Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. XCAT Male and Female Phantoms
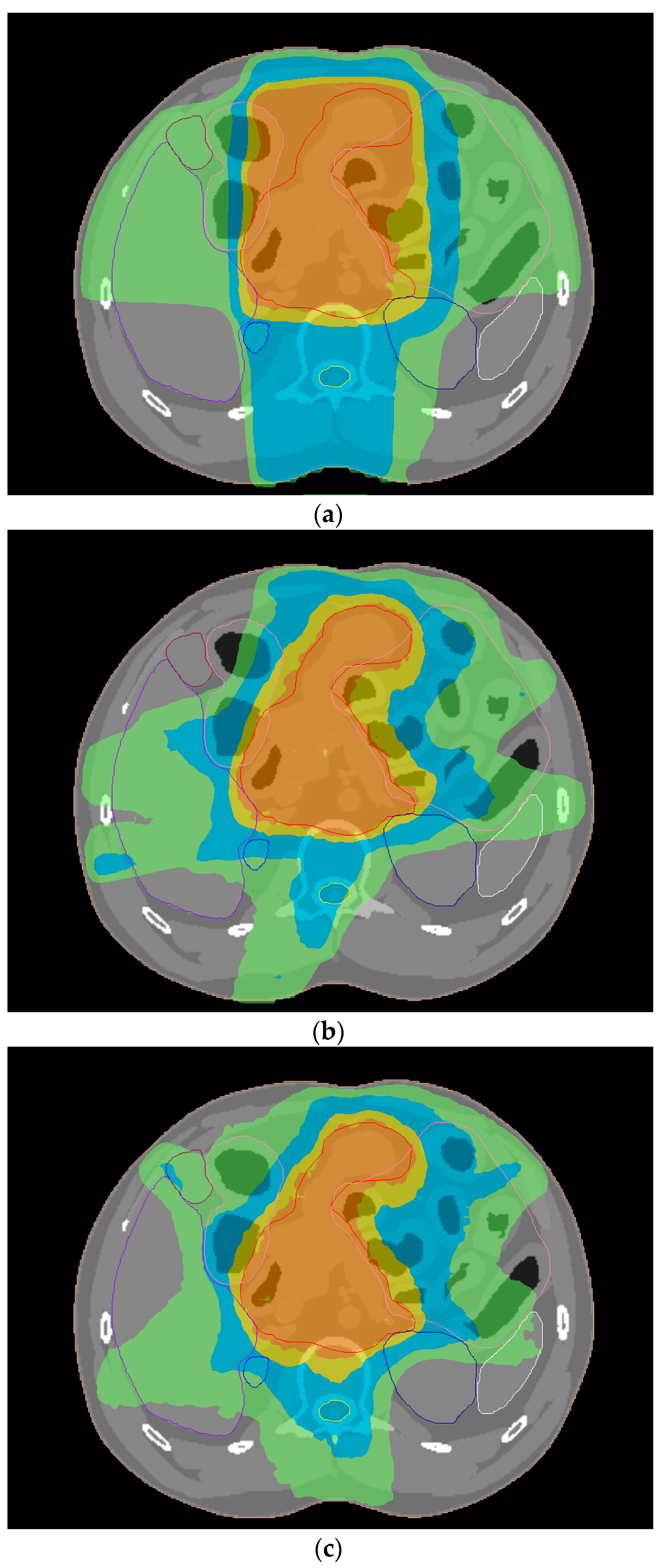
2.2. Treatment Planning for Gastric Cancer
2.3. TCP and NTCP Calculations
2.4. Development of the Software Tool
3. Results
3.1. Dosimetric Comparison of Treatment Plans
3.2. Radiobiological Comparison of Treatment Plans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gzito, B.G.; Palta, M.; Willett, C.G. Stomach cancer. In Perez and Brady’s Principles and Practice of Radiation Oncology, 7th ed.; Halperin, E.C., Wazer, D.A., Perez, C.A., Brady, L.W., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 1418–1449. [Google Scholar]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgey compared with surgey alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer Version 2. 2022. Available online: www.nccn.org (accessed on 8 February 2022).
