Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Searching Strategies and Data Sources
2.2. Study Selection and Eligibility Criteria
2.3. Objectives of the Study
2.4. Data Extraction
2.5. Statistical Analysis
2.6. RISK of BIAS (Quality) Assessment
3. Results
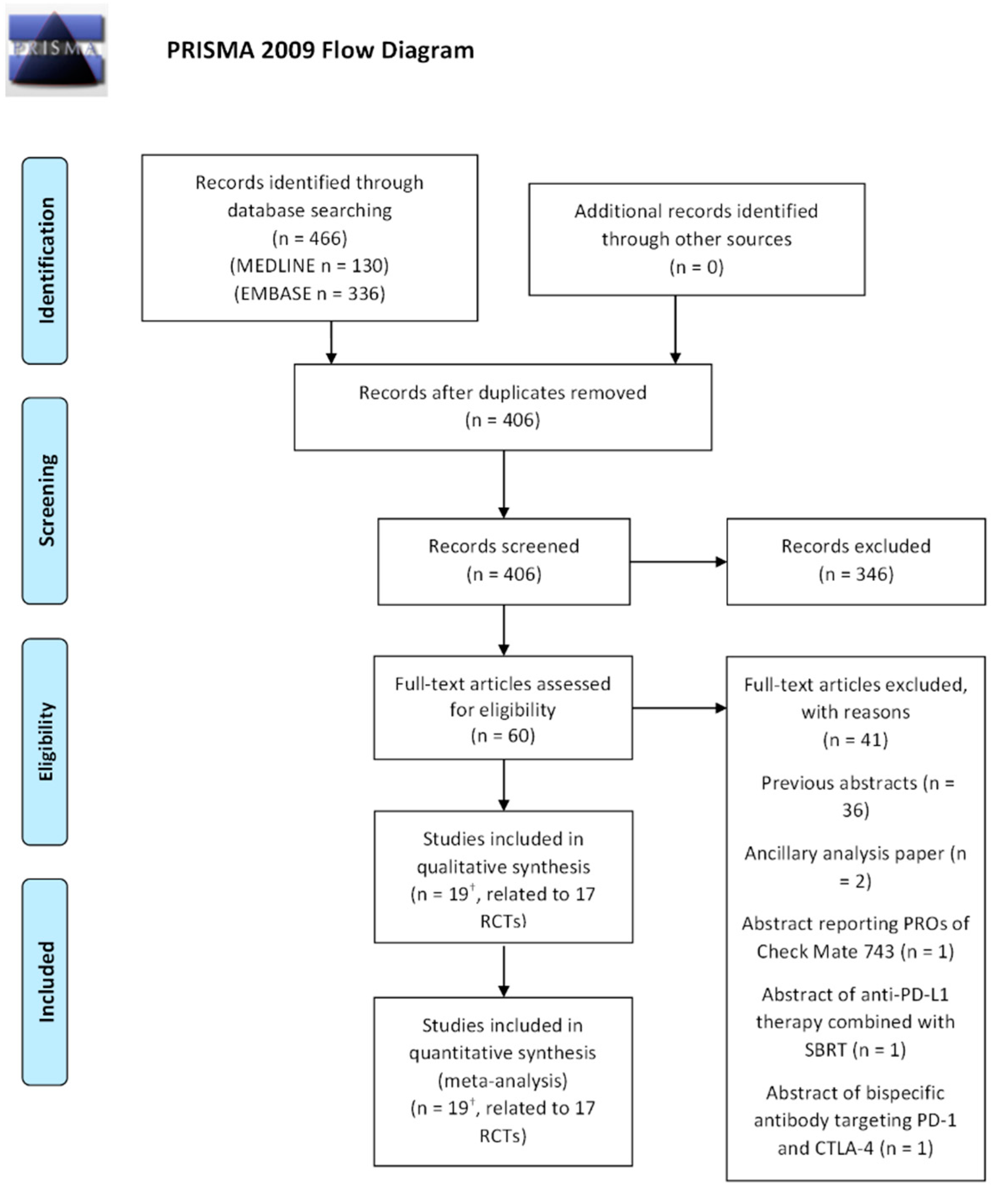
3.1. Literature Research
3.2. Systematic Review: First-Line Treatments
3.3. Systematic Review: Second-Line Treatments
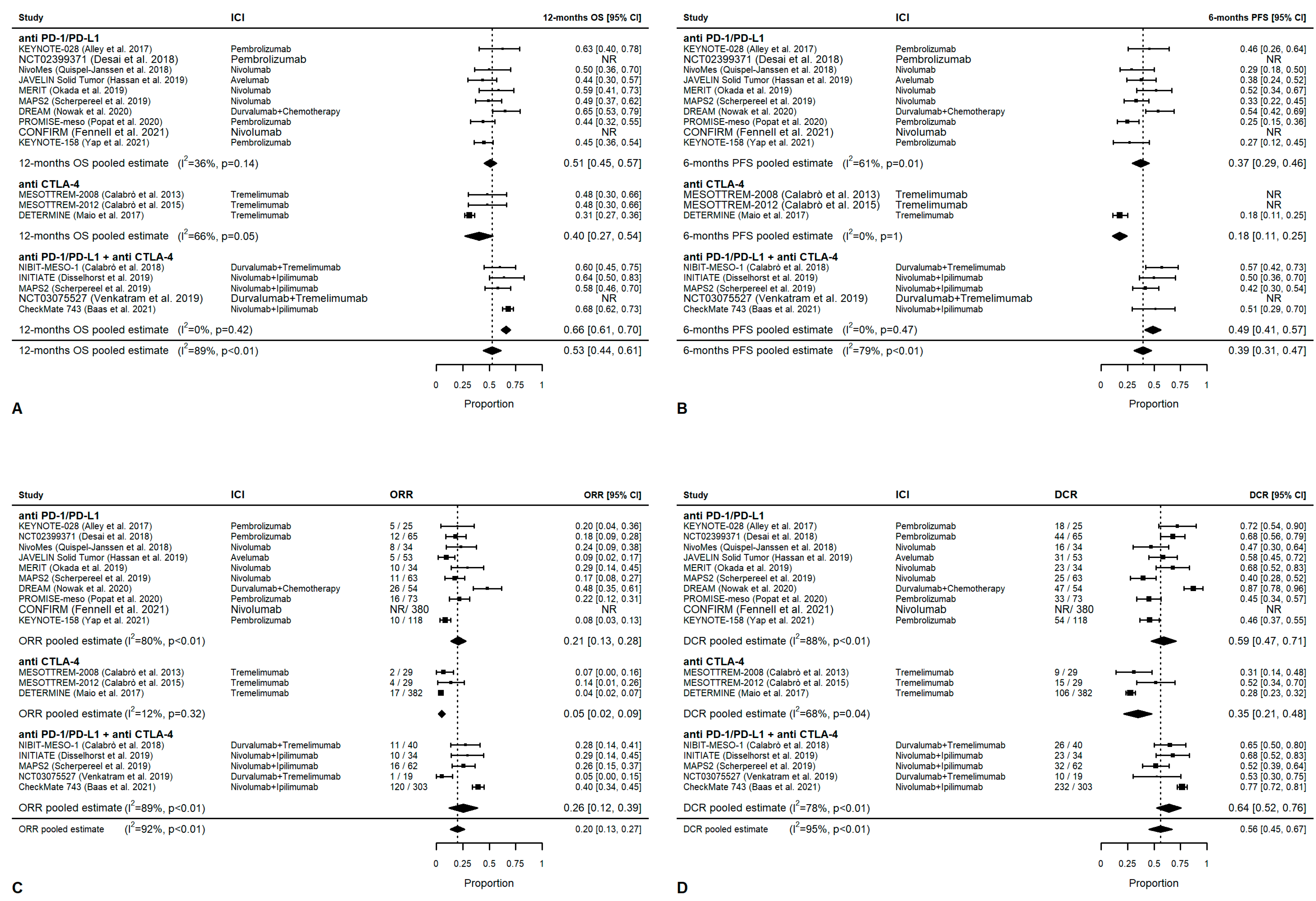
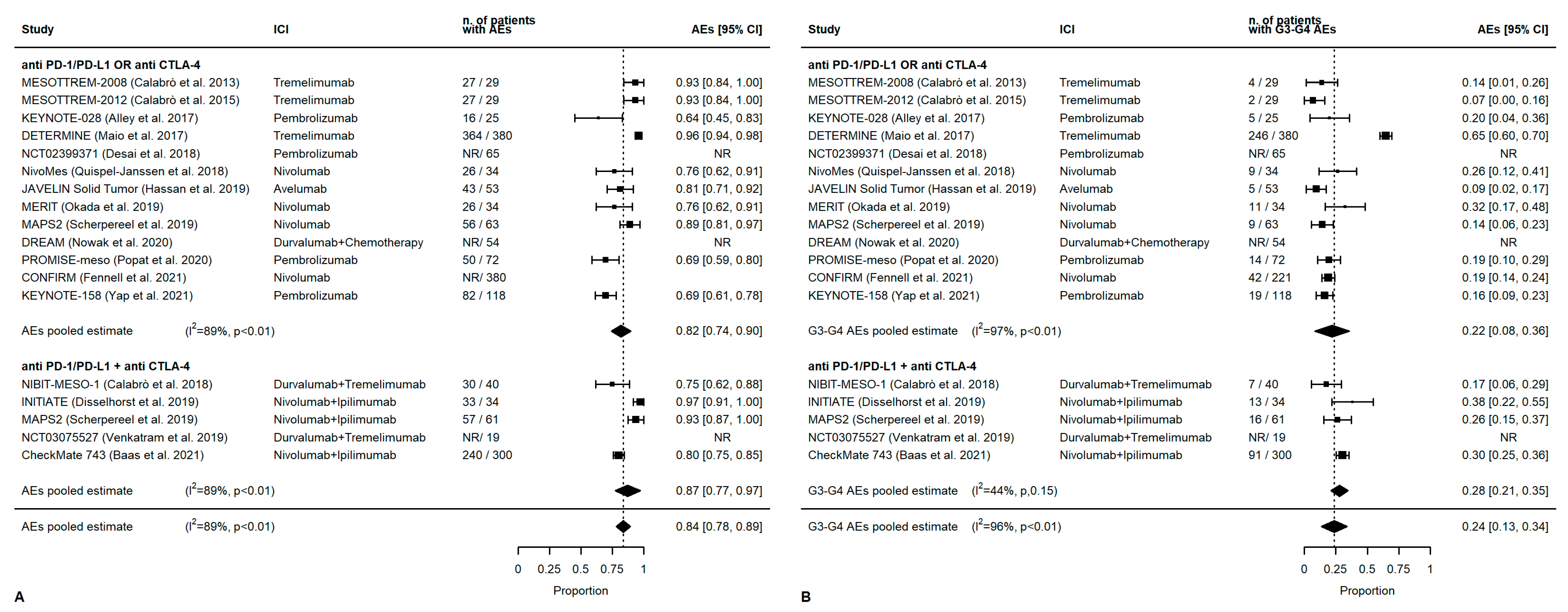
3.4. Meta-Analysis: Overall Outcomes
3.5. Meta-Analysis: First-Line Outcomes
3.6. Meta-Analysis: Second-Line Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becklake, M.; Bagatin, E.; Neder, J. Asbestos-related diseases of the lungs and pleura: Uses, trends and management over the last century. Int. J. Tuberc. Lung Dis. 2007, 11, 356–369. [Google Scholar] [PubMed]
