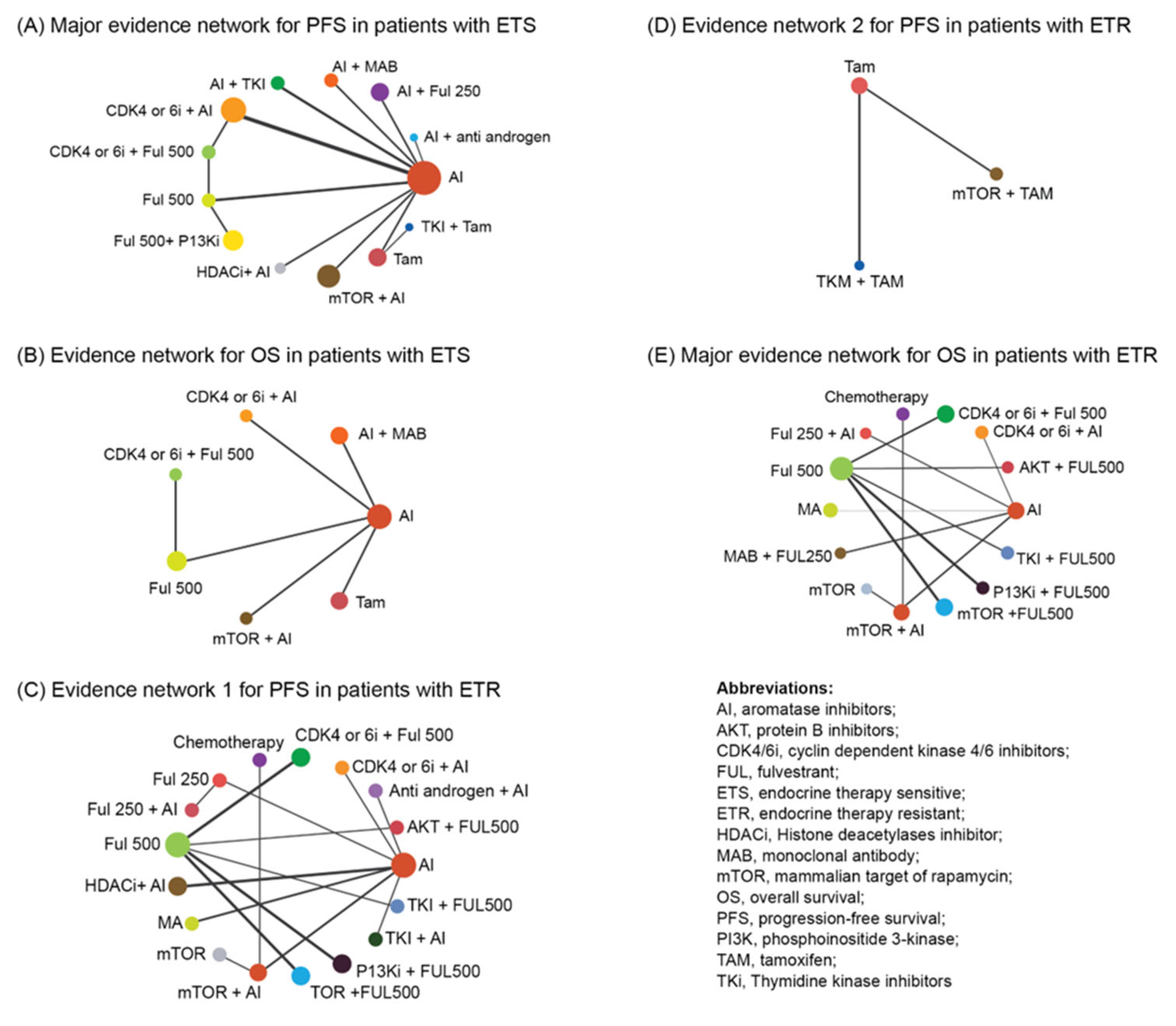
2.4. Network Geometry
2.5. Risk Bias in Individual Studies
2.6. Publication Bias and Sensitivity Analysis
3. Results
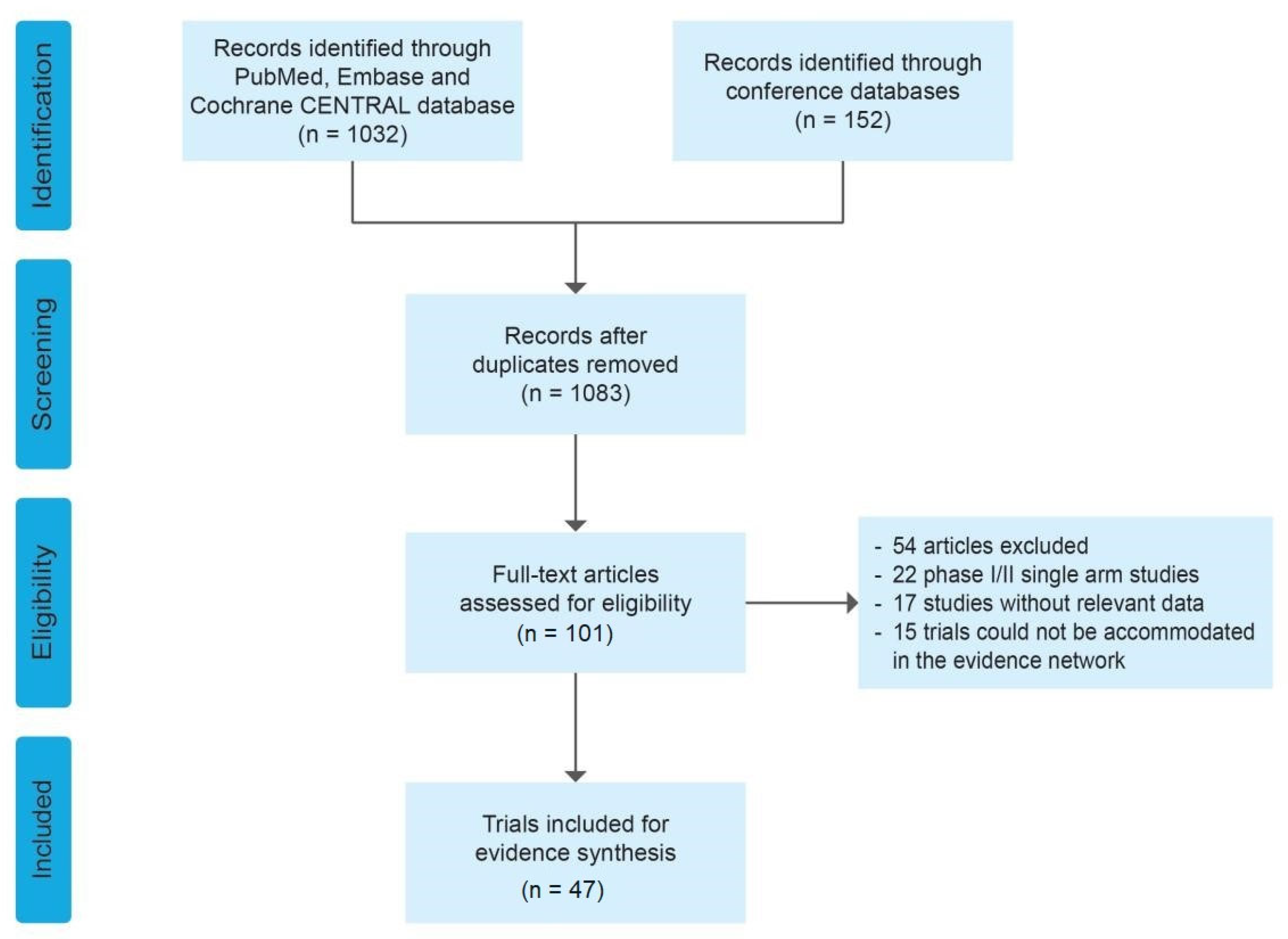
3.1. Systematic Review and Characteristics
3.2. Quality of the Evidence
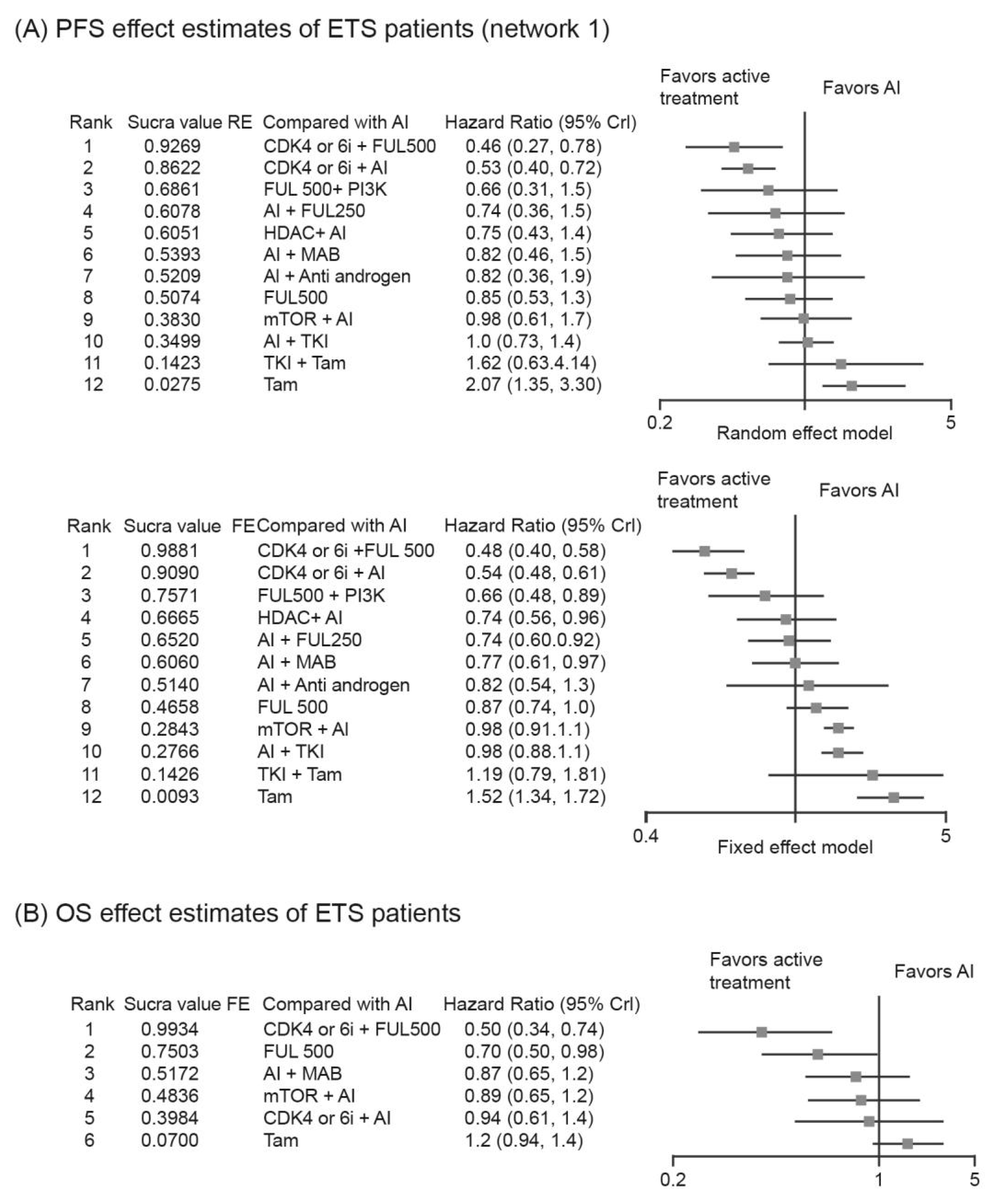
3.3. Analysis of PFS/OS in Patients with ETS
3.3.1. Bone-Only and Visceral Metastasis
3.3.2. Relative Efficacy of Targeted-Based Regimens
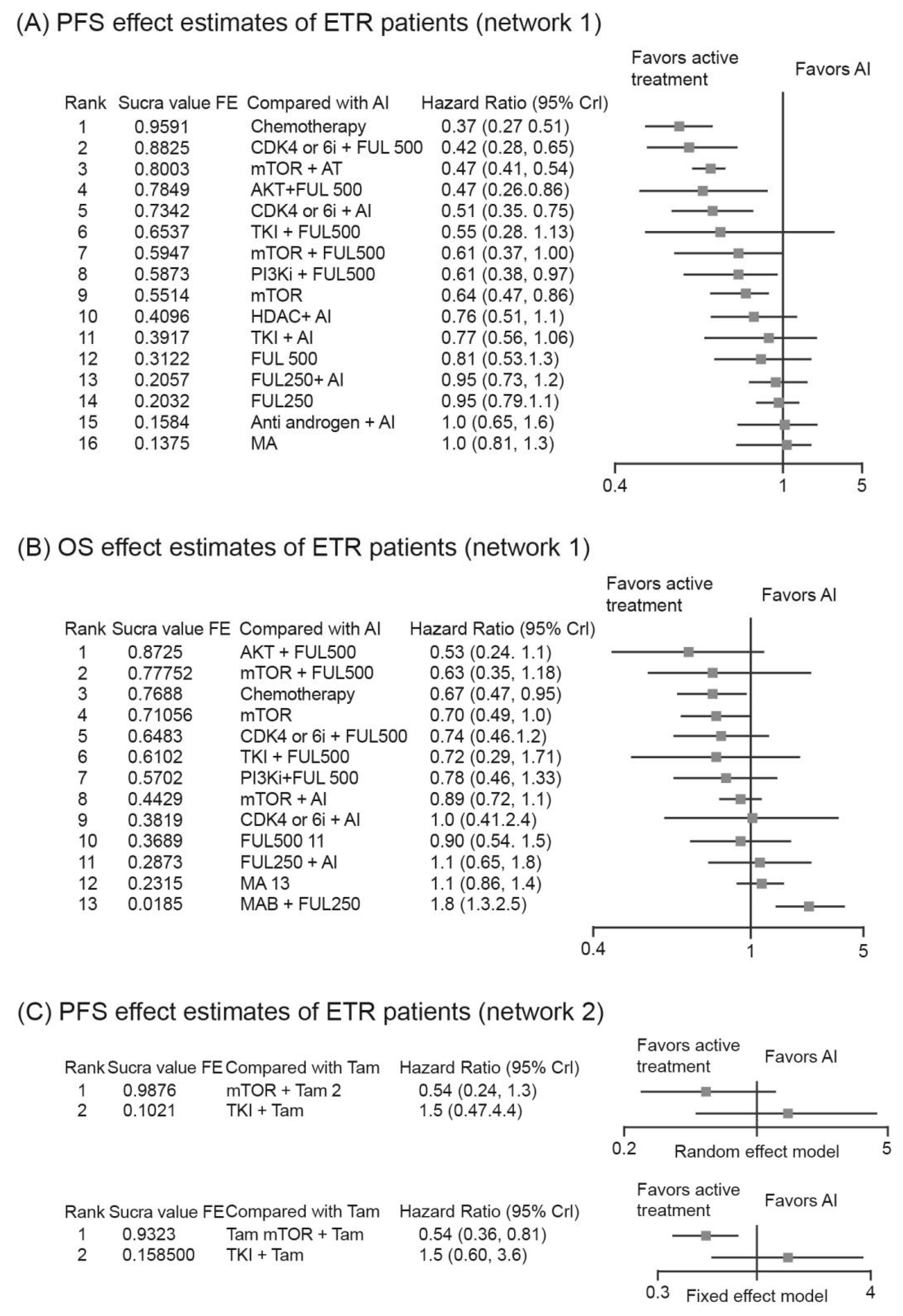
3.4. Analysis of PFS/OS in Patients with ETR
3.4.1. Bone-Only and Visceral Metastasis
3.4.2. Relative Efficacy of Targeted-Based Regimens
3.4.3. Menopausal Status
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10 (Suppl. S3), 20–29. [Google Scholar] [CrossRef] [PubMed]
- Anurag, M.; Ellis, M.J.; Haricharan, S. DNA Damage Repair Defects as a New Class of Endocrine Treatment Resistance Driver. Oncotarget 2018, 9, 36252–36253. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.-M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 Mutations in Hormone-Resistant Metastatic Breast Cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.T.; Shao, J.; Zhang, J.; Iglesia, M.; Chan, D.W.; Cao, J.; Anurag, M.; Singh, P.; He, X.; Kosaka, Y.; et al. Functional Annotation of ESR1 Gene Fusions in Estrogen Receptor-Positive Breast Cancer. Cell Rep. 2018, 24, 1434–1444.e7. [Google Scholar] [CrossRef]
- Newby, J.C.; Johnston, S.R.; Smith, I.E.; Dowsett, M. Expression of Epidermal Growth Factor Receptor and C-ErbB2 during the Development of Tamoxifen Resistance in Human Breast Cancer. Clin. Cancer Res. 1997, 3, 1643–1651. [Google Scholar]
- Borg, A.; Baldetorp, B.; Fernö, M.; Killander, D.; Olsson, H.; Rydén, S.; Sigurdsson, H. ERBB2 Amplification Is Associated with Tamoxifen Resistance in Steroid-Receptor Positive Breast Cancer. Cancer Lett. 1994, 81, 137–144. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Brandão, M.; Maurer, C.; Ziegelmann, P.K.; Pondé, N.F.; Ferreira, A.; Martel, S.; Piccart, M.; de Azambuja, E.; Debiasi, M.; Lambertini, M. Endocrine Therapy-Based Treatments in Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer: Systematic Review and Network Meta-Analysis. ESMO Open 2020, 5, e000842. [Google Scholar] [CrossRef]
- Messina, C.; Cattrini, C.; Buzzatti, G.; Cerbone, L.; Zanardi, E.; Messina, M.; Boccardo, F. CDK4/6 Inhibitors in Advanced Hormone Receptor-Positive/HER2-Negative Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Breast Cancer Res. Treat. 2018, 172, 9–21. [Google Scholar] [CrossRef]
- de Sire, A.; Moggio, L.; Demeco, A.; Fortunato, F.; Spanò, R.; Aiello, V.; Marotta, N.; Ammendolia, A. Efficacy of Rehabilitative Techniques in Reducing Hemiplegic Shoulder Pain in Stroke: Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101602. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Marinaro, C.; Pino, I.; Barletta, M.; Petraroli, A.; Pepe, D.; Lavano, F.; Ammendolia, A. Comparative Effectiveness of Breathing Exercises in Patients with Chronic Obstructive Pulmonary Disease. Complement Ther. Clin. Pract. 2020, 41, 101260. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Yariz, K.O.; Bondarenko, I.; Manikhas, A.; Semiglazov, V.; Alyasova, A.; Komisarenko, V.; Shparyk, Y.; Murray, J.L.; Jones, D.; et al. Randomized Phase II Trial of Letrozole plus Anti-MUC1 Antibody AS1402 in Hormone Receptor-Positive Locally Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2011, 17, 6822–6830. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Sun, T.; Yin, Y.M.; Li, H.P.; Yan, M.; Tong, Z.S.; Oppermann, C.P.; Liu, Y.P.; Costa, R.; Li, M.; et al. MONARCH plus: Abemaciclib plus Endocrine Therapy in Women with HR+/HER2- Advanced Breast Cancer: The Multinational Randomized Phase III Study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920963925. [Google Scholar] [CrossRef]
