NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacologic Agents
2.2. Cell Culture
2.3. MTT Assay
2.4. Immunoblots
2.5. Fluorescence-Activated Cell Sorting
2.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. RNA Sequencing (RNA-Seq)
2.8. In Vivo Experiments
2.8.1. Experiments in Mice
2.8.2. Experiments in Rats
2.9. Statistical Analysis
3. Results
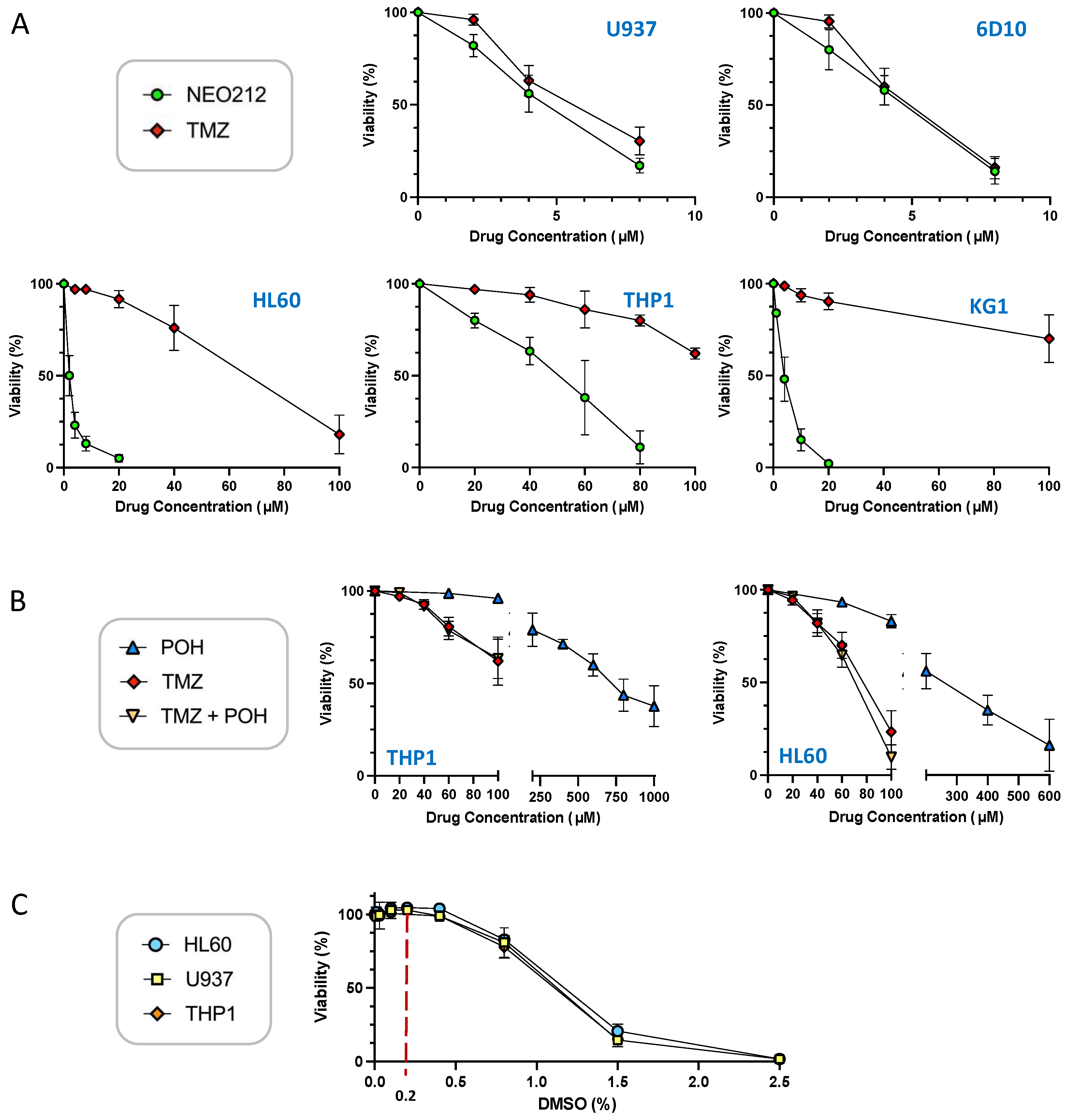
3.1. NEO212 Is Cytotoxic against AML Cells In Vitro
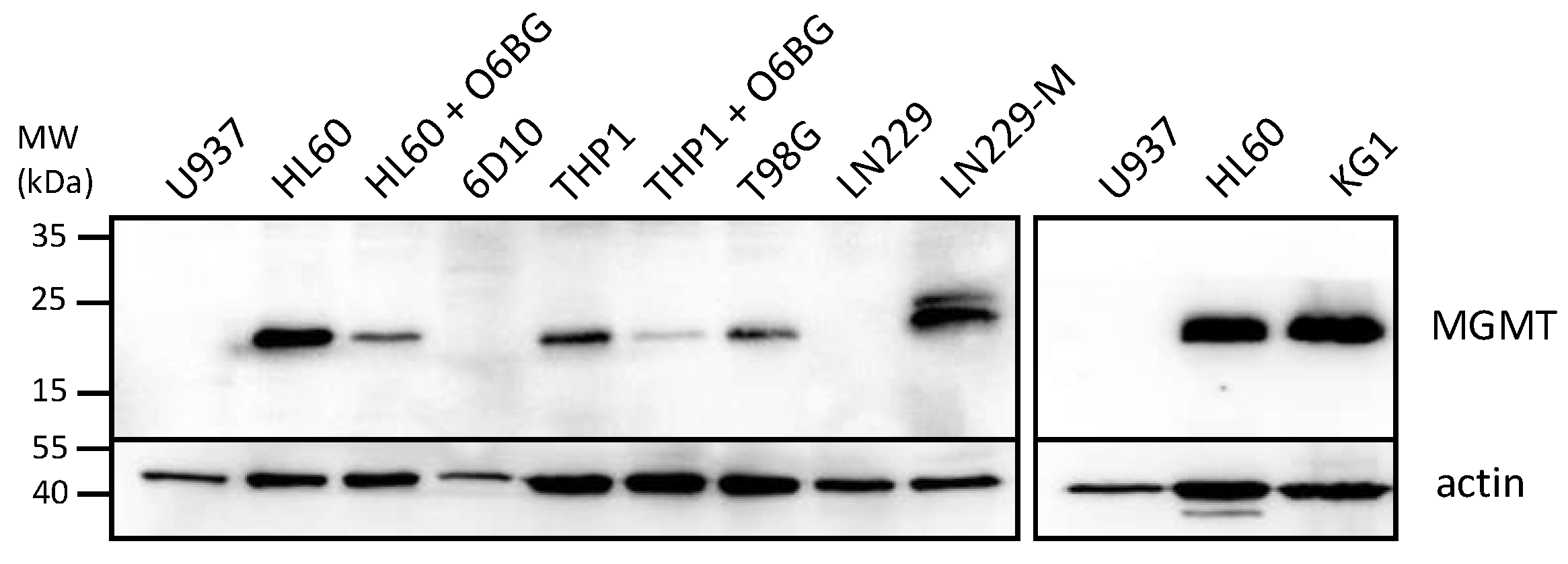
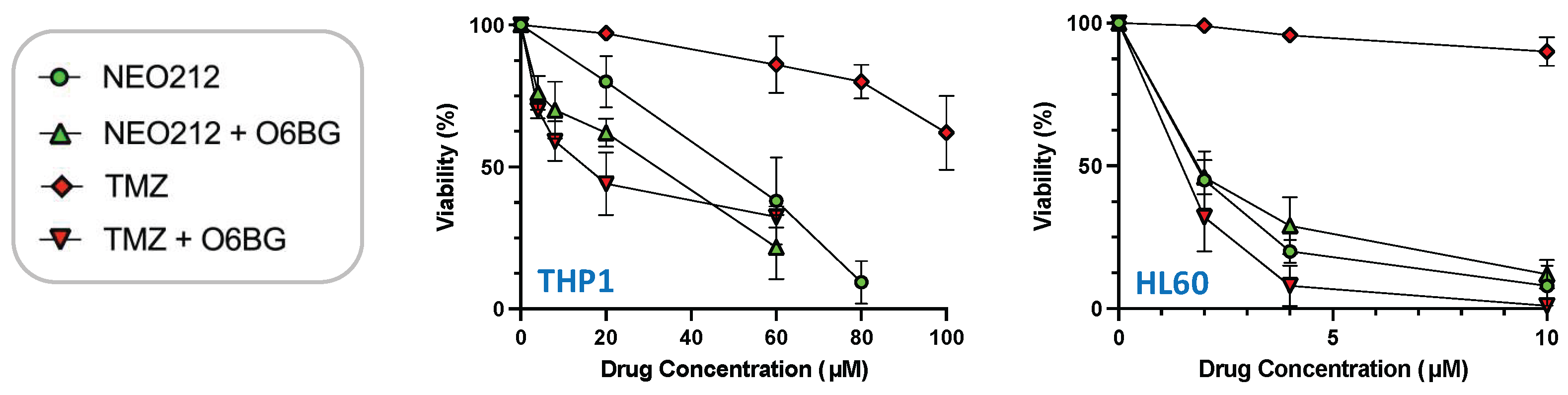
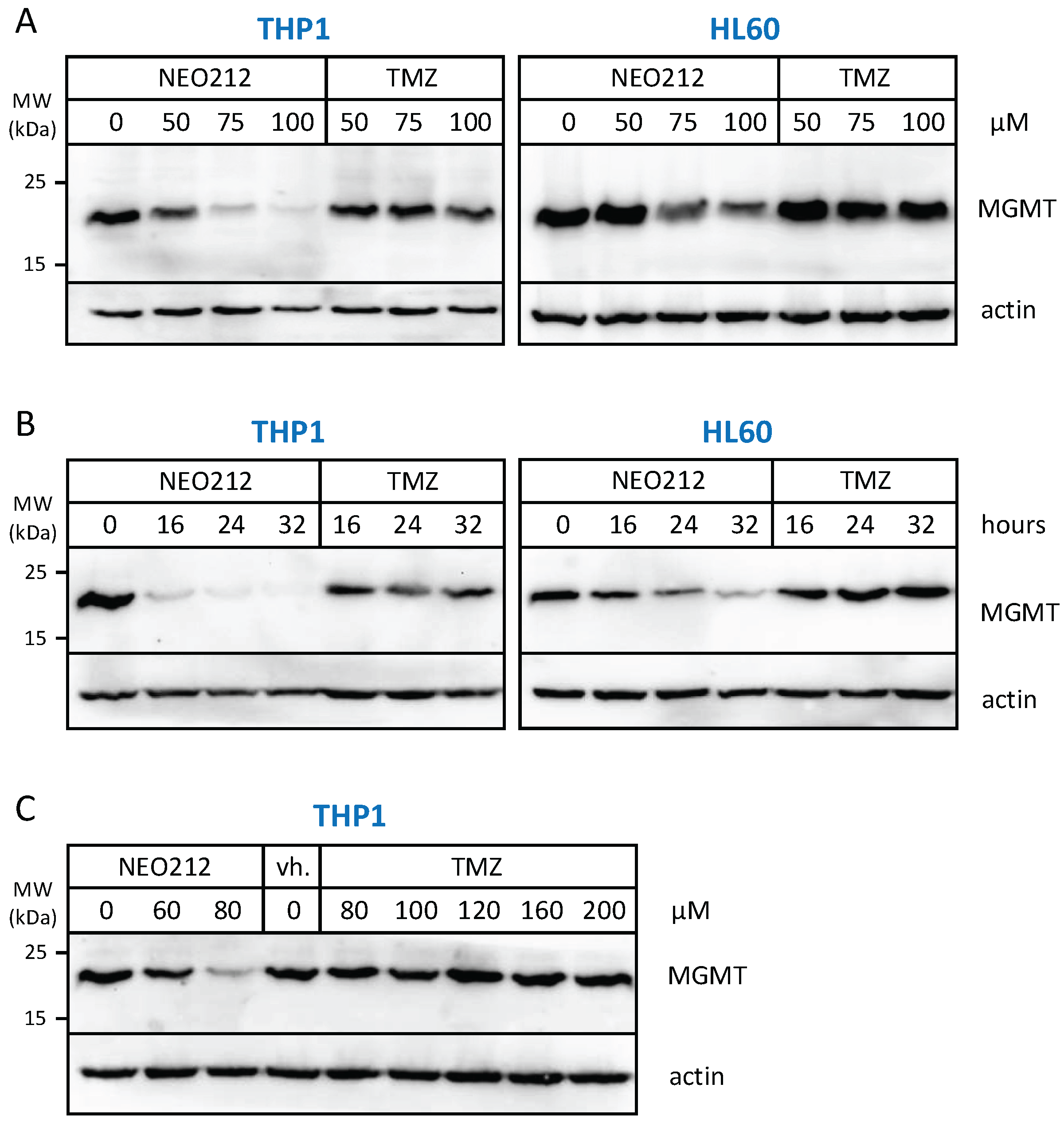
3.2. NEO212 Overcomes MGMT-Mediated Drug Resistance
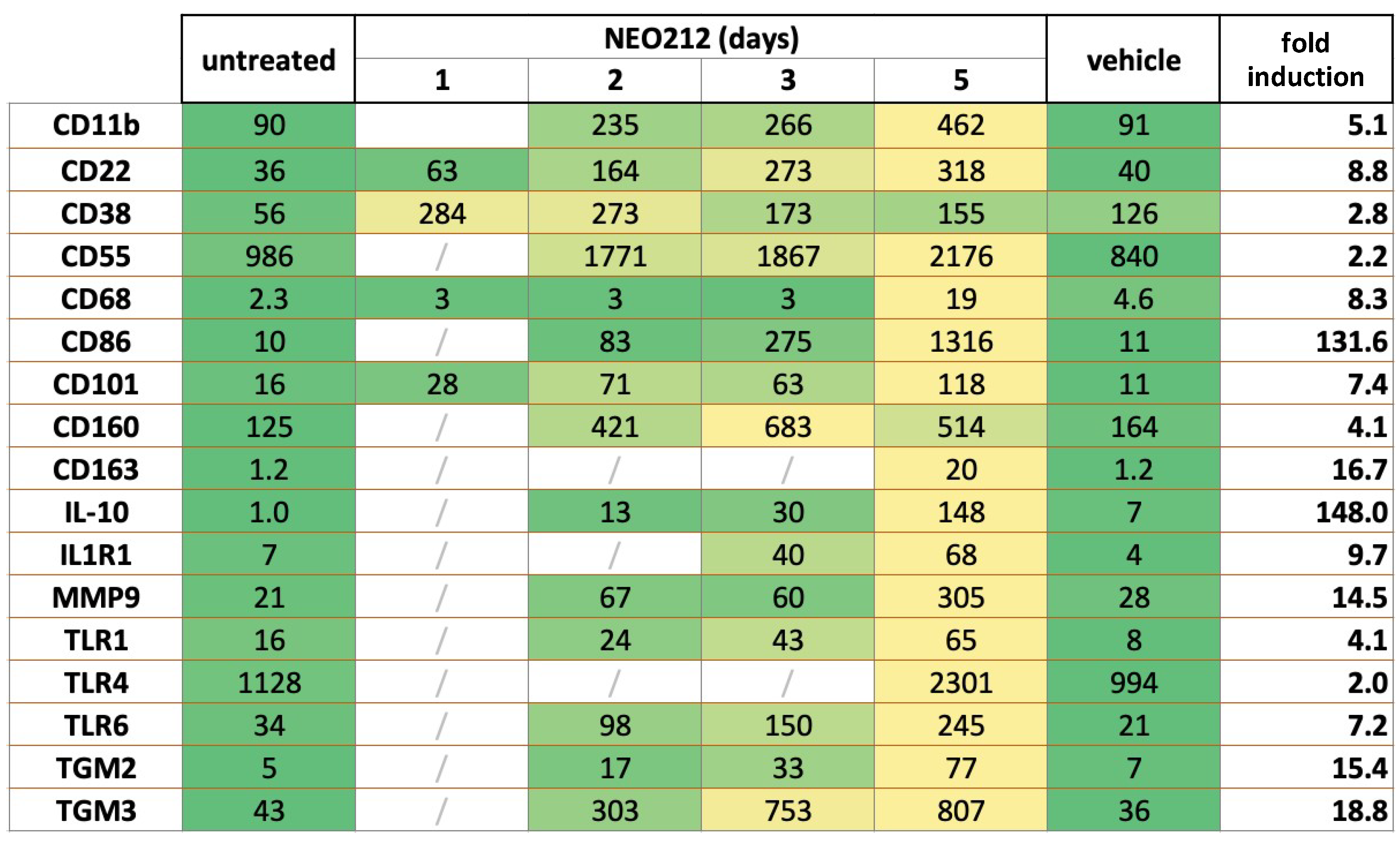
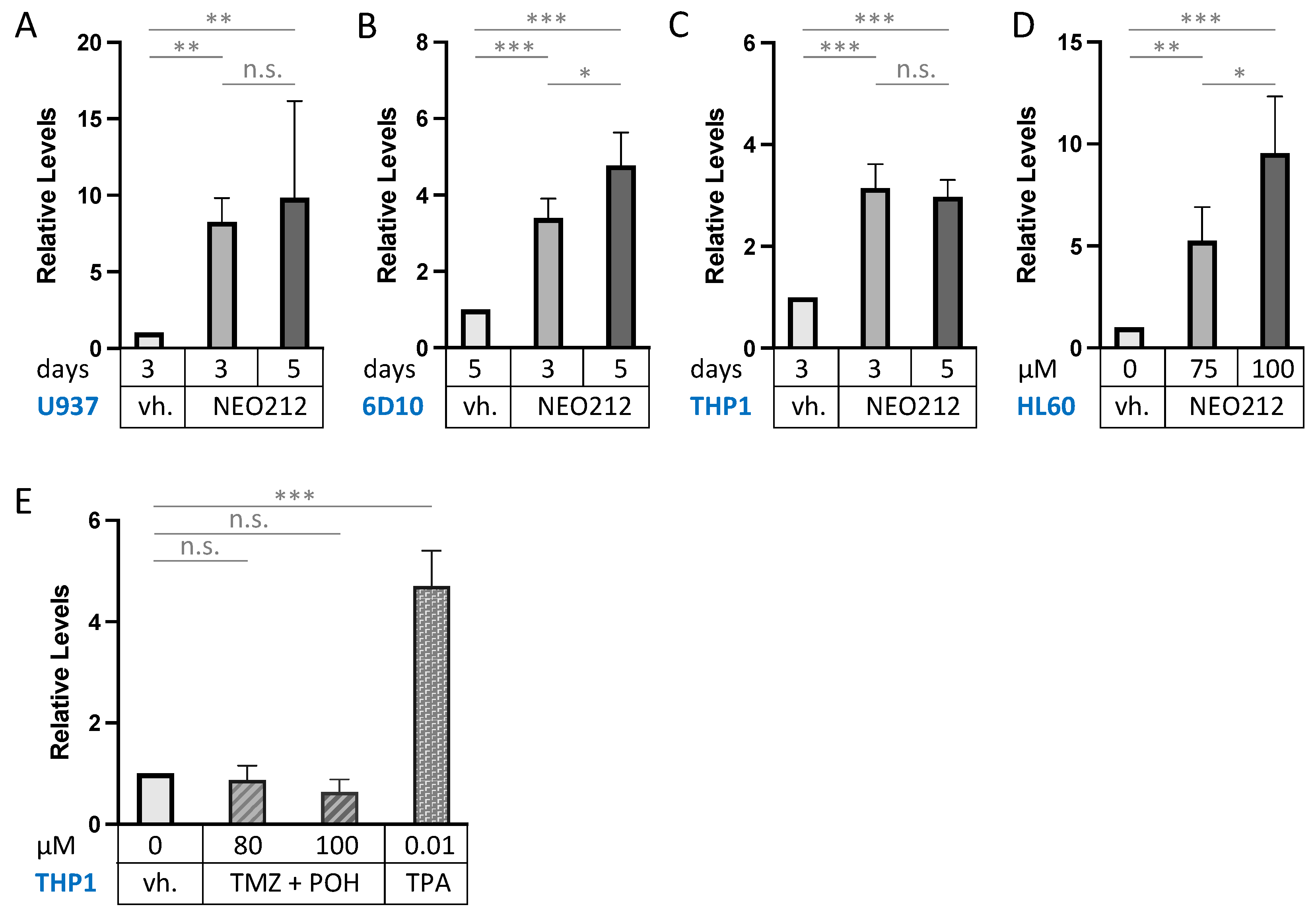
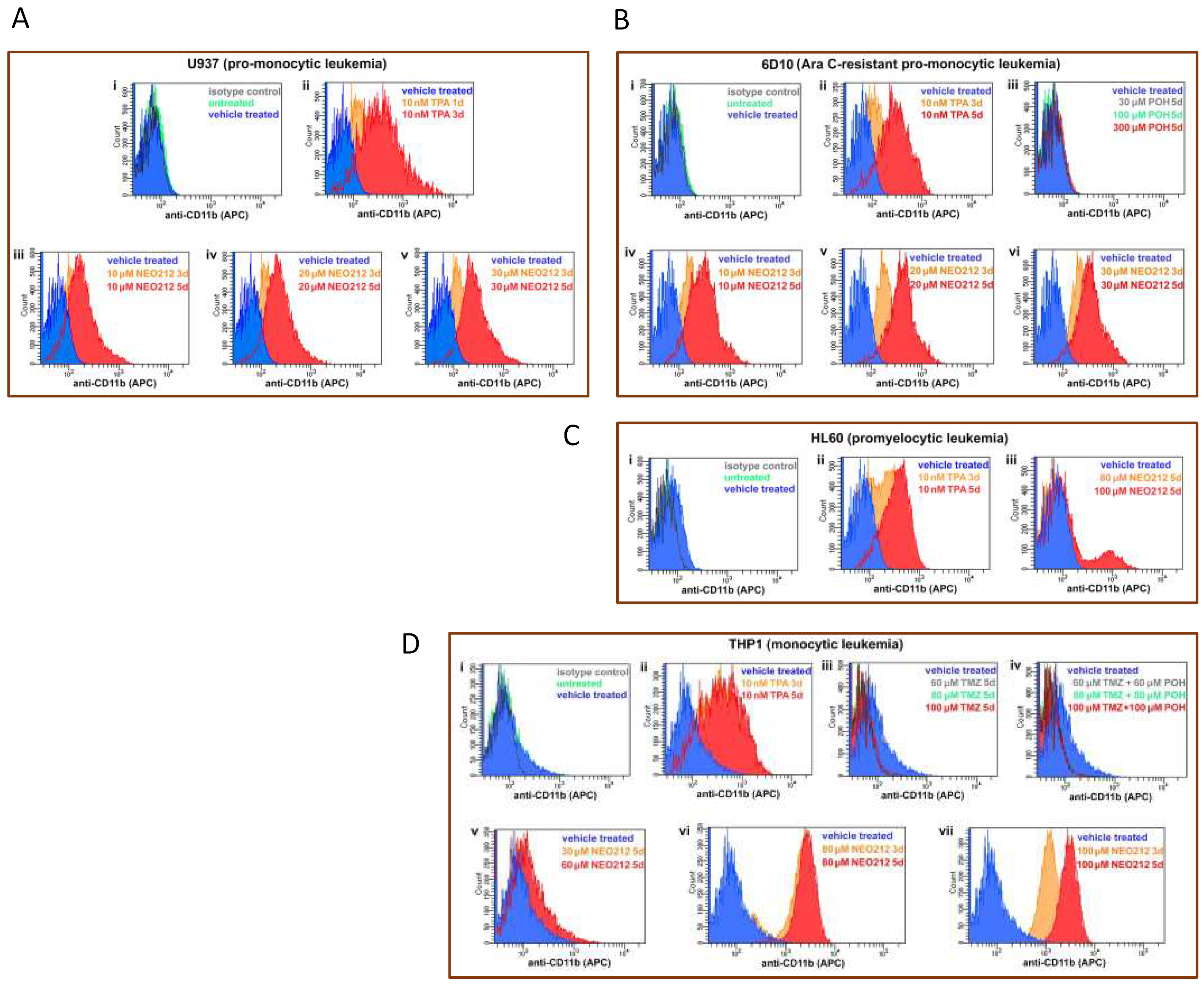
3.3. NEO212 Triggers Macrophage Differentiation
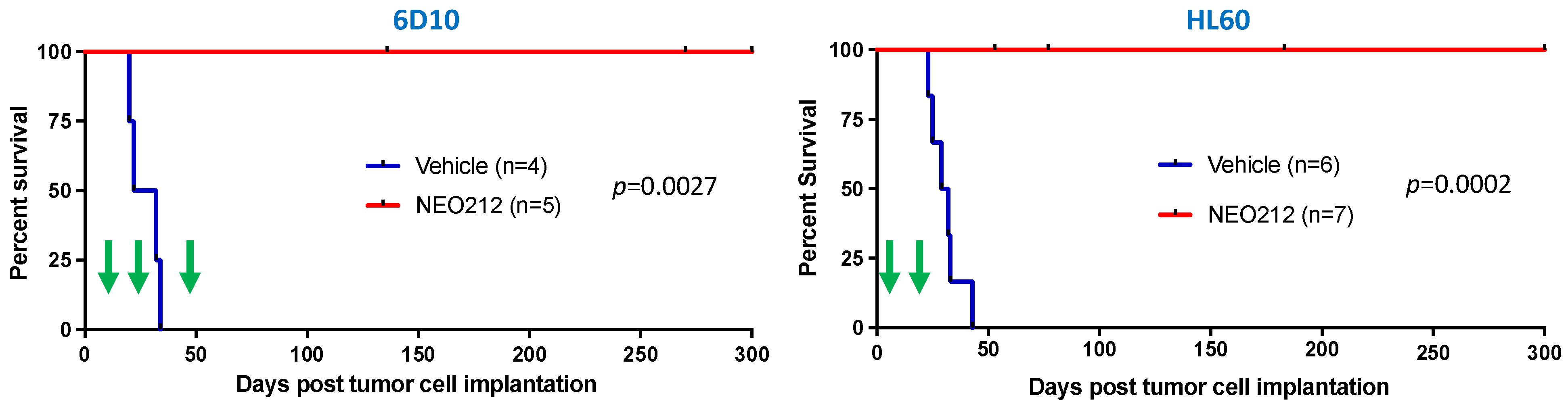
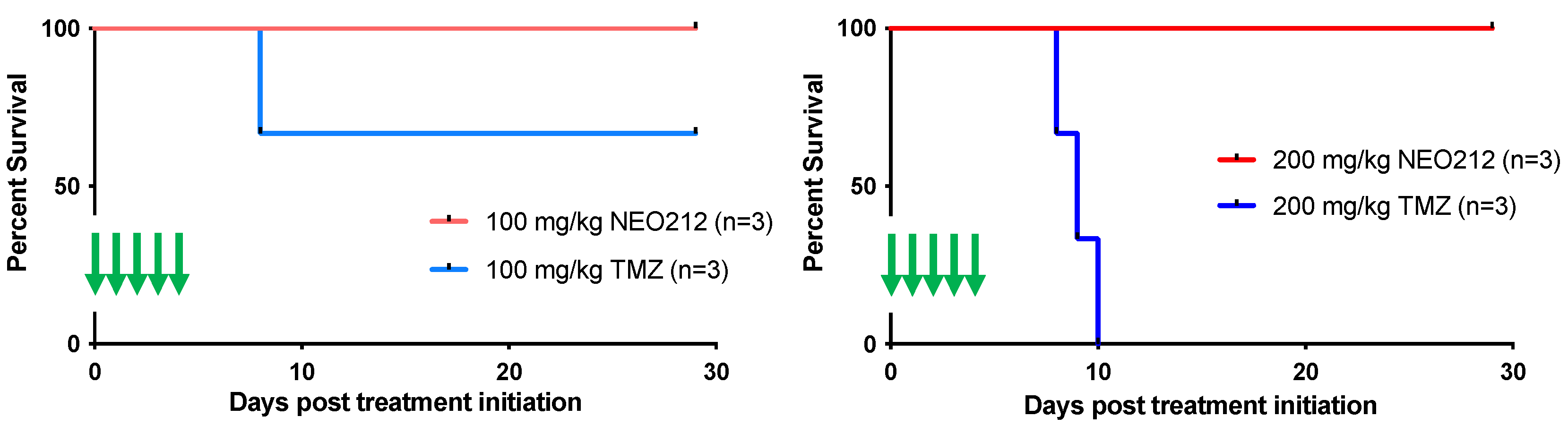
3.4. NEO212 Exerts Striking Therapeutic Activity In Vivo
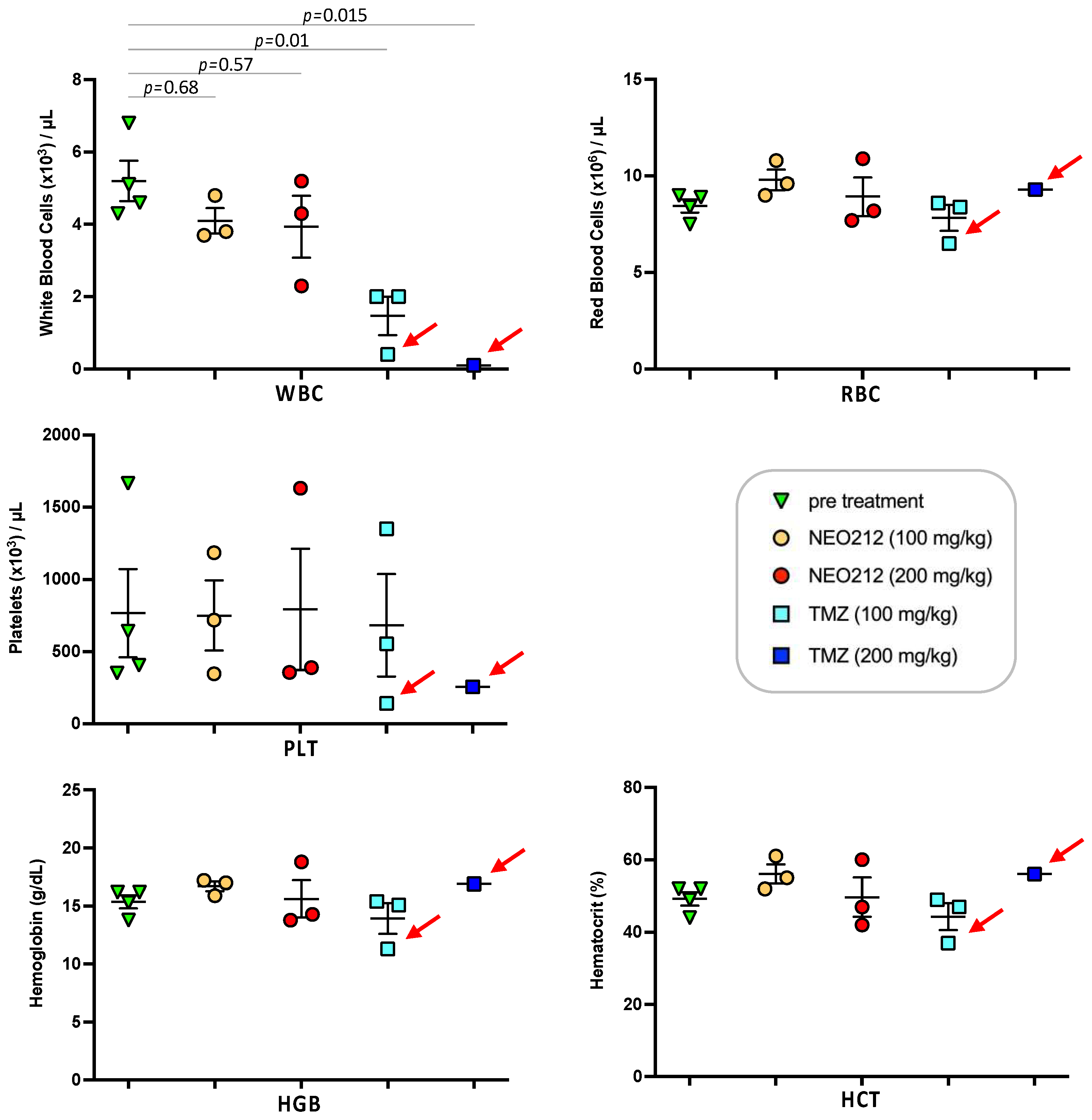
3.5. NEO212 Is Well Tolerated
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Numan, Y.; Abaza, Y.; Altman, J.K.; Platanias, L.C. Advances in the pharmacological management of acute myeloid leukemia in adults. Expert Opin. Pharmacother. 2022, 23, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Huerga-Domínguez, S.; Villar, S.; Prósper, F.; Alfonso-Piérola, A. Updates on the management of acute myeloid leukemia. Cancers 2022, 14, 4756. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute myeloid leukemia: Current progress and future directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, F.; Zare-Khormizi, M.R.; Hekmatimoghaddam, S.; Hashemi, A.S. Molecular targeting and rational chemotherapy in acute myeloid leukemia. J. Exp. Pharmacol. 2020, 12, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 2019, 12, 1937–1945. [Google Scholar] [CrossRef]