- Baas, P.; Fennell, D.; Kerr, K.M.; Van Schil, P.E.; Haas, R.L.; Peters, S. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v31–v39. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.; Musk, A.; Lake, R. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef]
- Yap, T.; Aerts, J.; Popat, S.; Al, E. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.; Rusthoven, J.; Symanowski, J.; Al, E. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Al, E. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase III trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Zauderer, M.G.; Kass, S.L.; Woo, K.; Sima, C.S.; Ginsberg, M.S.; Krug, L.M. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014, 84, 271–274. [Google Scholar] [CrossRef]
- Fennell, D.A.; Casbard, A.C.; Porter, C.; Rudd, R.; Lester, J.F.; Nicolson, M.; VIM Trial Group. A randomized phase II trial of oral vinorelbine as second-line therapy for patients with malignant pleural mesothelioma. J. Clin. Oncol. 2021, 39, 8507. [Google Scholar] [CrossRef]
- Guazzelli, A.; Bakker, E.; Krstic-Demonacos, M.; Lisanti, M.P.; Sotgia, F.; Mutti, L. Anti-CTLA-4 therapy for malignant mesothelioma. Immunotherapy 2017, 9, 273–280. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Malignant Pleural Mesothelioma. Volume Version 2. 2018. Available online: https://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiUnba8j-j7AhWPi_0HHWloBbMQFnoECAsQAQ&url=https%3A%2F%2Fwww.nccn.org%2Fguidelines%2Fguidelines-process%2Ftransparency-process-and-recommendations%2FGetFileFromFileManager%3FfileManagerId%3D3521&usg=AOvVaw3NXSFGHNNJ2KrnTcYtxS2o (accessed on 10 September 2022).