- Li, C.; Wang, J.; Hu, W.; Zhang, Z. Radiation-induced liver injury in three-dimensional conformal radiation therapy (3D-CRT) for postoperative or locoregional recurrent gastric cancer: Risk factors and dose limitations. PLoS ONE 2015, 10, e0136288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.A.; Alber, M.; Deasy, J.O.; Jackson, A.; Jee, K.W.K.; Marks, L.B.; Martel, M.K.; Mayo, C.; Moiseenko, V.; Niemierko, A.; et al. The use and QA of biologically related models for treatment planning: Short report of the TG-166 of the therapy physics committee of the AAPM. Med. Phys. 2012, 39, 1386–1409. [Google Scholar]
- Mondlane, G.; Ureba, A.; Gubanski, M.; Lind, P.A.; Siegbahn, A. Estimation of risk of normal-tissue following gastric cancer radiotherapy with photon- or scanned proton-beams. Anticancer Res. 2018, 38, 2619–2625. [Google Scholar]
- Sharfo, A.W.M.; Stieler, F.; Kupfer, O.; Heijmen, B.J.M.; Dirkx, M.L.P.; Breedveld, S.; Wenz, F.; Lohr, F.; Boda-Heggemann, J.; Buergy, D. Automated VMAT planning for postoperative adjuvant treatment of advanced gastric cancer. Radiat. Oncol. 2018, 13, 74. [Google Scholar] [CrossRef]
- Segars, W.P.; Sturgeon, G.; Mendonca, S.; Grimes, J.; Tsui, B.M.W. 4D XCAT phantom for multimodality imaging research. Med. Phys. 2010, 37, 4902–4915. [Google Scholar] [CrossRef]
- Mazonakis, M.; Lyraraki, E.; Tolia, M.; Damilakis, J. 3D-CRT, IMRT and VMAT for flank irradiation due to pediatric Wilms tumor: A comparative planning study with XCAT phantoms. Phys. Med. 2022, 103, 89–97. [Google Scholar] [CrossRef]
- Shahzadeh, S.; Gholami, S.; Aghamiri, S.M.R.; Mahami, H.; Nabavi, M.; Kalantari, F. Evaluation of normal tissue complication probability in gated and conventional radiotherapy using 4D XCAT digital phantom. Comp. Biol. Med. 2018, 97, 21–29. [Google Scholar] [CrossRef]
- Taylor, S.; Lim, P.; Ahmad, R.; Alhadi, A.; Harris, W.; Rompokos, V.; D’Souza, D.; Gaze, M.; Gains, J.; Veiga, C. Risk of radiation-induced second malignant neoplasms from photon and proton radiotherapy in paediatric abdominal neuroblastoma. Phys. Imag. Radiat. Oncol. 2021, 19, 45–52. [Google Scholar] [CrossRef]
- Chung, H.T.; Lee, B.; Park, E.; Lu, J.J.; Xia, P. Can all centers plan intensity-modulate radiotherapy (IMRT) effectively? An external audit of dosimetric comparisons between three-dimensional conformal radiotherapy and IMRT for adjuvant chemoradiation for gastric cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Mazonakis, M.; Kachris, S.; Damilakis, J. Secondary bladder and rectal cancer risk estimates following standard fractionated and moderately hypofractionated VMAT for prostate carcinoma. Med. Phys. 2020, 47, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Mazonakis, M.; Lyraraki, E.; Damilakis, J. Lifetime radiation-induced sarcoma risk in patients subjected to IMRT or VMAT for uterine cervix carcinoma. Phys. Eng. Sci. Med. 2021, 44, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef]