- Chen, T.C.; Cho, H.Y.; Wang, W.; Barath, M.; Sharma, N.; Hofman, F.M.; Schönthal, A.H. A novel temozolomide-perillyl alcohol conjugate exhibits superior activity against breast cancer cells in vitro and intracranial triple-negative tumor growth in vivo. Mol. Cancer Ther. 2014, 13, 1181–1193. [Google Scholar] [CrossRef]
- Chen, T.C.; Cho, H.Y.; Wang, W.; Nguyen, J.; Jhaveri, N.; Rosenstein-Sisson, R.; Hofman, F.M.; Schönthal, A.H. A novel temozolomide analog, neo212, with enhanced activity against mgmt-positive melanoma in vitro and in vivo. Cancer Lett. 2015, 358, 144–151. [Google Scholar] [CrossRef]
- Jhaveri, N.; Agasse, F.; Armstrong, D.; Peng, L.; Commins, D.; Wang, W.; Rosenstein-Sisson, R.; Vaikari, V.P.; Santiago, S.V.; Santos, T.; et al. A novel drug conjugate, neo212, targeting proneural and mesenchymal subtypes of patient-derived glioma cancer stem cells. Cancer Lett. 2016, 371, 240–250. [Google Scholar] [CrossRef]
- Chua, J.; Nafziger, E.; Leung, D. Evidence-based practice: Temozolomide beyond glioblastoma. Curr Oncol. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair. 2019, 78, 128–141. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Hainfellner, J.A.; Marosi, C.; Preusser, M. Assessing mgmt methylation status and its current impact on treatment in glioblastoma. CNS Oncol. 2015, 4, 47–52. [Google Scholar] [CrossRef]
- Agnihotri, S.; Gajadhar, A.S.; Ternamian, C.; Gorlia, T.; Diefes, K.L.; Mischel, P.S.; Kelly, J.; McGown, G.; Thorncroft, M.; Carlson, B.L.; et al. Alkylpurine-DNA-n-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Investig. 2012, 122, 253–266. [Google Scholar] [CrossRef]
- Rao, V.; Kumar, G.; Vibhavari, R.J.A.; Nandakumar, K.; Thorat, N.; Chamallamudi, M.R.; Kumar, N. Temozolomide resistance: A multifarious review on mechanisms beyond o6-methylguanine-dna methyltransferase. CNS Neurol. Disord. Drug Targets 2022. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Rao, A.; Ramani, N.; Stoppacher, R.; Coyle, T. Complete response to temozolomide in chronic lymphocytic leukemia. Clin. Case Rep. 2017, 5, 1130–1131. [Google Scholar] [CrossRef]
