Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods—Search Strategy
3. Results
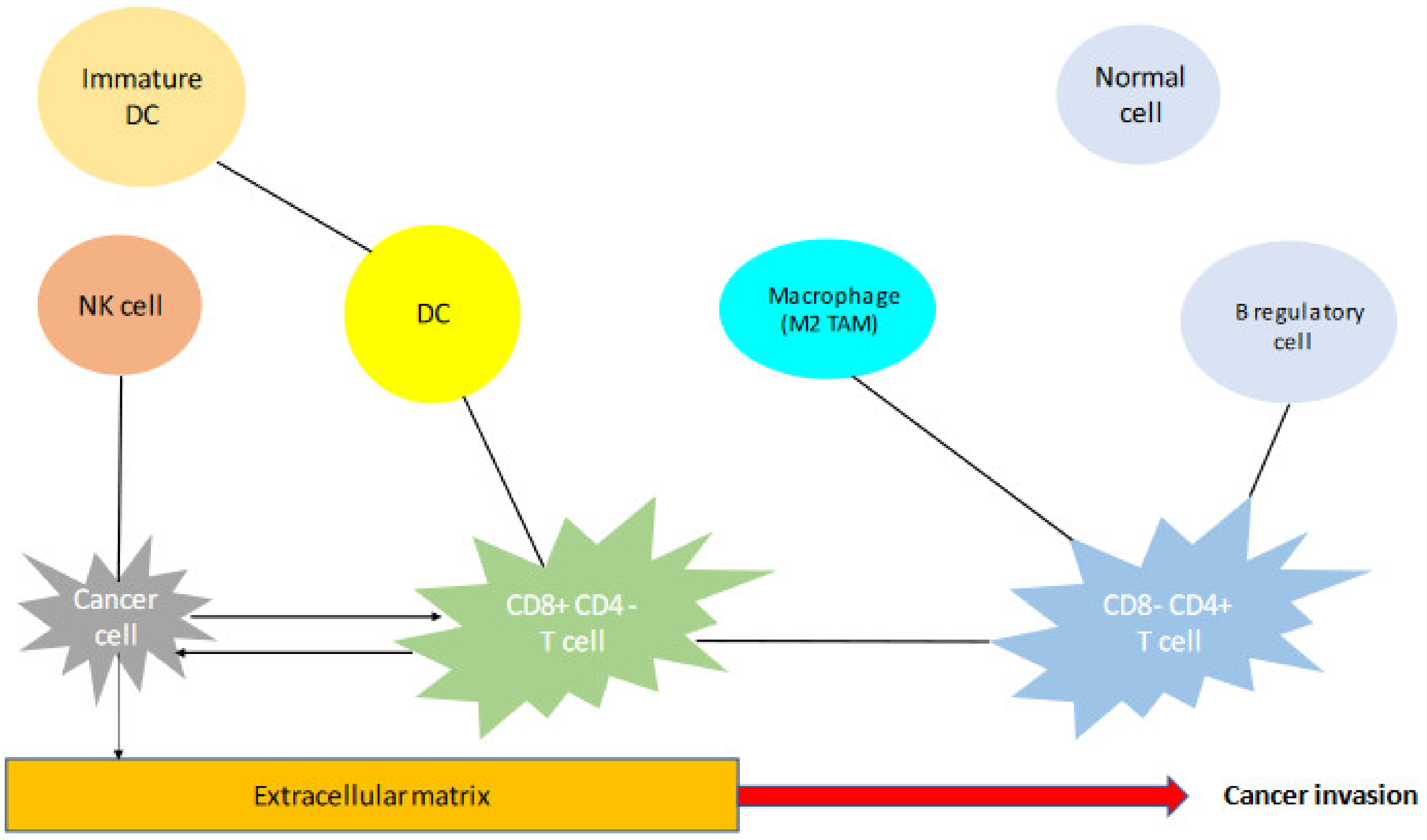
3.1. The Particularities of TME of TET and the Limitations in the Use of Immunotherapy
3.2. The Impact of PD-L1 Expression and the Presence of Different Cell Types in the TME of TET
3.3. Tumor Mutational Burden (TMB) and Immune Infiltration
3.4. Tumor-Associated Macrophages (TAMs)
3.5. The Heat Shock Protein 27 and 70 (HSP27 and 70) Expression
3.6. Fibronectin
3.7. SOX (SRY-Related High-Mobility Group Box) 9
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E.A. Tumor Acidity as Evolutionary Spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Ramishetti, S.; Huang, L. Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy. Ther. Deliv. 2012, 3, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Wright, C.D. Management of thymomas. Crit Rev. Oncol. Hematol. 2008, 65, 109–120. [Google Scholar] [CrossRef]
- Meng, F.; Wang, S.; Zhang, J.; Yan, Y.; Wang, C.; Yang, C.; Guan, Z.; Wang, C. Alteration in gene expression profiles of thymoma: Genetic differences and potential novel targets. Thorac. Cancer 2019, 10, 1129–1135. [Google Scholar] [CrossRef]
- Hou, X.; Lin, S.; Liu, Y.; Wang, K.; Yu, Z.; Jia, J.; Yu, J.; Zheng, W.; Bai, J.; Chang, L.; et al. Analysis of the tumor microen-vironment and mutation burden identifies prognostic features in thymic epithelial tumors. Am. J. Cancer Res. 2022, 12, 2387–2396. [Google Scholar]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Arbour, K.C.; Naidoo, J.; Steele, K.E.; Ni, A.; Moreira, A.L.; Rekhtman, N.; Robbins, P.B.; Karakunnel, J.; Rimner, A.; Huang, J.; et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS ONE 2017, 12, e0182665. [Google Scholar] [CrossRef]
- Giaccone, G.; Thompson, J.; McGuire, C.; Manning, M.; Kallakury, B.; Chahine, J.J.; Subramaniam, D.S.; Liu, S.V.; Gibney, G.T.; Kim, C.; et al. Pembrolizumab in patients with recurrent thymic carcinoma: Results of a phase II study. J. Clin. Oncol. 2017, 35, 8573. [Google Scholar] [CrossRef]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [CrossRef]
- Tateo, V.; Manuzzi, L.; De Giglio, A.; Parisi, C.; Lamberti, G.; Campana, D.; Pantaleo, M. Immunobiology of Thymic Epithelial Tumors: Implications for Immunotherapy with Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 9056. [Google Scholar] [CrossRef] [PubMed]
- Bedekovics, J.; Beke, L.; Mokanszki, A.; Szilagyi, S.; Mehes, G. Programmed Death-ligand 1 (PD-L1) Expression in Thymic Epithelial Tumors. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 1–9. [Google Scholar] [CrossRef]
- Padda, S.K.; Riess, J.W.; Schwartz, E.J.; Tian, L.; Kohrt, H.E.; Neal, J.W.; West, R.B.; Wakelee, H.A. Diffuse High Intensity PD–L1 Staining in Thymic Epithelial Tumors. J. Thorac. Oncol. 2015, 10, 500–508. [Google Scholar] [CrossRef]
- Katsuya, Y.; Fujita, Y.; Horinouchi, H.; Ohe, Y.; Watanabe, S.-I.; Tsuta, K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015, 88, 154–159. [Google Scholar] [CrossRef]
- Yokoyama, S.; Miyoshi, H.; Nakashima, K.; Shimono, J.; Hashiguchi, T.; Mitsuoka, M.; Takamori, S.; Akagi, Y.; Ohshima, K. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin. Cancer Res. 2016, 22, 4727–4734. [Google Scholar] [CrossRef]