- Wolff, A.C.; Lazar, A.A.; Bondarenko, I.; Garin, A.M.; Brincat, S.; Chow, L.; Sun, Y.; Neskovic-Konstantinovic, Z.; Guimaraes, R.C.; Fumoleau, P.; et al. Randomized Phase III Placebo-Controlled Trial of Letrozole plus Oral Temsirolimus as First-Line Endocrine Therapy in Postmenopausal Women with Locally Advanced or Metastatic Breast Cancer. J. Clin. Oncol. 2013, 31, 195–202. [Google Scholar] [CrossRef]
- Dickler, M.N.; Barry, W.T.; Cirrincione, C.T.; Ellis, M.J.; Moynahan, M.E.; Innocenti, F.; Hurria, A.; Rugo, H.S.; Lake, D.E.; Hahn, O.; et al. Phase III Trial Evaluating Letrozole As First-Line Endocrine Therapy With or Without Bevacizumab for the Treatment of Postmenopausal Women With Hormone Receptor-Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance). J. Clin. Oncol. 2016, 34, 2602–2609. [Google Scholar] [CrossRef]
- Goss, P.; Bondarenko, I.N.; Manikhas, G.N.; Pendergrass, K.B.; Miller, W.H.; Langecker, P.; Blanchett, D. Phase III, Double-Blind, Controlled Trial of Atamestane plus Toremifene Compared with Letrozole in Postmenopausal Women with Advanced Receptor-Positive Breast Cancer. J. Clin. Oncol. 2007, 25, 4961–4966. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Valero, V.; Mangalik, A.; Royce, M.; Rabinowitz, I.; Arena, F.P.; Kroener, J.F.; Curcio, E.; Watkins, C.; Bacus, S.; et al. Phase II, Randomized Trial to Compare Anastrozole Combined with Gefitinib or Placebo in Postmenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2010, 16, 1904–1914. [Google Scholar] [CrossRef]
- Krop, I.; Abramson, V.; Colleoni, M.; Traina, T.; Holmes, F.; Garcia-Estevez, L.; Hart, L.; Awada, A.; Zamagni, C.; Morris, P.G.; et al. A Randomized Placebo Controlled Phase II Trial Evaluating Exemestane with or without Enzalutamide in Patients with Hormone Receptor-Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 6149–6157. [Google Scholar] [CrossRef] [PubMed]
- Albanell, J.; Martinez, M.T.M.; Ramos, M.; Connor, M.O’.; la Cruz-Merino, L.D.; Bertran, A.S.; Martínez-Jáñez, N.; Moreno, F.; Pérez, I.F.; Company, J.A.; et al. LBA19 GEICAM/2014-12 (FLIPPER) Study: First Analysis from a Randomized Phase II Trial of Fulvestrant (F)/Palbociclib (P) versus (vs) F/Placebo (PL) as First-Line Therapy in Postmenopausal Women with HR (Hormone Receptor)+/HER2– Endocrine Sensitive Advanced Breast Cancer (ABC). Ann. Oncol. 2020, 31, S1151. [Google Scholar] [CrossRef]
- Milla-Santos, A.; Milla, L.; Portella, J.; Rallo, L.; Pons, M.; Rodes, E.; Casanovas, J.; Puig-Gali, M. Anastrozole versus Tamoxifen as First-Line Therapy in Postmenopausal Patients with Hormone-Dependent Advanced Breast Cancer: A Prospective, Randomized, Phase III Study. Am. J. Clin. Oncol. 