- Zhai, Y.; Shang, J.; Yao, W.; Wu, D.; Fu, C.; Yan, L. Successful eradication of central nervous system infiltration of primary plasma cell leukemia by temozolomide. Ann. Hematol. 2022, 101, 2555–2557. [Google Scholar] [CrossRef]
- Singh, R.; Mehrotra, S.; Gopalakrishnan, M.; Gojo, I.; Karp, J.E.; Greer, J.M.; Chen, A.; Piekarz, R.; Kiesel, B.F.; Gobburu, J.; et al. Population pharmacokinetics and exposure-response assessment of veliparib co-administered with temozolomide in patients with myeloid leukemias. Cancer Chemother. Pharmacol. 2019, 83, 319–328. [Google Scholar] [CrossRef]
- Brandwein, J.M.; Yang, L.; Schimmer, A.D.; Schuh, A.C.; Gupta, V.; Wells, R.A.; Alibhai, S.M.; Xu, W.; Minden, M.D. A phase ii study of temozolomide therapy for poor-risk patients aged >or=60 years with acute myeloid leukemia: Low levels of mgmt predict for response. Leukemia 2007, 21, 821–824. [Google Scholar] [CrossRef]
- Horton, T.M.; Thompson, P.A.; Berg, S.L.; Adamson, P.C.; Ingle, A.M.; Dolan, M.E.; Delaney, S.M.; Hedge, M.; Weiss, H.L.; Wu, M.F.; et al. Phase i pharmacokinetic and pharmacodynamic study of temozolomide in pediatric patients with refractory or recurrent leukemia: A children’s oncology group study. J. Clin. Oncol. 2007, 25, 4922–4928. [Google Scholar] [CrossRef] [PubMed]
- Seiter, K.; Katragadda, S.; Ponce, D.; Rasul, M.; Ahmed, N. Temozolomide and cisplatin in relapsed/refractory acute leukemia. J. Hematol. Oncol. 2009, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Seiter, K.; Liu, D.; Loughran, T.; Siddiqui, A.; Baskind, P.; Ahmed, T. Phase i study of temozolomide in relapsed/refractory acute leukemia. J. Clin. Oncol. 2002, 20, 3249–3253. [Google Scholar] [CrossRef]