- Kehwar, T.S. Analytical approach to estimate normal tissue complication probability using best fit of normal tissue tolerance doses into the NTCP equation of the linear quadratic model. J. Cancer Res. Ther. 2005, 1, 168–179. [Google Scholar] [CrossRef]
- Mehri-Kakavand, G.; Pursamidi, M.; Parwaie, W.; Ghorbani, M.; Khosravi, M.; Hosseini, S.M.; Meigooni, A.S. Assessment of field-in-field, 3-field and 4-field treatment planning methods for radiotherapy of gastro-esophageal junction cancer. J. Biomed. Phys. Eng. 2022, 12, 439–454. [Google Scholar]
- Mesbahi, A.; Rasouli, N.; Motlagh, B.N.; Mohammadzadeh, M. Radiobiological model-based comparison of three-dimensional conformal and intensity-modulated radiation therapy plans for nasopharyngeal carcinoma. Ir. J. Med. Phys. 2017, 14, 190–196. [Google Scholar]
- Puzhakal, N.; Kallikuzhiyil Kochunny, A.; Manthala Padannayil, N.; Singh, N.; Elavan Chalil, J.; Kulangarakath Umer, J. Comparison of treatment plans: A retrospective study by the method of radiobiological evaluation. Pol. J. Med. Phys. Eng. 2016, 22, 61–68. [Google Scholar] [CrossRef][Green Version]
- Taylor, M.L.; Yeo, U.A.; Supple, J.; Keehan, S.; Siva, S.; Kron, T.; Pham, D.; Haworth, A.; Franich, R.D. The importance of quasi-4D path-integrated dose accumulation for more accurate risk estimation in stereotactic liver radiotherapy. Technol. Cancer Res. Treat. 2016, 15, 428–436. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Meng, X.; Li, C.; Sun, X.; Shang, D.; Pang, L.; Li, Y.; Lu, J.; Yu, J. Dosimetric and radiobiological comparison of external beam radiotherapy using simultaneous integrated boost technique for esophageal cancer in different location. Front. Oncol. 2019, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.; Chin, R.K.; Cook, K.A.; Sheng, K.; Kishan, A.U.; Hedge, J.V.; Tenn, S.; Steinberg, M.L.; Cao, M. Automated non-coplanar VMAT for dose escalation in recurrent head and neck cancer patients. Cancers 2021, 13, 1910. [Google Scholar] [CrossRef] [PubMed]
- Okunief, P.; Niemierko, A.; Suit, H.D. Radiation dose-response of human tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G. The Python Library Reference, Release 3.8.2; Python Software Foundation: Wilmington, DE, USA, 2020. [Google Scholar]
- Gao, J.; Xu, B.; Lin, Y.; Xu, Z.; Huang, M.; Li, X.; Wu, X.; Chen, Y. Stereotactic body radiotherapy boost with the cyberknife for locally advanced cervical cancer: Dosimetric analysis and potential clinical benefits. Cancers 2022, 14, 5166. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, A.; Russo, S.; Bonora, M.; Vischioni, B.; Camarda, A.M.; Ingargiola, R.; Molinelli, S.; Ronchi, S.; Rossi, E.; Vai, A.; et al. A patient selection approach based on NTCP models and DVH parameters for definitive proton therapy in locally advanced sinonasal cancer patients. Cancers 2022, 14, 2678. [Google Scholar] [CrossRef]