2003, 26, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Pérez-Carrión, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jänicke, F.; et al. Superior Efficacy of Letrozole versus Tamoxifen as First-Line Therapy for Postmenopausal Women with Advanced Breast Cancer: Results of a Phase III Study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001, 19, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Paridaens, R.J.; Dirix, L.Y.; Beex, L.V.; Nooij, M.; Cameron, D.A.; Cufer, T.; Piccart, M.J.; Bogaerts, J.; Therasse, P. Phase III Study Comparing Exemestane with Tamoxifen as First-Line Hormonal Treatment of Metastatic Breast Cancer in Postmenopausal Women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J. Clin. Oncol. 2008, 26, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Vukelja, S.J.; Ann Holmes, F.; Blum, J.L.; McIntyre, K.J.; Lindquist, D.L.; Osborne, C.R.; Sanchez, I.J.; Goldschmidt, J.H.; Wang, Y.; et al. Randomized Phase-II Evaluation of Letrozole plus Dasatinib in Hormone Receptor Positive Metastatic Breast Cancer Patients. npj Breast Cancer 2019, 5, 36. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Livingston, R.B.; et al. Combination Anastrozole and Fulvestrant in Metastatic Breast Cancer. N. Engl. J. Med. 2012, 367, 435–444. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Linden, H.M.; et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Engl. J. Med. 2019, 380, 1226–1234. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The Cyclin-Dependent Kinase 4/6 Inhibitor Palbociclib in Combination with Letrozole versus Letrozole Alone as First-Line Treatment of Oestrogen Receptor-Positive, HER2-Negative, Advanced Breast Cancer (PALOMA-1/TRIO-18): A Randomised Phase 2 Study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall Survival Results from the Randomized Phase 2 Study of Palbociclib in Combination with Letrozole versus Letrozole Alone for First-Line Treatment of ER+/HER2− Advanced Breast Cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus Letrozole as First-Line Therapy in Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer with Extended Follow-Up. Breast Cancer Res. Treat. 2019, 174, 719. [Google Scholar] [CrossRef]
- Finn, R.S.; Dieras, V.; Rugo, H.S.; Joy, A.A.; Moulder, S.L.; Walshe, J.M.; Mukai, H.; Shparyk, Y.V.; Park, I.H.; Mori, A.; et al. Palbociclib (PAL) + Letrozole (L) as First-Line (1L) Therapy (Tx) in Estrogen Receptor-Positive (ER+)/Human Epidermal Growth Factor Receptor 2-Negative (HER2−) Advanced Breast Cancer (ABC): Efficacy and Safety across Patient (Pt) Subgroups. JCO 2017, 35, 1039-1039. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus Palbociclib versus Fulvestrant plus Placebo for Treatment of Hormone-Receptor-Positive, HER2-Negative Metastatic Breast Cancer That Progressed on Previous Endocrine Therapy (PALOMA-3): Final Analysis of the Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated Results from MONALEESA-2, a Phase III Trial of First-Line Ribociclib plus Letrozole versus Placebo plus Letrozole in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus Fulvestrant for Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer in the Phase III Randomized MONALEESA-3 Trial: Updated Overall Survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Hurvitz, S.; Lee, S.C.; Jerusalem, G.; Im, S.; Chia, S.K.L.; Campos, S.; Sonke, G.S.; Lteif, A. 329P-Ribociclib (RIB) in Patients (Pts) with HR+/HER2− Advanced Breast Cancer (ABC) and Resistance to Prior Endocrine Therapy (ET) in the MONALEESA (ML)...|OncologyPRO. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/ribociclib-rib-in-patients-pts-with-hr-her2-advanced-breast-cancer-abc-and-resistance-to-prior-endocrine-therapy-et-in-the-monaleesa-ml (accessed on 20 September 2022).