- Rizzieri, D.; LoRusso, S.; Tse, W.; Khan, K.; Advani, A.; Moore, J.; Karsten, V.; Cahill, A.; Gerson, S.L. Phase i study of temozolomide and laromustine (vnp40101m) in patients with relapsed or refractory leukemia. Clin. Lymphoma Myeloma Leuk. 2010, 10, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, J.M.; Kassis, J.; Leber, B.; Hogge, D.; Howson-Jan, K.; Minden, M.D.; Galarneau, A.; Pouliot, J.F. Phase ii study of targeted therapy with temozolomide in acute myeloid leukaemia and high-risk myelodysplastic syndrome patients pre-screened for low o(6) -methylguanine DNA methyltransferase expression. Br. J. Haematol. 2014, 167, 664–670. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Kohrt, H.E.; Gotlib, J.; Coutre, S.E.; Zhang, B.; Arber, D.A.; Zehnder, J.L. Tailored temozolomide therapy according to mgmt methylation status for elderly patients with acute myeloid leukemia. Am. J. Hematol. 2012, 87, 45–50. [Google Scholar] [CrossRef]
- D’Atri, S.; Piccioni, D.; Castellano, A.; Tuorto, V.; Franchi, A.; Lu, K.; Christiansen, N.; Frankel, S.; Rustum, Y.M.; Papa, G.; et al. Chemosensitivity to triazene compounds and o6-alkylguanine-DNA alkyltransferase levels: Studies with blasts of leukaemic patients. Ann. Oncol. 1995, 6, 389–393. [Google Scholar] [CrossRef]
- Arai, H.; Yamauchi, T.; Uzui, K.; Ueda, T. Leukemia cells are sensitized to temozolomide, carmustine and melphalan by the inhibition of o(6)-methylguanine-DNA methyltransferase. Oncol. Lett. 2015, 10, 845–849. [Google Scholar] [CrossRef][Green Version]
- Crowell, P.L.; Elson, C.E. Isoprenoids, health and disease. In Neutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 31–54. [Google Scholar]
- Chen, T.C.; Fonseca, C.O.; Schönthal, A.H. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am. J. Cancer Res. 2015, 5, 1580–1593. [Google Scholar]
- Chen, T.C.; da Fonseca, C.O.; Levin, D.; Schönthal, A.H. The monoterpenoid perillyl alcohol: Anticancer agent and medium to overcome biological barriers. Pharmaceutics 2021, 13, 2167. [Google Scholar] [CrossRef]
- da Fonseca, C.O.; Teixeira, R.M.; Silva, J.C.; Fischer, J.D.S.D.G.; Meirelles, O.C.; Landeiro, J.A.; Quirico-Santos, T. Long-term outcome in patients with recurrent malignant glioma treated with perillyl alcohol inhalation. Anticancer Res. 2013, 33, 5625–5631. [Google Scholar]