- Monti, S.; Xu, T.; Mohan, R.; Liao, Z.; Palma, G.; Cella, L. Radiation-induced esophagitis in non-small-cell lung cancer patients: Voxel-based analysis and NTCP modeling. Cancers 2022, 14, 1883. [Google Scholar] [CrossRef]
- Jansen, E.P.M.; Nijkamp, J.; Gubanski, M.; Lind, P.A.R.M.; Verheij, M. Interobserver variation of clinical target volume delineation in gastric cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1166–1170. [Google Scholar] [CrossRef]
- Hansen, C.R.; Crijns, W.; Hussein, M.; Rossi, L.; Gallego, P.; Verbakel, W.; Unkelbach, J.; Thwaites, D.; Heijmen, B. Radiotherapy treatment planning study Guidelines (RATING): A framework for setting up and reporting on scientific treatment planning studies. Radiother. Oncol. 2020, 153, 67–78. [Google Scholar] [CrossRef]
- Valentin, J. Basic anatomical and physiological data for use in radiological protection: Reference values. ICRP Publication 89. Ann. ICRP 2002, 32, 1–277. [Google Scholar] [CrossRef]



| Organ-at-Risk | Parameter |
|---|---|
| Lungs | Dav < 20 Gy |
| V30Gy < 15% | |
| V20Gy < 20% | |
| V10Gy < 40% | |
| Heart | Dav < 30 Gy |
| V30Gy < 30% | |
| Kidneys | Dav < 18 Gy |
| V20Gy < 33% | |
| Liver | Dav < 25 Gy |
| V30Gy < 33% | |
| Bowel | V45Gy < 195 cc |
| Spinal cord | Dmax < 45 Gy |
| Organ-at-Risk | α | γ50 | TD50 (Gy) | α/β (Gy) | Endpoint |
|---|---|---|---|---|---|
| Brain stem | 7 | 3 | 65 | 3 | Necrosis |
| Parotid | 0.5 | 4 | 46 | 2 | Xerostomia |
| Ear (mid/ext) | 31 | 3 | 40 | 10 | Acute serious otitis |
| Ear (mid/ext) | 31 | 4 | 65 | 3 | Chronic serious otitis |
| TMJ | 14 | 4 | 72 | 3 | Limited joint function |
| Larynx | 12.5 | 4 | 70 | 3.8 | Laryngeal edema |
| Mandible | 14 | 4 | 72 | 3 | Limited joint function |
| Optic chiasm | 25 | 3 | 65 | 3 | Blindness |
| Optic nerve | 25 | 3 | 65 | 3 | Blindness |
| Eye lens | 3 | 1 | 18 | 1.2 | Cataract |
| Cochlea | 31 | 3 | 65 | 3 | Chronic serious otitis |
| Brain | 5 | 3 | 60 | 2.1 | Necrosis |
| Lung | 1 | 2 | 24.5 | 3 | Pneumonitis |
| Heart | 3 | 3 | 50 | 2.5 | Pericarditis |
| Liver | 3 | 3 | 40 | 1.5 | Liver failure |
| Kidney | 1 | 3 | 28 | 2.5 | Nephritis |
| Bowel | 6 | 4 | 55 | 3 | Obstruction |
| Stomach | 14 | 3 | 65 | 5 | Perforation |
| Esophagus | 19 | 4 | 68 | 3 | Perforation |
| Rectum | 8 | 4 | 80 | 3.9 | Necrosis/Stenosis/fistula |
| Bladder | 2 | 4 | 80 | 8 | Bladder contracture/volume loss |
| Femoral heads | 4 | 4 | 65 | 0.85 | Necrosis |
| Spinal cord | 7.4 | 4 | 66.5 | 3 | Myelitis/necrosis |
| Structure | Parameter | 3D-CRT | IMRT | VMAT | ||||
|---|---|---|---|---|---|---|---|---|
| 6 MV | 10 MV | 15 MV | 6 MV | 10 MV | 6 MV | 10 MV | ||
| PTV | V42.75Gy(%) | 98.0 | 98.3 | 97.3 | 100.0 | 99.9 | 99.9 | 100.0 |
| HI | 1.10 | 1.09 | 1.09 | 1.03 | 1.04 | 1.04 | 1.03 | |
| CN | 0.55 | 0.61 | 0.58 | 0.86 | 0.87 | 0.87 | 0.87 | |