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. PopeA multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef]
- Fennell, D.; Ottensmeier, C.; Califano, R.; Hanna, G.; Ewings, S.; Hill, K.; Lester, J. PS01.11 Nivolumab Versus Placebo in Relapsed Malignant Mesothelioma: The CONFIRM Phase 3 Trial. J. Thorac. Oncol. 2021, 16, S62. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 30, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Meirson, T.; Pentimalli, F.; Cerza, F.; Baglio, G.; Gray, S.G.; Correale, P.; Krstic-Demonacos, M.; Markel, G.; Giordano, A.; Bomze, D.; et al. Comparison of 3 Randomized Clinical Trials of Frontline Therapies for Malignant Pleural Mesothelioma. MA Netw. Open 2022, 5, e221490. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Tripoli, S. Current treatments for inoperable mesothelioma: Indirect comparisons based on individual patient data reconstructed retrospectively from 4 trials. J. Chemother 2022, 12, 1–5. [Google Scholar] [CrossRef]
- Kerrigan, K.; Jo, Y.; Chipman, J.; Haaland, B.; Puri, S.; Akerley, W.; Patel, S. A Real-World Analysis of the Use of Systemic Therapy in Malignant Pleural Mesothelioma and the Differential Impacts on Overall Survival by Practice Pattern. JTO Clin. Res. Rep. 2022, 3, 100280. [Google Scholar] [CrossRef]
- Ujiie, H.; Kadota, K.; Nitadori, J.-I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The Tumoral and Stromal Immune Microenvironment in Malignant Pleural Mesothelioma: A Comprehensive Analysis Reveals Prognostic Immune Markers. Oncoimmunology 2015, 4, e1009285. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Mannarino, L.; Kirschner, M.B.; Opitz, I.; Rigutto, A.; Laure, A.; Lia, M.; Nozza, P.; Maconi, A.; Marchini, S.; et al. Tumor Immune Microenvironment and Genetic Alterations in Mesothelioma. Front. Oncol. 2021, 11, 660039. [Google Scholar] [CrossRef]
- Pinto, C.; Zucali, P.A.; Pagano, M.; Grosso, F.; Pasello, G.; Garassino, M.C.; Tiseo, M.; Parra, H.S.; Grossi, F.; Cappuzzo, F.; et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021, 22, 1438–1447. [Google Scholar] [CrossRef]
- Petrelli, F.; Ardito, R.; Conti, B.; Coinu, A.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Barni, S.; Ghidini, A. A systematic review and meta-analysis of second-line therapies for treatment of mesothelioma. Respir. Med. 2018, 141, 72–80. [Google Scholar] [CrossRef]
- Parikh, K.; Hendriks, L.E.L.; Bironzo, P.; Remon, J. Immune checkpoint inhibitors a new player in the therapeutic game of mesothelioma: New reality with new challenges. Cancer Treat. Rev. 2021, 99, 102250. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 7, 16. [Google Scholar] [CrossRef]
- Der Simonian, X.R.; Laird, N. Meta-analysis in clinical trials. Control. Clin Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Okada, M.; Kijima, T.; Aoe, K.; Kato, T.; Fujimoto, N.; Nakagawa, K.; Takeda, Y.; Hida, T.; Kanai, K.; et al. Three-Year Follow-up Results of the MERIT Trial: A Japanese Phase II Study of Nivolumab in Malignant Pleural Mesothelioma. Ann. Oncol. 2020, 31, S1076. [Google Scholar] [CrossRef]
- Calabrò, L.; Morra, A.; Giannarelli, D.; Amato, G.; D’Incecco, A.; Covre, A.; Maio, M. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): An open-label, non-randomised, phase 2 study. Lancet Respir. Med. 2018, 6, 451–460. [Google Scholar] [CrossRef]