- Su, Y.; Ou, Y.; Chen, Y.; Ma, X. Construction of immune-related LncRNAs classifier to predict prognosis and immunotherapy response in thymic epithelial tumors. Biosci. Rep. 2022, 42, BSR20220317. [Google Scholar] [CrossRef]
- Katsuya, Y.; Horinouchi, H.; Seto, T.; Umemura, S.; Hosomi, Y.; Satouchi, M.; Nishio, M.; Kozuki, T.; Hida, T.; Sukigara, T.; et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur. J. Cancer 2019, 113, 78–86. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.S.; Ku, B.M.; Choi, Y.-L.; Cristescu, R.; Han, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J. Clin. Oncol. 2019, 37, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Miyoshi, H.; Nishi, T.; Hashiguchi, T.; Mitsuoka, M.; Takamori, S.; Akagi, Y.; Kakuma, T.; Ohshima, K. Clinicopathologic and Prognostic Implications of Programmed Death Ligand 1 Expression in Thymoma. Ann. Thorac. Surg. 2016, 101, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Wu, L.X.; Li, X.F.; Zhu, Y.C.; Pan, W.W.; Wang, W.X.; Xu, C.W.; Huang, J.H.; Wu, M.H.; Du, K.Q. PD-L1 Ex-pression Level in Different Thymoma Stages and Thymic Carcinoma: A Meta-Analysis. Tumori 2020, 106, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Miyoshi, H. Thymic tumors and immune checkpoint inhibitors. J. Thorac. Dis. 2018, 10 (Suppl. S13), S1509–S1515. [Google Scholar] [CrossRef]
- Weissferdt, A.; Fujimoto, J.; Kalhor, N.; Rodriguez, J.; Bassett, R.; Wistuba, I.; Moran, C. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod. Pathol. 2017, 30, 826–833. [Google Scholar] [CrossRef]
- Rajan, A.; Heery, C.R.; Thomas, A.; Mammen, A.L.; Perry, S.; O’Sullivan Coyne, G.; Guha, U.; Berman, A.; Szabo, E.; Madan, R.A.; et al. Efficacy and Tolerability of Anti-programmed Death-Ligand 1 (PD-L1) Antibody (Avelumab) Treatment in Advanced Thymoma. J. Immunother. Cancer 2019, 7, 269. [Google Scholar] [CrossRef]
- Masaoutis, C.; Palamaris, K.; Kokkali, S.; Levidou, G.; Theocharis, S. Unraveling the Immune Microenvironment of Thymic Epithelial Tumors: Implications for Autoimmunity and Treatment. Int. J. Mol. Sci. 2022, 23, 7864. [Google Scholar] [CrossRef]
- Fend, F.; Kirchner, T.; Marx, A.; Müller-Hermelink, H.-K. B-cells in thymic epithelial tumours. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 63, 241–247. [Google Scholar] [CrossRef]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.L.; Smyth, M.J. Anti-TIM3 Antibody Promotes T Cell IFN-γ–Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef]
- Parkhurst, M.; Gros, A.; Pasetto, A.; Prickett, T.; Crystal, J.S.; Robbins, P.; Rosenberg, S.A. Isolation of T-Cell Receptors Specifically Reactive with Mutated Tumor-Associated Antigens from Tumor-Infiltrating Lymphocytes Based on CD137 Ex-pression. Clin. Cancer Res. 2017, 23, 2491–2505. [Google Scholar] [CrossRef]