| Left Lung | V10Gy(%) | 21.5 | 21.5 | 21.3 | 29.3 | 31.3 | 28.3 | 27.5 |
| V20Gy(%) | 13.0 | 12.4 | 11.7 | 13.8 | 13.5 | 12.4 | 11.9 | |
| V30Gy(%) | 6.0 | 5.9 | 5.7 | 7.0 | 6.8 | 6.6 | 6.1 | |
| Dav (Gy) | 6.8 | 6.6 | 6.4 | 8.4 | 8.2 | 8.1 | 7.9 | |
| Right Lung | V10Gy(%) | 13.6 | 13.5 | 13.3 | 24.5 | 22.8 | 27.4 | 27.9 |
| V20Gy(%) | 6.9 | 5.9 | 4.8 | 7.6 | 7.6 | 7.7 | 8.1 | |
| V30Gy(%) | 1.1 | 1.1 | 1.1 | 2.9 | 2.8 | 2.9 | 3.0 | |
| Dav (Gy) | 4.6 | 4.4 | 4.2 | 6.8 | 6.5 | 7.0 | 6.8 | |
| Heart | V30Gy(%) | 12.9 | 13.2 | 11.2 | 8.2 | 8.2 | 8.3 | 7.9 |
| Dav (Gy) | 17.6 | 17.5 | 17.2 | 16.5 | 16.4 | 16.2 | 16.0 | |
| Left Kidney | V20Gy(%) | 26.0 | 25.4 | 24.8 | 15.4 | 14.6 | 14.4 | 14.0 |
| Dav (Gy) | 15.7 | 15.5 | 15.1 | 12.2 | 11.3 | 12.8 | 12.6 | |
| Right Kidney | V20Gy(%) | 21.7 | 20.2 | 16.9 | 15.5 | 15.2 | 10.5 | 10.8 |
| Dav (Gy) | 12.6 | 12.4 | 12.0 | 10.5 | 9.9 | 11.0 | 10.9 | |
| Liver | V30Gy(%) | 20.4 | 20.4 | 20.3 | 19.2 | 20.0 | 17.7 | 18.2 |
| Dav (Gy) | 22.6 | 22.4 | 21.9 | 20.8 | 21.2 | 21.1 | 21.0 | |
| Bowel | V45Gy (cc) | 151.8 | 165.7 | 134.4 | 69.7 | 68.2 | 68.2 | 71.7 |
| Spinal cord | Dmax(Gy) | 35.6 | 35.1 | 34.5 | 36.0 | 37.7 | 36.3 | 36.7 |
| Structure | Parameter | 3D-CRT | IMRT | VMAT | ||||
|---|---|---|---|---|---|---|---|---|
| 6 MV | 10 MV | 15 MV | 6 MV | 10 MV | 6 MV | 10 MV | ||
| PTV | V42.75Gy(%) | 96.7 | 97.4 | 95.8 | 100.0 | 100.0 | 99.9 | 99.9 |
| HI | 1.09 | 1.08 | 1.08 | 1.03 | 1.03 | 1.04 | 1.04 | |
| CN | 0.35 | 0.52 | 0.44 | 0.84 | 0.84 | 0.85 | 0.86 | |
| Left Lung | V10Gy(%) | 12.3 | 12.4 | 12.3 | 17.9 | 17.9 | 18.2 | 18.1 |
| V20Gy(%) | 7.2 | 7.2 | 7.1 | 10.8 | 10.2 | 10.5 | 9.5 | |
| V30Gy(%) | 3.9 | 3.8 | 3.4 | 5.7 | 5.5 | 5.4 | 5.2 | |
| Dav (Gy) | 4.3 | 4.3 | 4.1 | 5.8 | 5.6 | 5.7 | 5.5 | |
| Right Lung | V10Gy(%) | 5.8 | 5.8 | 5.7 | 13.0 | 12.6 | 14.3 | 14.1 |
| V20Gy(%) | 1.7 | 1.6 | 1.5 | 5.1 | 4.8 | 5.7 | 6.0 | |
| V30Gy(%) | 0.4 | 0.4 | 0.4 | 2.0 | 1.7 | 1.9 | 1.9 | |
| Dav (Gy) | 2.5 | 2.4 | 2.3 | 4.2 | 4.0 | 4.4 | 4.4 | |
| Heart | V30Gy(%) | 3.7 | 3.9 | 3.3 | 5.4 | 5.7 | 6.0 | 5.4 |
| Dav (Gy) | 9.9 | 9.7 | 9.4 | 10.9 | 10.8 | 10.9 | 10.2 | |
| Left Kidney | V20Gy(%) | 20.9 | 20.9 | 20.1 | 11.7 | 11.3 | 10.2 | 9.1 |
| Dav (Gy) | 12.3 | 12.3 | 12.0 | 10.8 | 10.6 | 11.6 | 11.4 | |
| Right Kidney | V20Gy(%) | 18.8 | 18.5 | 17.9 | 15.3 | 15.6 | 10.5 | 10.7 |
| Dav (Gy) | 10.0 | 9.9 | 9.9 | 9.6 | 9.3 | 9.8 | 9.8 | |
| Liver | V30Gy(%) | 18.7 | 19.0 | 18.6 | 18.4 | 18.3 | 17.6 | 17.6 |
| Dav (Gy) | 22.1 | 22.0 | 21.5 | 19.8 | 19.5 | 19.9 | 19.9 | |
| Bowel | V45Gy (cc) | 85.4 | 94.7 | 69.6 | 52.7 | 52.2 | 52.3 | 50.8 |
| Spinal cord | Dmax(Gy) | 31.7 | 31.1 | 30.4 | 33.1 | 33.3 | 32.3 | 35.8 |
| Structure | Parameter | 3D-CRT | IMRT | VMAT | ||||
|---|---|---|---|---|---|---|---|---|
| 6 MV | 10 MV | 15 MV | 6 MV | 10 MV | 6 MV | 10 MV | ||
| PTV | TCP (%) | 50.0 | 50.2 | 49.7 | 51.4 | 51.3 | 51.3 | 51.3 |