- Johnston, S.R.; Kilburn, L.S.; Ellis, P.; Dodwell, D.; Cameron, D.; Hayward, L.; Im, Y.-H.; Braybrooke, J.P.; Brunt, A.M.; Cheung, K.-L.; et al. Fulvestrant plus Anastrozole or Placebo versus Exemestane Alone after Progression on Non-Steroidal Aromatase Inhibitors in Postmenopausal Patients with Hormone-Receptor-Positive Locally Advanced or Metastatic Breast Cancer (SoFEA): A Composite, Multicentre, Phase 3 Randomised Trial. Lancet Oncol. 2013, 14, 989–998. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.-A.; Kalev, D.; Egle, D.; et al. Buparlisib plus Fulvestrant in Postmenopausal Women with Hormone-Receptor-Positive, HER2-Negative, Advanced Breast Cancer Progressing on or after MTOR Inhibition (BELLE-3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, T.; Shao, Z.; Zhang, Q.; Ouyang, Q.; Tong, Z.; Wang, S.; Luo, Y.; Teng, Y.; Wang, X.; et al. Effectiveness of Adding Everolimus to the First-Line Treatment of Advanced Breast Cancer in Premenopausal Women Who Experienced Disease Progression While Receiving Selective Estrogen Receptor Modulators: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, e213428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, W.; Hu, X.; Zhang, Q.; Sun, T.; Cui, S.; Wang, S.; Ouyang, Q.; Yin, Y.; Geng, C.; et al. Tucidinostat plus Exemestane for Postmenopausal Patients with Advanced, Hormone Receptor-Positive Breast Cancer (ACE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Ismail-Khan, R.; Klein, P. Results of ENCORE 301, a Randomized, Phase II, Double-Blind, Placebo-Controlled Study of Exemestane with or without Entinostat in Postmenopausal Women with Locally Recurrent or Metastatic Estrogen Receptor-Positive (ER+) Breast Cancer Progressing on a Nonsteroidal Aromatase Inhibitor (AI). JCO 2011, 29, 268-268. [Google Scholar] [CrossRef]
- Xu, B.; Hu, X.; Li, W.; Sun, T.; Shen, K.; Wang, S.; Cheng, Y.; Zhang, Q.; Cui, S.; Tong, Z.; et al. 228MO PALOMA-4: Primary Results from a Phase III Trial of Palbociclib (PAL) + Letrozole (LET) vs Placebo (PBO) + LET in Asian Postmenopausal Women with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative (ER+/HER2–) Advanced Breast Cancer (ABC). Ann. Oncol. 2021, 32, S457. [Google Scholar] [CrossRef]
- Treilleux, I.; Arnedos, M.; Cropet, C.; Wang, Q.; Ferrero, J.-M.; Abadie-Lacourtoisie, S.; Levy, C.; Legouffe, E.; Lortholary, A.; Pujade-Lauraine, E.; et al. Translational Studies within the TAMRAD Randomized GINECO Trial: Evidence for MTORC1 Activation Marker as a Predictive Factor for Everolimus Efficacy in Advanced Breast Cancer. Ann. Oncol. 2015, 26, 120–125. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.-A.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus Fulvestrant versus Placebo plus Fulvestrant in Postmenopausal, Hormone Receptor-Positive, HER2-Negative, Advanced Breast Cancer (BELLE-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Campone, M.; Im, S.-A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiełło-Gruszfeld, A.; Pistilli, B.; Tseng, L.-M.; et al. Buparlisib plus Fulvestrant versus Placebo plus Fulvestrant for Postmenopausal, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, Advanced Breast Cancer: Overall Survival Results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus Exemestane for Hormone-Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Overall Survival Results from BOLERO-2†. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus Capivasertib versus Placebo after Relapse or Progression on an Aromatase Inhibitor in Metastatic, Oestrogen Receptor-Positive Breast Cancer (FAKTION): A Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef]