- Ros-Martínez, S.; Navas-Carrillo, D.; Alonso-Romero, J.L.; Orenes-Piñero, E. Immunoscore: A novel prognostic tool. Association with clinical outcome, response to treatment and survival in several malignancies. Crit. Rev. Clin. Lab. Sci. 2020, 57, 432–443. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–16. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, L.; Li, C.; Liu, L.; Ye, X.; Han, J. Prognostic Model of Eleven Genes Based on the Immune Microenvironment in Patients With Thymoma. Front. Genet. 2022, 13. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Kaul, D.; Ismail, M.; Badakhshi, H. Significance of tumor mutation burden and immune infiltration in thymic epithelial tumors. Thorac. Cancer 2021, 12, 1995–2006. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Buttner, R.; Longshore, J.W.; Lopez-Rios, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Imple-menting TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef]
- Sato, J.; Kitano, S.; Motoi, N.; Ino, Y.; Yamamoto, N.; Watanabe, S.; Ohe, Y.; Hiraoka, N. CD20 + Tumor-infiltrating Immune Cells and CD204 + M2 Macrophages Are Associated with Prognosis in Thymic Carcinoma. Cancer Sci. 2020, 111, 1921–1932. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef]
- Migita, T.; Sato, E.; Saito, K.; Mizoi, T.; Shiiba, K.-I.; Matsuno, S.; Nagura, H.; Ohtani, H. Differing expression of MMPs-1 and -9 and urokinase receptor between diffuse- and intestinal-type gastric carcinoma. Int. J. Cancer 1999, 84, 74–79. [Google Scholar] [CrossRef]
- Souza-Moreira, L.; Soares, V.C.; Dias, S.; Bozza, P.T. Adipose-derived mesenchymal stromal cells modulate lipid metabolism and lipid droplet biogenesis via AKT/mTOR -PPARgamma Signalling in macrophages. Sci. Rep. 2019, 9, 20304. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, T.; Shen, X.N.; Xia, X.F.; Xu, G.D.; Bai, X.L.; Liang, T.B. Macrophage-induced tumor angiogenesis is reg-ulated by the TSC2-mTOR pathway. Cancer Res. 2012, 72, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Lazaris, A.C.; Theodoropoulos, G.E.; Davaris, P.S.; Panoussopoulos, D.; Nakopoulou, L.; Kittas, C.; Golematis, B.C. Heat shock protein 70 and HLA-DR molecules tissue expression. Dis. Colon Rectum 1995, 38, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Glaessgen, A.; Jonmarker, S.; Lindberg, A.; Nilsson, B.; Lewensohn, R.; Ekman, P.; Valdman, A.; Egevad, L. Heat shock proteins 27, 60 and 70 as prognostic markers of prostate cancer. Apmis 2008, 116, 888–895. [Google Scholar] [CrossRef]
- Joo, M.; Chi, J.G.; Lee, H. Expressions of HSP70 and HSP27 in Hepatocellular Carcinoma. J. Korean Med Sci. 2005, 20, 829–834. [Google Scholar] [CrossRef]
- Małusecka, E.; Krzyzowska-Gruca, S.; Gawrychowski, J.; Fiszer-Kierzkowska, A.; Kołosza, Z.; Krawczyk, Z. Stress proteins HSP27 and HSP70i predict survival in non-small cell lung carcinoma. Anticancer Res. 2008, 28, 501–506. [Google Scholar]
- Mohtasham, N.; Babakoohi, S.; Montaser-Kouhsari, L.; Memar, B.; Salehinejad, J.; Rahpeyma, A.; Khageh-Ahmady, S.; Marouzi, P.; Firooz, A.; Pazoki-Toroudi, H.; et al. The expression of heat shock proteins 27 and 105 in squamous cell carci-noma of the tongue and relationship with clinicopathological index. Med. Oral. Pathol. Oral. Cir. Bucal. 2011, 16, 730–735. [Google Scholar] [CrossRef]
- Janik, S.; Schiefer, A.I.; Bekos, C.; Hacker, P.; Haider, T.; Moser, J.; Klepetko, W.; Müllauer, L.; Ankersmit, H.J.; Moser, B. HSP27 and 70 expression in thymic epithelial tumors and benign thymic alterations: Diagnostic, prognostic and physiologic implications. Sci. Rep. 2016, 6, 24267. [Google Scholar] [CrossRef]