- Schönthal, A.H.; Peereboom, D.M.; Wagle, N.; Lai, R.; Mathew, A.J.; Hurth, K.M.; Simmon, V.F.; Howard, S.P.; Taylor, L.P.; Chow, F.; et al. Phase i trial of intranasal neo100, highly purified perillyl alcohol, in adult patients with recurrent glioblastoma. Neurooncol. Adv. 2021, 3, vdab005. [Google Scholar] [CrossRef]
- Schönthal, A.H.; Swenson, S.; Bonney, P.A.; Wagle, N.; Simmon, V.F.; Mathew, A.J.; Hurth, K.M.; Chen, T.C. Detection of perillyl alcohol and its metabolite perillic acid in postsurgical glioblastoma tissue after intranasal administration of neo100: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22215. [Google Scholar] [CrossRef]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Perillyl alcohol and its drug-conjugated derivatives as potential novel methods of treating brain metastases. Int. J. Mol. Sci. 2016, 17, 1463. [Google Scholar] [CrossRef]
- Cho, H.Y.; Wang, W.; Jhaveri, N.; Lee, D.J.; Sharma, N.; Dubeau, L.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C. Neo212, temozolomide conjugated to perillyl alcohol, is a novel drug for effective treatment of a broad range of temozolomide-resistant gliomas. Mol. Cancer Ther. 2014, 13, 2004–2017. [Google Scholar] [CrossRef]
- Silva-Hirschberg, C.; Hartman, H.; Stack, S.; Swenson, S.; Minea, R.O.; Davitz, M.A.; Chen, T.C.; Schönthal, A.H. Cytotoxic impact of a perillyl alcohol-temozolomide conjugate, neo212, on cutaneous t-cell lymphoma in vitro. Ther. Adv. Med. Oncol. 2019, 11, 1758835919891567. [Google Scholar] [CrossRef]
- Schönthal, A.H.; Swenson, S.; Minea, R.O.; Kim, H.N.; Cho, H.; Mohseni, N.; Kim, Y.M.; Chen, T.C. Potentially curative therapeutic activity of neo212, a perillyl alcohol-temozolomide conjugate, in preclinical cytarabine-resistant models of acute myeloid leukemia. Cancers 2021, 13, 3385. [Google Scholar] [CrossRef]