- Krop, I.E.; Mayer, I.A.; Ganju, V.; Dickler, M.; Johnston, S.; Morales, S.; Yardley, D.A.; Melichar, B.; Forero-Torres, A.; Lee, S.C.; et al. Pictilisib for Oestrogen Receptor-Positive, Aromatase Inhibitor-Resistant, Advanced or Metastatic Breast Cancer (FERGI): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef]
- Jerusalem, G.; de Boer, R.H.; Hurvitz, S.; Yardley, D.A.; Kovalenko, E.; Ejlertsen, B.; Blau, S.; Özgüroglu, M.; Landherr, L.; Ewertz, M.; et al. Everolimus Plus Exemestane vs Everolimus or Capecitabine Monotherapy for Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer: The BOLERO-6 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1367–1374. [Google Scholar] [CrossRef]
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women With Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1556–1564. [Google Scholar] [CrossRef]
- Musolino, A.; Campone, M.; Neven, P.; Denduluri, N.; Barrios, C.H.; Cortes, J.; Blackwell, K.; Soliman, H.; Kahan, Z.; Bonnefoi, H.; et al. Phase II, Randomized, Placebo-Controlled Study of Dovitinib in Combination with Fulvestrant in Postmenopausal Patients with HR+, HER2- Breast Cancer That Had Progressed during or after Prior Endocrine Therapy. Breast Cancer Res. 2017, 19, 18. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in Combination with Endocrine Therapy versus Capecitabine in Hormonal Receptor-Positive, Human Epidermal Growth Factor 2-Negative, Aromatase Inhibitor-Resistant Metastatic Breast Cancer: A Phase III Randomised Controlled Trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, P.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; et al. Dalpiciclib versus Placebo plus Fulvestrant in HR+/HER2- Advanced Breast Cancer That Relapsed or Progressed on Previous Endocrine Therapy (DAWNA-1): A Multicenter, Randomized, Phase 3 Study. J. Clin. Oncol. 2021, 39, 1002-1002. [Google Scholar] [CrossRef]
- Buzdar, A.; Hayes, D.; El-Khoudary, A.; Yan, S.; Lønning, P.; Lichinitser, M.; Gopal, R.; Falkson, G.; Pritchard, K.; Lipton, A.; et al. Phase III Randomized Trial of Droloxifene and Tamoxifen as First-Line Endocrine Treatment of ER/PgR-Positive Advanced Breast Cancer. Breast Cancer Res. Treat. 2002, 73, 161–175. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- George, W.; Sledge, J.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116. [Google Scholar] [CrossRef]
- Shao, Z.; Cai, L.; Wang, S.; Hu, X.; Shen, K.; Wang, H.; Li, H.; Feng, J.; Liu, Q.; Cheng, J.; et al. 238P BOLERO-5: A Phase II Study of Everolimus and Exemestane Combination in Chinese Post-Menopausal Women with ER+/HER2- Advanced Breast Cancer. Ann. Oncol. 2021, 32, S463. [Google Scholar] [CrossRef]
- Johnston, S.; Pippen, J.; Pivot, X.; Lichinitser, M.; Sadeghi, S.; Dieras, V.; Gomez, H.L.; Romieu, G.; Manikhas, A.; Kennedy, M.J.; et al. Lapatinib Combined with Letrozole versus Letrozole and Placebo as First-Line Therapy for Postmenopausal Hormone Receptor-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 5538–5546. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.R.; Lindemann, J.P.O.; Llombart-Cussac, A.; Rolski, J.; Feltl, D.; Dewar, J.; Emerson, L.; Dean, A.; Ellis, M.J. Fulvestrant 500 Mg versus Anastrozole 1 Mg for the First-Line Treatment of Advanced Breast Cancer: Follow-up Analysis from the Randomized “FIRST” Study. Breast Cancer Res. Treat. 