- McConnell, J.R.; McAlpine, S.R. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg. Med. Chem. Lett. 2013, 23, 1923–1928. [Google Scholar] [CrossRef]
- Rick, J.W.; Chandra, A.; Ore, C.D.; Nguyen, A.T.; Yagnik, G.; Aghi, M.K. Fibronectin in malignancy: Cancer-specific alterations, protumoral effects, and therapeutic implications. Semin. Oncol. 2019, 46, 284–290. [Google Scholar] [CrossRef]
- Menrad, A.; Menssen, H.D. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin. Ther. Targets 2005, 9, 491–500. [Google Scholar] [CrossRef]
- Petrini, I.; Sollini, M.; Bartoli, F.; Barachini, S.; Montali, M.; Pardini, E.; Burzi, I.S.; Erba, P.A. ED-B-Containing Isoform of Fibronectin in Tumor Microenvironment of Thymomas: A Target for a Theragnostic Approach. Cancers 2022, 14, 2592. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, L.; Luo, W.; Zhao, Y.; Nashan, B.; Yu, F.; Liu, Y. Diagnostic and Prognostic Significances of SOX9 in Thymic Epithelial Tumor. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2019, 67, 122–153. [Google Scholar] [CrossRef]
- Panda, M.; Tripathi, S.K.; Biswal, B.K. SOX9: An emerging driving factor from cancer progression to drug resistance. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188517. [Google Scholar] [CrossRef]
- Matheu, A.; Collado, M.; Wise, C.; Manterola, L.; Cekaite, L.; Tye, A.J.; Canamero, M.; Bujanda, L.; Schedl, A.; Cheah, K.S.; et al. Oncogenicity of the Developmental Transcription Factor Sox9. Cancer Res. 2012, 72, 1301–1315. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
| Favorable Prognosis |
|---|
| High PD-L1 expression on tumor cells (controversial) [11] |
| Moderate and high levels of CD3+ TILs (IHC2 or 3 vs IHC1) [11] |
| Higher proportion of neutrophils, Th2 cells, TILs, iDCs, CD8+ T cells [31] |
| Dismal Prognosis |
| High PD-L1 expression on tumor cells (controversial) [16] |
| Lower expression level of HMGB1 [9] |
| Higher proportion of immune macrophages, NK cells, Treg, Type II IFN response, and aDCs [31] |
| High tumor mutational burden [35] |
| Tumor-associated macrophages (further investigation is needed) [38,39,40,41,42] |
| HSP27 and 70 expression [42,43,44,45,46,47] |
| High SOX9 expression [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrafiotis, A.C.; Siozopoulou, V.; Hendriks, J.M.H.; Pauwels, P.; Koljenovic, S.; Van Schil, P.E. Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review. Cancers 2022, 14, 6082. https://doi.org/10.3390/cancers14246082
Agrafiotis AC, Siozopoulou V, Hendriks JMH, Pauwels P, Koljenovic S, Van Schil PE. Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review. Cancers. 2022; 14(24):6082. https://doi.org/10.3390/cancers14246082
Chicago/Turabian StyleAgrafiotis, Apostolos C., Vasiliki Siozopoulou, Jeroen M. H. Hendriks, Patrick Pauwels, Senada Koljenovic, and Paul E. Van Schil. 2022. "Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review" Cancers 14, no. 24: 6082. https://doi.org/10.3390/cancers14246082
APA StyleAgrafiotis, A. C., Siozopoulou, V., Hendriks, J. M. H., Pauwels, P., Koljenovic, S., & Van Schil, P. E. (2022). Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review. Cancers, 14(24), 6082. https://doi.org/10.3390/cancers14246082