- Kurata, M.; Rathe, S.K.; Bailey, N.J.; Aumann, N.K.; Jones, J.M.; Veldhuijzen, G.W.; Moriarity, B.S.; Largaespada, D.A. Using genome-wide crispr library screening with library resistant dck to find new sources of ara-c drug resistance in aml. Sci. Rep. 2016, 6, 36199. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qian, F.; Arner, B.E.; Nettleford, S.K.; Paulson, R.F.; Prabhu, K.S. Intra-peritoneal transplantation for efficient and easy generation of experimental acute myeloid leukemia in mice. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sawyers, C.L.; Gishizky, M.L.; Quan, S.; Golde, D.W.; Witte, O.N. Propagation of human blastic myeloid leukemias in the scid mouse. Blood 1992, 79, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Minea, R.O.; Duc, T.C.; Swenson, S.D.; Cho, H.Y.; Huang, M.; Hartman, H.; Hofman, F.M.; Schonthal, A.H.; Chen, T.C. Developing a clinically relevant radiosensitizer for temozolomide-resistant gliomas. PLoS ONE 2020, 15, e0238238. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Jiang, S.X.; Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Rich, J.N.; Gururangan, S.; Friedman, A.H.; Bigner, D.D.; Sampson, J.H.; et al. Phase ii trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J. Clin. Oncol. 2009, 27, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Koeffler, H.P. Induction of differentiation of human acute myelogenous leukemia cells: Therapeutic implications. Blood 1983, 62, 709–721. [Google Scholar] [CrossRef]
- Funakoshi, K.; Bagheri, M.; Zhou, M.; Suzuki, R.; Abe, H.; Akashi, H. Highly sensitive and specific alu-based quantification of human cells among rodent cells. Sci. Rep. 2017, 7, 13202. [Google Scholar] [CrossRef]
- Cho, H.Y.; Swenson, S.; Thein, T.Z.; Wang, W.; Wijeratne, N.R.; Marin-Ramos, N.I.; Katz, J.E.; Hofman, F.M.; Schönthal, A.H.; Chen, T.C. Pharmacokinetic properties of the temozolomide perillyl alcohol conjugate (neo212) in mice. Neurooncol. Adv. 2020, 2, vdaa160. [Google Scholar] [CrossRef]