2012, 136, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.R.; Llombart-Cussac, A.; Rolski, J.; Feltl, D.; Dewar, J.; Macpherson, E.; Lindemann, J.; Ellis, M.J. Activity of Fulvestrant 500 Mg versus Anastrozole 1 Mg as First-Line Treatment for Advanced Breast Cancer: Results from the FIRST Study. J. Clin. Oncol. 2009, 27, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Hyams, D.M.; Chan, A.; de Oliveira, C.; Snyder, R.; Vinholes, J.; Audeh, M.W.; Alencar, V.M.; Lombard, J.; Mookerjee, B.; Xu, J.; et al. Cediranib in Combination with Fulvestrant in Hormone-Sensitive Metastatic Breast Cancer: A Randomized Phase II Study. Investig. New Drugs 2013, 31, 1345–1354. [Google Scholar] [CrossRef]
- Johnston, S.; Basik, M.; Hegg, R.; Lausoontornsiri, W.; Grzeda, L.; Clemons, M.; Dreosti, L.; Mann, H.; Stuart, M.; Cristofanilli, M. Inhibition of EGFR, HER2, and HER3 Signaling with AZD8931 in Combination with Anastrozole as an Anticancer Approach: Phase II Randomized Study in Women with Endocrine-Therapy-Naïve Advanced Breast Cancer. Breast Cancer Res. Treat. 2016, 160, 91–99. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 Mg versus Anastrozole 1 Mg for Hormone Receptor-Positive Advanced Breast Cancer (FALCON): An International, Randomised, Double-Blind, Phase 3 Trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Osborne, C.K.; Pippen, J.; Jones, S.E.; Parker, L.M.; Ellis, M.; Come, S.; Gertler, S.Z.; May, J.T.; Burton, G.; Dimery, I.; et al. Double-Blind, Randomized Trial Comparing the Efficacy and Tolerability of Fulvestrant versus Anastrozole in Postmenopausal Women with Advanced Breast Cancer Progressing on Prior Endocrine Therapy: Results of a North American Trial. J. Clin. Oncol. 2002, 20, 3386–3395. [Google Scholar] [CrossRef]
- Toss, A.; Venturelli, M.; Sperduti, I.; Molinaro, E.; Isca, C.; Barbieri, E.; Piacentini, F.; Omarini, C.; Cortesi, L.; Cascinu, S.; et al. First-Line Treatment for Endocrine-Sensitive Bone-Only Metastatic Breast Cancer: Systematic Review and Meta-Analysis. Clin. Breast Cancer 2019, 19, e701–e716. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine Treatment versus Chemotherapy in Postmenopausal Women with Hormone Receptor-Positive, HER2-Negative, Metastatic Breast Cancer: A Systematic Review and Network Meta-Analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Shimoi, T.; Sagara, Y.; Hara, F.; Toyama, T.; Iwata, H. First-Line Endocrine Therapy for Postmenopausal Patients with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Breast Cancer 2020, 27, 340–346. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Zhu, H.; Zhou, Y.; Mao, F.; Huang, X.; Sun, Q.; Li, C. Efficacy and Safety Profile of Histone Deacetylase Inhibitors for Metastatic Breast Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 901152. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, W.; Li, W.; Guo, C.; Luo, L.; Gao, Y.; Cao, Y. Comparison of a Histone Deacetylase Inhibitor plus Exemestane with Exemestane Alone in Hormone Receptor-Positive Advanced Breast Cancer That Progressed on Prior Endocrine Therapy: A Meta-Analysis. Exp. Ther. Med. 2022, 24, 575. [Google Scholar] [CrossRef]
| S. No | Study Name | Treatment Regimen | Menopausal Status | Patients (N) | Phase | Centers | Previous CT | Endocrine Status s/r/m | 1st ET |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ibrahim et al., 2011 [13] | SAI + MAB | Post | 110 | II | multi | no | s | no |