| Left Lung | NTCP (%) | 2.4 × 10−3 | 2.0 × 10−3 | 1.5 × 10−3 | 1.3 × 10−2 | 1.2 × 10−2 | 9.5 × 10−3 | 7.5 × 10−3 |
| Right Lung | NTCP (%) | 9.9 × 10−5 | 7.2 × 10−5 | 4.8 × 10−5 | 2.4 × 10−3 | 1.6 × 10−3 | 3.0 × 10−3 | 2.6 × 10−3 |
| Heart | NTCP (%) | 9.2 × 10−3 | 5.6 × 10−3 | 4.8 × 10−3 | 1.6 × 10−3 | 1.5 × 10−3 | 1.4 × 10−3 | 1.2 × 10−3 |
| Left Kidney | NTCP (%) | 5.1 × 10−2 | 4.5 × 10−2 | 3.4 × 10−2 | 2.3 × 10−3 | 9.3 × 10−4 | 4.1 × 10−3 | 3.5 × 10−3 |
| Right Kidney | NTCP (%) | 3.6 × 10−3 | 2.9 × 10−3 | 2.2 × 10−3 | 3.5 × 10−4 | 1.7 × 10−4 | 6.7 × 10−4 | 6.7 × 10−4 |
| Liver | NTCP (%) | 7.4 × 10−1 | 7.3 × 10−1 | 6.2 × 10−1 | 3.0 × 10−1 | 3.3 × 10−1 | 2.7 × 10−1 | 2.7 × 10−1 |
| Bowel | NTCP (%) | 6.2 × 10−3 | 6.5 × 10−3 | 5.2 × 10−3 | 3.8 × 10−3 | 3.8 × 10−3 | 3.6 × 10−3 | 3.8 × 10−3 |
| Spinal cord | NTCP (%) | 9.1 × 10−5 | 7.2 × 10−5 | 5.0 × 10−5 | 9.2 × 10−6 | 1.4 × 10−5 | 1.5 × 10−5 | 1.3 × 10−5 |
| Structure | Parameter | 3D-CRT | IMRT | VMAT | ||||
|---|---|---|---|---|---|---|---|---|
| 6 MV | 10 MV | 15 MV | 6 MV | 10 MV | 6 MV | 10 MV | ||
| PTV | TCP (%) | 48.7 | 49.3 | 48.5 | 51.5 | 51.4 | 51.4 | 51.3 |
| Left Lung | NTCP (%) | 6.5 × 10−5 | 5.4 × 10−5 | 4.3 × 10−5 | 6.2 × 10−4 | 5.0 × 10−4 | 6.1 × 10−4 | 4.1 × 10−4 |
| Right Lung | NTCP (%) | 8.3 × 10−7 | 5.5 × 10−7 | 3.4 × 10−7 | 5.1 × 10−5 | 3.4 × 10−5 | 6.8 × 10−5 | 6.8 × 10−5 |
| Heart | NTCP (%) | 1.4 × 10−4 | 1.5 × 10−4 | 1.3 × 10−4 | 1.9 × 10−4 | 2.0 × 10−4 | 2.1 × 10−4 | 1.7 × 10−4 |
| Left Kidney | NTCP (%) | 3.1 × 10−3 | 2.7 × 10−3 | 1.8 × 10−3 | 5.3 × 10−4 | 4.1 × 10−4 | 1.2 × 10−3 | 1.1 × 10−3 |
| Right Kidney | NTCP (%) | 2.1 × 10−4 | 1.9 × 10−4 | 1.8 × 10−4 | 9.1 × 10−5 | 7.7 × 10−5 | 1.4 × 10−4 | 1.6 × 10−4 |
| Liver | NTCP (%) | 5.1 × 10−1 | 5.2 × 10−1 | 4.5 × 10−1 | 2.9 × 10−1 | 2.7 × 10−1 | 2.4 × 10−1 | 2.4 × 10−1 |
| Bowel | NTCP (%) | 6.2 × 10−3 | 6.4 × 10−3 | 5.2 × 10−3 | 4.3 × 10−3 | 4.2 × 10−3 | 4.2 × 10−3 | 4.0 × 10−3 |
| Spinal cord | NTCP (%) | 3.0 × 10−5 | 2.5 × 10−5 | 1.8 × 10−5 | 5.3 × 10−6 | 6.5 × 10−6 | 9.4 × 10−6 | 5.9 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazonakis, M.; Tzanis, E.; Lyraraki, E.; Damilakis, J. Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer. Cancers 2022, 14, 6098. https://doi.org/10.3390/cancers14246098
Mazonakis M, Tzanis E, Lyraraki E, Damilakis J. Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer. Cancers. 2022; 14(24):6098. https://doi.org/10.3390/cancers14246098
Chicago/Turabian StyleMazonakis, Michalis, Eleftherios Tzanis, Efrossyni Lyraraki, and John Damilakis. 2022. "Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer" Cancers 14, no. 24: 6098. https://doi.org/10.3390/cancers14246098
APA StyleMazonakis, M., Tzanis, E., Lyraraki, E., & Damilakis, J. (2022). Automatic Radiobiological Comparison of Radiation Therapy Plans: An Application to Gastric Cancer. Cancers, 14(24), 6098. https://doi.org/10.3390/cancers14246098