- Calabrò, L.; Rossi, G.; Morra, A.; Rosati, C.; Cutaia, O.; Daffinà, M.G.; Maio, M. Tremelimumab plus durvalumab retreatment and 4-year outcomes in patients with mesothelioma: A follow-up of the open label, non-randomised, phase 2 NIBIT-MESO-1 study. Lancet Respir. Med. 2021, 9, 969–976. [Google Scholar] [CrossRef]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.; Brown, C.; Hughes, B.G.M.; Karikios, D.J. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): A multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, L.; Morra, A.; Fonsatti, E.; Cutaia, O.; Amato, G.; Giannarelli, D.; Di Giacomo, A.M.; Danielli, R.; Altomonte, M.; Mutti, L.; et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2013, 14, 1104–1111. [Google Scholar] [CrossRef]
- Calabrò, L.; Morra, A.; Fonsatti, E.; Cutaia, O.; Fazio, C.; Annesi, D.; Lenoci, M.; Amato, G.; Danielli, R.; Altomonte, M.; et al. Effi cacy and safety of an intensifi ed schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: An open-label, single-arm, phase 2 study. Lancet Respir. Med. 2015, 3, 301–309. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Disselhorst, M.J.; Quispel-Janssen, J.; Lalezari, F.; Monkhorst, K.; de Vries, J.F.; van der Noort, V.; Baas, P. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): Results of a prospective, single-arm, phase 2 trial. Lancet Respir. Med. 2019, 7, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Thomas, A.; Nemunaitis, J.J.; Patel, M.R.; Bennouna, J.; Chen, F.L.; Delord, J.-P.; Dowlati, A.; Kochuparambil, S.T.; Taylor, M.H.; et al. Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): Preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Yap, T.A.; Nakagawa, K.; Fujimoto, N.; Kuribayashi, K.; Guren, T.K.; Calabrò, L.; Kindler, H.L. Efficacy and safety of pembrolizumab in patients with advanced mesothelioma in the open-label, single-arm, phase 2 KEYNOTE-158 study. Lancet Respir. Med. 2021, 9, 613–621. [Google Scholar] [CrossRef]
- Scherpereel, A.; Mazières, J.; Greillier, L.; Lantuéjoul, S.; Do, P.; Bylicki, O.; Carmier, D. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kijima, T.; Aoe, K.; Kato, T.; Fujimoto, N.; Nakagawa, K.; Takeda, Y.; Hida, T.; Kanai, K.; Imamura, F.; et al. Clinical Efficacy and Safety of Nivolumab: Results of a Multicenter, Open-label, Single-arm, Japanese Phase II study in Malignant Pleural Mesothelioma (MERIT). Clin. Cancer Res. 2019, 25, 5485–5492. [Google Scholar] [CrossRef]
- Quispel-Janssen, J.; van der Noort, V.; de Vries, J.F.; Zimmerman, M.; Lalezari, F.; Thunnissen, E.; Monkhorst, K.; Schouten, R.; Schunselaar, L.; Disselhorst, M.; et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1569–1576. [Google Scholar] [CrossRef]
- Desai, A.; Karrison, T.; Rose, B.; Tan, Y.; Hill, B.; Pemberton, E.; Straus, C.; Seiwert, T.; Kindler, H.L. Phase II Trial of Pembrolizumab (NCT02399371) In Previously-Treated Malignant Mesothelioma (MM): Final Analysis. J. Thorac. Oncol. 2018, 13, S339. [Google Scholar] [CrossRef]
- Venkatraman, D.; Anderson, A.; Digumarthy, S.; Lizotte, P.H.; Awad, M.M. Phase 2 study of tremelimumab plus durvalumab for previously-treated malignant pleural mesothelioma (MPM). J. Clin. Oncol. 2019, 37, 8549. [Google Scholar] [CrossRef]
- Desai, A.; Karrison, T.; Rose, B.; Pemberton, E.; Hill, B.; Straus, C.M.; Tan, Y.-H.C.; Seiwert, T.Y.; Kindler, H.L. Phase II trial of pembrolizumab (P) in patients (pts) with previously-treated mesothelioma (MM). J. Clin. Oncol. 2018, 36, 8565. [Google Scholar] [CrossRef]