- Chen, T.C.; Cho, H.Y.; Wang, W.; Wetzel, S.J.; Singh, A.; Nguyen, J.; Hofman, F.M.; Schönthal, A.H. Chemotherapeutic effect of a novel temozolomide analog on nasopharyngeal carcinoma in vitro and in vivo. J. Biomed. Sci. 2015, 22, 71–80. [Google Scholar] [CrossRef]
- Madan, V.; Koeffler, H.P. Differentiation therapy of myeloid leukemia: Four decades of development. Haematologica 2021, 106, 26–38. [Google Scholar] [CrossRef]
- Sachs, L. Constitutive uncoupling of the controls for growth and differentiation in myeloid leukemia and the development of cancer. J. Natl. Cancer Inst. 1980, 65, 675–679. [Google Scholar] [CrossRef][Green Version]
- Stöckbauer, P.; Malaskova, V.; Soucek, J.; Chudomel, V. Differentiation of human myeloid leukemia cell lines induced by tumor-promoting phorbol ester (tpa). I. Changes of the morphology, cytochemistry and the surface differentiation antigens analyzed with monoclonal antibodies. Neoplasma 1983, 30, 257–272. [Google Scholar]
- Prieto, J.; Eklund, A.; Patarroyo, M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994, 156, 191–211. [Google Scholar] [CrossRef]
- Pahl, H.L.; Rosmarin, A.G.; Tenen, D.G. Characterization of the myeloid-specific cd11b promoter. Blood 1992, 79, 865–870. [Google Scholar] [CrossRef]
- Rubio, M.A.; Lopez-Rodriguez, C.; Nueda, A.; Aller, P.; Armesilla, A.L.; Vega, M.A.; Corbi, A.L. Granulocyte-macrophage colony-stimulating factor, phorbol ester, and sodium butyrate induce the cd11c integrin gene promoter activity during myeloid cell differentiation. Blood 1995, 86, 3715–3724. [Google Scholar] [CrossRef]
- Yamada, M.; Kurahashi, K. Regulation of myeloperoxidase gene expression during differentiation of human myeloid leukemia hl-60 cells. J. Biol. Chem. 1984, 259, 3021–3025. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Song, X.; Liu, L.; Chang, M.; Geng, X.; Wang, X.; Wang, W.; Chen, T.C.; Xie, L.; Song, X. Neo212 induces mitochondrial apoptosis and impairs autophagy flux in ovarian cancer. J. Exp. Clin. Cancer Res. 2019, 38, 239. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Libutti, S.K.; Tamarkin, L.; Nilubol, N. Targeting the invincible barrier for drug delivery in solid cancers: Interstitial fluid pressure. Oncotarget 2018, 9, 35723–35725. [Google Scholar] [CrossRef]