| SAI | |||||||||
| 2 | Goetz et al., 2017 [15] | CDK4/6i + NSAI | Post | 493 | III | multi | no | s | yes |
| NSAI | |||||||||
| 3 | Zhang et al., 2020 [16] | CDK4/6i + NSAI | Post | 306 | III | multi | no | s | no |
| NSAI | |||||||||
| CDK4/6i + FUL | Post | 157 | III | multi | no | r | yes | ||
| FUL500 | |||||||||
| 4 | Antonio C. Wolff et al., 2013 [17] | MTOR + NSAI | Post | 1112 | III | multi | no | s | no |
| NSAI | |||||||||
| 5 | Dickler et al., 2016 [18] | NSAI + MAB | Post | 343 | II | multi | ≤1 line | s | yes: ≤4 weeks |
| NSAI | |||||||||
| 6 | Goss et al., 2007 [19] | SAI + TOR | Post | 865 | III | multi | yes | s | no |
| SAI | |||||||||
| 7 | Cristofanilli et al., 2010 [20] | NSAI + TKI | Post | 93 | II | multi | no | s | no |
| NSAI | |||||||||
| 8 | Krop et al., 2020 [21] | AI + anti-androgen | Post | 127 | II | multi | ≤1 line | s | yes |
| AI | |||||||||
| 9 | Albanell et al., 2020 [22] | CDK4/6i + Ful 500 | Post | 189 | II | multi | no | s | no |
| Ful 500 | |||||||||
| 10 | Llombart-Cussac et al., 2021 [23] | CDK4/6i + Ful 500 | Multi | 486 | II | muti | no | s | yes |
| CDK4/6i + NSAI | |||||||||
| 11 | Mouridsen et al., 2001 [24] | NSAI | Post | 907 | III | multi | ≤1 line | s | no |
| Tam | |||||||||
| 12 | Paridaens et al., 2008 [25] | SAI | Post | 371 | III | multi | ≤1 line | s | no |
| Tam | |||||||||
| 13 | Milla-Santos et al., 2003 [23] | NSAI | Post | 238 | III | multi | no | s | no |
| Tam | |||||||||
| 14 | Paul et al., 2019 [26] | AI + TKI | Post | 120 | II | multi | ≤1 line | m | no |
| AI | |||||||||
| 15 | Mehta et al., 2012 [27,28] | AI + Ful 250 | Post | 694 | III | multi | yes | m | yes |
| AI | |||||||||
| 16 | Finn et al., 2015, Finn et al., 2020 [29,30] | CDK4/6i + AI | Post | 165 | II | multi | no | m | no |
| AI | |||||||||
| 17 | Finn et al., 2016 [31], Rugo et al., 2019 [32], Finn et al., 2017 [33] | CDK4/6i + AI | Post | 666 | III | multi | no | m | yes |
| AI | |||||||||
| 18 | Cristofanilli et al., 2016 [34] | CDK4/6i + ful500 | Both | 521 | III | multi | ≤1 line | m | yes |
| Ful 500 | |||||||||
| 19 | Hortobagyi et al., 2018 [35] | CDK4/6i + AI | Post | 668 | III | multi | no | m | yes |
| AI | |||||||||
| 20 | Slamon et al., 2021 [36], Slamon et al., 2018 [37], Slamon et al., 2020 [38], Hurvitz et al., [39] | CDK4/6i + ful500 | Post | 726 | III | multi | no | m | yes |
| Ful 500 | |||||||||
| 21 | Johnston et al., 2013 [40] | AI + Ful 250 | Post | 723 | III | multi | yes | m | yes |
| Ful 250 | |||||||||
| Ful 250 | Post | 723 | III | multi | yes | m | yes | ||
| AI | |||||||||
| 22 | André et al., 2019 [41] | Ful500 + PI3K | Post | 341 | III | multi | yes | m | yes |
| Ful 500 | |||||||||
| 23 | Leo et al., 2018 [42] | Ful500 + PI3K | Post | 432 | III | multi | allowed | m | yes |
| Ful 500 | |||||||||
| 24 | Fan et al., 2021 [43] | NSAI + mTOR | Pre | 199 | II | multi | yes ≤1 line | m | yes: TAM |
| NSAI | |||||||||
| 25 | Jiang et al., 2019 [44] | HDAC + SAI | Post | 365 | III | multi | ≤1 line | m | yes |
| SAI | |||||||||
| 26 | Yardley et al., 2011 [45] | HDAC + AI | Post | 130 | II | multi | ≤1 line | m | yes: NSAI |
| AI | |||||||||
| 27 | Xu et al., 2021 [46] | CDK4_or_6i_plus_AI | Post | 340 | III | multi | no | s | |
| AI | |||||||||
| 28 | Treilleux et al., 2015 [47] | mTOR + TAM | Post | 111 | II | multi | ≤1 line | r | yes: AI |
| TAM | |||||||||