- Tagliamento, M.; Bironzo, P.; Curcio, H.; De Luca, E.; Pignataro, D.; Rapetti, S.G.; Audisio, M.; Bertaglia, V.; Paratore, C.; Bungaro, M.; et al. A systematic review and meta-analysis of trials assessing PD-1/PD-L1 immune checkpoint inhibitors activity in pre-treated advanced stage malignant mesothelioma. Critical Reviews in Oncology. Hematology 2022, 172, 103639. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Signori, A.; Curioni-Fontecedro, A.; Cortellini, A.; Ponzano, M.; Giunta, E.F.; Rebuzzi, S.E.; Chan, S.; Gebbia, V.; van Eeden, R.; et al. Systemic therapy for pre-treated malignant mesothelioma: A systematic review, meta-analysis and network meta-analysis of randomised controlled trials. Eur. J. Cancer 2022, 166, 287–299. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Li, N.; Zheng, B.; Liu, M.; Cai, H. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221116604. [Google Scholar] [CrossRef]
- Ye, Z.-M.; Tang, Z.-Q.; Xu, Z.; Zhou, Q.; Li, H. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front. Public Health 2022, 22, 947375. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Brown, R.J.; Sammon, C.; Daumont, M.J.; McKenna, M.; Sanzari, J.K.; Forde, P.M. The Predictive and Prognostic Nature of Programmed Death-Ligand 1 in Malignant Pleural Mesothelioma: A Systematic Literature Review. JTO Clin. Res. Rep. 2022, 22, 100315. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Montgomery, R.; Fadia, M.; Wang, J.; Ali, S. PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma. Asia Pac. J. Clin. Oncol. 2018, 14, 69–73. [Google Scholar] [CrossRef]
- Ladanyi, M.; Vega, F.S.; Zauderer, M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med. 2019, 26, 18. [Google Scholar] [CrossRef]
- Louw, A.; Panou, V.; Szejniuk, W.M.; Meristoudis, C.; Chai, S.M.; van Vliet, C.; Lee, Y.C.G.; Dick, I.M.; Firth, T.; Lynggaard, L.A.; et al. BAP1 loss by immunohistochemistry predicts improved survival to first line platinum/pemetrexed chemotherapy for pleural mesothelioma patients: A validation study. J. Thorac. Oncol. 2022, 27, 921–930. [Google Scholar] [CrossRef]
- Marcq, E.; Siozopoulou, V.; De Waele, J.; Van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Prognostic and Predictive Aspects of the Tumor Immune Microenvironment and Immune Checkpoints in Malignant Pleural Mesothelioma. Oncoimmunology 2017, 6, e1261241. [Google Scholar] [CrossRef]
- Lievense, L.A.; Bezemer, K.; Cornelissen, R.; Kaijen-Lambers, M.E.; Hegmans, J.P.; Aerts, J.G. Precision Immunotherapy; Dynamics in the Cellular Profile of Pleural Effusions in Malignant Mesothelioma Patients. Lung Cancer 2017, 107, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative Analysis of the Role of Fiber Length on Phagocytosis and Inflammatory Response by Alveolar Macrophages. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef]
- Forde, P.M.; Anagnostou, V.; Sun, Z.; Dahlberg, S.E.; Kindler, H.L.; Niknafs, N.; Purcell, T.; Santana-Davila, R.; Dudek, A.Z.; Borghaei, H.; et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: Survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat. Med. 2021, 27, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Peikert, T.; Smadbeck, J.B.; Udell, J.B.; Garcia-Rivera, E.; Elsbernd, L.; Erskine, C.L.; Van Keulen, V.P.; Kosari, F.; Murphy, S.J.; et al. Neoantigenic Potential of Complex Chromosomal Rearrangements in Mesothelioma. J. Thorac. Oncol. 2019, 14, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Z.; Wang, X.; Li, H.; Liu, X.-S. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife 2019, 26, e49020. [Google Scholar] [CrossRef] [PubMed]