- Mori, T.; Koga, T.; Shibata, H.; Ikeda, K.; Shiraishi, K.; Suzuki, M.; Iyama, K. Interstitial fluid pressure correlates clinicopathological factors of lung cancer. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 201–208. [Google Scholar] [CrossRef]
- Schmidt, T.; Carmeliet, P. Angiogenesis: A target in solid tumors, also in leukemia? Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kurtin, S. Myeloid toxicity of cancer treatment. J. Adv. Pract. Oncol. 2012, 3, 209–224. [Google Scholar] [PubMed]
- Scaringi, C.; De Sanctis, V.; Minniti, G.; Enrici, R.M. Temozolomide-related hematologic toxicity. Onkologie 2013, 36, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Pyakuryal, B.; Pudasainee, P.; Rajasurya, V.; Gundabolu, K.; Bhatt, V.R. Treatment strategies for therapy-related acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2020, 20, 147–155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.C.; Minea, R.O.; Swenson, S.; Yang, Z.; Thein, T.Z.; Schönthal, A.H. NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity. Cancers 2022, 14, 6065. https://doi.org/10.3390/cancers14246065
Chen TC, Minea RO, Swenson S, Yang Z, Thein TZ, Schönthal AH. NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity. Cancers. 2022; 14(24):6065. https://doi.org/10.3390/cancers14246065
Chicago/Turabian StyleChen, Thomas C., Radu O. Minea, Steve Swenson, Zhuoyue Yang, Thu Zan Thein, and Axel H. Schönthal. 2022. "NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity" Cancers 14, no. 24: 6065. https://doi.org/10.3390/cancers14246065
APA StyleChen, T. C., Minea, R. O., Swenson, S., Yang, Z., Thein, T. Z., & Schönthal, A. H. (2022). NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity. Cancers, 14(24), 6065. https://doi.org/10.3390/cancers14246065