| 29 | Baselga et al., 2017 [48,49] | PI3Ki + Ful500 | Post | 1147 | III | multi | ≤1 line | m | yes |
| FUL500 | |||||||||
| 30 | Piccart et al., 2014 [50] | mTOR + AI | Post | 724 | III | multi | ≤1 line | r | yes: AI |
| AI | |||||||||
| 31 | Jones et al., 2020 [51] | AKT + FUL | Post | 140 | II | multi | ≤1 line | r | yes: AI |
| FUL500 | |||||||||
| 32 | Krop et al., 2016 [52] | PI3Ki + Ful500 | Post | 168 | II | multi | ≤1 line | r | AI |
| FUL500 | |||||||||
| PI3Ki + Ful500 | Post | 61 | II | multi | ≤1 line | r | AI | ||
| FUL500 | |||||||||
| 33 | Jerusalem et al., 2018 [53] | mTOR + AI | Post | 309 | II | multi | ≤1 line | r | yes |
| Mtor | |||||||||
| 34 | Schmid et al., 2019 [54] | mTOR + FUL500 | Post | 326 | II | multi | ≤1 line | r | yes |
| FUL500 | |||||||||
| 35 | Musolino et al., 2017 [55] | TKI + FUL500 | Post | 97 | II | multi | no | r | yes |
| FUL500 | |||||||||
| 36 | Martin et al., 2021 [56] | CDK4/6i + FUL | Post | 601 | III | multi | ≤1 line | r | yes: NSAI |
| Chemotherapy | |||||||||
| 37 | Kornblum et al., 2018 [57] | mTOR + FUL500 | Post | 131 | II | multi | ≤1 line | r | yes: AI |
| FUL500 | |||||||||
| 38 | Xu et al., 2021 [58] | CDK4/6i + FUL | Both | 361 | III | multi | ≤1 line | r | yes |
| FUL500 | |||||||||
| 39 | Buzdar et al., 2002 [59] | AI | Post | 602 | II | multi | ≤1 line | r | yes |
| MA | |||||||||
| 40 | Sledge et al., 2017 [60,61] | CDK4/6i + FUL | Both | 669 | III | multi | no | r | yes |
| FUL500 | |||||||||
| 41 | Shao et al., 2021 [62] | mTOR + AI | Post | 159 | Ⅱ | multi | yes | r | yes |
| AI | |||||||||
| 42 | Johnston et al., 2009 [63] | TKI + AI | Post | 1286 | III | multi | no | m | yes |
| AI | |||||||||
| 43 | Robertson et al., 2012 [64], Robertson et al., 2009 [65] Roberson et al., 2012 [64] | FUL500 | Post | 205 | III | multi | yes | s | no |
| AI | |||||||||
| 44 | Hyams et al., 2013 [66] | TKI + FUL250 | Post | 62 | III | multi | ≤1 line | s | yes |
| FUL250 | |||||||||
| 45 | Johnston et al., 2016 [67] | TKI + AI | Post | 359 | II | multi | ≤1 line | s | yes |
| AI | |||||||||
| 46 | Robertson et al., 2016 [68] | FUL | Post | 462 | III | multi | allowed | s | yes |
| AI | |||||||||
| 47 | Osborne et al., 2002 [69] | FUL | Post | 400 | III | multi | no | m | yes |
| AI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Han, Y.; Wang, J.; Li, Q.; Xu, B. Endocrine Therapy-Based Strategies for Metastatic Breast Cancer with Different Endocrine Sensitivity Statuses: A Systematic Review and Network Meta-Analysis. Cancers 2022, 14, 6100. https://doi.org/10.3390/cancers14246100
Wang J, Han Y, Wang J, Li Q, Xu B. Endocrine Therapy-Based Strategies for Metastatic Breast Cancer with Different Endocrine Sensitivity Statuses: A Systematic Review and Network Meta-Analysis. Cancers. 2022; 14(24):6100. https://doi.org/10.3390/cancers14246100
Chicago/Turabian StyleWang, Jiani, Yiqun Han, Jiayu Wang, Qing Li, and Binghe Xu. 2022. "Endocrine Therapy-Based Strategies for Metastatic Breast Cancer with Different Endocrine Sensitivity Statuses: A Systematic Review and Network Meta-Analysis" Cancers 14, no. 24: 6100. https://doi.org/10.3390/cancers14246100
APA StyleWang, J., Han, Y., Wang, J., Li, Q., & Xu, B. (2022). Endocrine Therapy-Based Strategies for Metastatic Breast Cancer with Different Endocrine Sensitivity Statuses: A Systematic Review and Network Meta-Analysis. Cancers, 14(24), 6100. https://doi.org/10.3390/cancers14246100