| Trial | Phase | Blinding | Treatment | Dose | Control Arm | Primary EPs | Stratification Factors |
|---|---|---|---|---|---|---|---|
| Calabrò et al., 2018, NIBIT-MESO-1 [27,28] | II | open label, single arm | Durvalumab + tremelimumab | Durva: 20 mg/kg 1q28 ipi: 1 mg/kg 1q28 | / | ORR | / |
| Calabro’ et al., 2013 [30] | II | open label, single arm | Tremelimumab | 15 mg/kg ev 1q90 | / | ORR | EORTC prognostic score |
| Calabro’ et al., 2015 [31] | II | open label, single arm | Tremelimumab | 10 mg/kg 1q28 for 4 cycles, then every 12 weeks | / | ORR | EORTC prognostic score |
| Nowak A.K et al., 2020, DREAM [29] | II | open label, single arm | Durvalumab + chemotherapy | Durvalumab 1125 mg 1q21 cisplatin 75 mg/m2 or carboplatin AUC5 + pemetrexed 500 mg/m2 | / | PFS | / |
| Baas P. et al., 2021, CheckMate 743 [13] | III | open label, randomized | Nivolumab + ipilimumab | Nivolumab 3 mg/kg 1q14 ipilimumab 1 mg/kg 1q42 | CT cisplatin or carboplatin + pemetrexed | OS | Sex, Histology |
| Quispel-Janssen J. et al., 2018, NivoMes [39] | II | open label, single arm | Nivolumab | 3 mg/kg 1q14 | / | DCR at 12 weeks | / |
| Popat A. et al., 2020, PROMISE-meso [11] | III | open label, randomised | Pembrolizumab | 200 mg 1q21 | gemcitabine/vinorelbine | PFS | Histology |
| Fennel DA et al., 2021, CONFIRM [12] | III | Double blind, randomized | Nivolumab | 3 mg/kg 1q14 | placebo | OS e PFS (IA, investigator assessed) | Histology |
| Okada M. et al., 2019, MERIT [38] | II | open label, single arm | Nivolumab | 240 mg 1q14 | / | ORR (CA, centrally assessed) | / |
| Maio M. et al., 2017, DETERMINE [32] | IIb | Double blind | Tremelimumab | 10 mg/kg 1q28 for 7 doses, then every 12 weeks | placebo | OS | EORTC status, line of therapy, anatomic site |
| Alley W et al., 2017, KEYNOTE-028 [35] | Ib | open label, single arm | Pembrolizumab | 10 mg/kg 1q14 o 1q21 or 2 mg/kg 1q21 | / | ORR | / |
| Yap T. et al., 2021, KEYNOTE-158 [36] | II | open label, single arm | Pembrolizumab | 200 mg 1 q21 | / | ORR (CA) | / |
| Hassan R. et al., 2019, JAVELIN [34] | Ib | open label, single arm | Avelumab | 10 mg/kg 1q14 | / | ORR (IA) | / |
| Desai et al., 2018 [40] | II | open label, single arm | Pembrolizumab | 200 mg 1q21 | / | ORR | / |
| Sherpereel A et al., 2019, MAPS-2 [37] | II | open label, randomized | Nivolumab or Nivlumab + Ipilimumab | Nivolumab 3 mg/kg ipilimumab 1 mg/kg | / | DCR | / |
| Venkatraman D et al., 2019 [41] | II | open label, single arm | Durvalumab + Tremelimumab | Durval: 1500 mg 1q28 tremel: 75 mg 1q28 | / | ORR | / |
| Disselhorst MJ et al., 2019 INITIATE [33] | II | open label, single arm | Nivolumab + Ipilimumab | Nivo: 240 mg 1q14 ipi: 1 mg/kg 1q42 | / | DCR IA 12 W | / |
| Trial | Pts n (Experimental Arm) | mFUP (Months; IQR) | mPFS (Months) (95%CI) | mOS (Months) (95%CI) | ORR (%) (IQR) | mDOR (Months) (95%CI) | DCR (%) (IQR) |
|---|---|---|---|---|---|---|---|
| Calabrò et al., 2018, NIBIT-MESO-126, [28] | 40 | 19.2 (13.8–20.5) | 8.0 (6.7–9.3) | 16.6 (13.1–20.1) | 28 (15–44) | 16.1 (IQR 11.5–20.5) | 65 (48–79) |
| Calabro’ et al., 2013 [30] | 29 | 27 (23–35) | 6.2 (1.3–11.1) | 10.7 (0–21.9) | 6.9% (0.0–16.1) | 12.4 (6–30) | 31.0 (14.2–47.9) |
| Calabro’ et al., 2015 [31] | 29 | 21.3 (18.7–25.9) | 6.2 (5.7–6.7) | 11.3 (3.4–19.2) | 3.4%; 0–10.0) | NE | 37.9 (20.2–55.6) |
| Nowak A.K et al., 2020, DREAM [29] | 54 | 28.2 (26.5–30.2) | 6.9 (5.5–9.0) | 18.4 (13.1–24.8) | 48 (35–61) | 5.6 (4.9–12.3) | 87 (80–91) |
| Baas P. et al., 2021, CheckMate 743 [13] | 605 (303) | 29.7 (26.7–32.9), | 6.8 (5.6–7.4) | 18.1 (16.9–22.0) | 40 (34.1–45.4) | 11 (1.45–3.27) | 77 (71.4–81.2) |
| Quispel-Janssen J. et al., 2018 NivoMes [39] | 34 | 27.5 (19.3–NR) | 2.6 (2.23–5.49) | 11.8 (9.7–15.7) | 24 (NR) | 7.0 (>3) | 47 (NR) |
| Popat A. et al., 2020, PROMISE-meso [11] | 144 (73) | 17.5 (9.9–14.5) | 2.5 (2.1–4.2) | 10.7 (7.6–15.0) | 22 (13–33) | 4.6 (2.1–NR) | 45 (39–55) |
| Fennel DA et al., 2021, CONFIRM [12] | 332 (221) | 11.6 (7.2–16.8) | 3.0 (2.8–4.1) | 10.2 (8.5–12.1) | 11 (NR) | 4.6 (3.0–6.9) | 12 (NR) |
| Okada M. et al., 2019, MERIT [38] | 34 | 16.8 (1.8–20.2) | 6.1 (2.9–9.9) | 17.3 (11.5–NR) | 29 (16.8–46.2) | 11.1 (3.5–16.2) | 68 (50.8–80.9) |
| Maio M. et al., 2017, DETERMINE [32] | 571 (382) | NE | NE | 7.7 (6.8–8.9) | 4.5 (2.6–7.0) | 4.8 (26–8.3) | 27.7 (16.0–28.3) |
| Alley W et al., 2017, KEYNOTE-028 [35] | 25 | 18.7 (10.4–24.0) | 5.4(3.4–7.5) | 18 (9.4-NR) | 20 (6.8–40.7) | 12.0 (3.7-NR) | 72 (NE) |
| Yap T. et al., 2021, KEYNOTE-158 [36] | 118 | 38.5 (37.5–39.2) | 2.1 (2.1–3.9). | 10.0 (7.6–13.4) | 8 (4–15) | 14.3 (4.0–33.9) | 46 (NE) |
| Hassan R. et al., 2019, JAVELIN [34] | 53 | 24.8 (16.8–27.8) | 4.1 (1.4–6.2) | 10.7 (6.4–20.2) | 19 (3.1–20.7) | 15.2 (11.1–NR) | 58 |
| Desai et al., 2018 [40] | 65 | NR | 4.5 (2.3–6.2) | 11.5 (7.6–14) | 19 | NE | 66 |
| Sherpereel A et al., 2019, MAPS-2 [37] (nivo cohort) | 125 (63) | 20.1 (19.6–20.3) | 4.1 (2.8–5.7) | 11.9 (6.7–17.7) | 19 (8–29) | 7.4 (4.1–11.9) | 40 (28–52) |
| Sherpereel A et al., 2019, MAPS-231 (nivo-ipi cohort) [37] | 125 (62) | 20.1 (19.6–20.3) | 5.6 (3.1–8.3) | 15.9 (10.7-NR) | 28 (16–40) | 8.3 (3.0–14.0) | 52 (39–64) |
| Venkatraman D et al., 2019 [41] | 19 | 7.1 (NE) | 2.8 (2.0–5.7) | 7.8 (6.2-NR) | 5 (NE) | NE | 52.6 (NE) |
| Disselhorst MJ et al., 2019 INITIATE [33] | 35 | 14.3 (12.7–15.7) | 6.2 (4.1–NR) | NR (12.7-NR) | 29 (NE) | 14.3 (6.4–NR) | 68 (NE) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemelli, M.; Cortinovis, D.L.; Baggi, A.; di Mauro, P.; Calza, S.; Berruti, A.; Grisanti, S.; Rota, M. Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 6063. https://doi.org/10.3390/cancers14246063
Gemelli M, Cortinovis DL, Baggi A, di Mauro P, Calza S, Berruti A, Grisanti S, Rota M. Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(24):6063. https://doi.org/10.3390/cancers14246063
Chicago/Turabian StyleGemelli, Maria, Diego Luigi Cortinovis, Alice Baggi, Pierluigi di Mauro, Stefano Calza, Alfredo Berruti, Salvatore Grisanti, and Matteo Rota. 2022. "Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis" Cancers 14, no. 24: 6063. https://doi.org/10.3390/cancers14246063
APA StyleGemelli, M., Cortinovis, D. L., Baggi, A., di Mauro, P., Calza, S., Berruti, A., Grisanti, S., & Rota, M. (2022). Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis. Cancers, 14(24), 6063. https://doi.org/10.3390/cancers14246063










