Prognostic Impact and Clinical Implications of Unfavorable Upgrading in Low-Risk Prostate Cancer after Robot-Assisted Radical Prostatectomy: Results of a Single Tertiary Referral Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection, Patient Selection, and Evaluation of Parameters
2.2. Study Design and Outcome of Interest
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Study Population
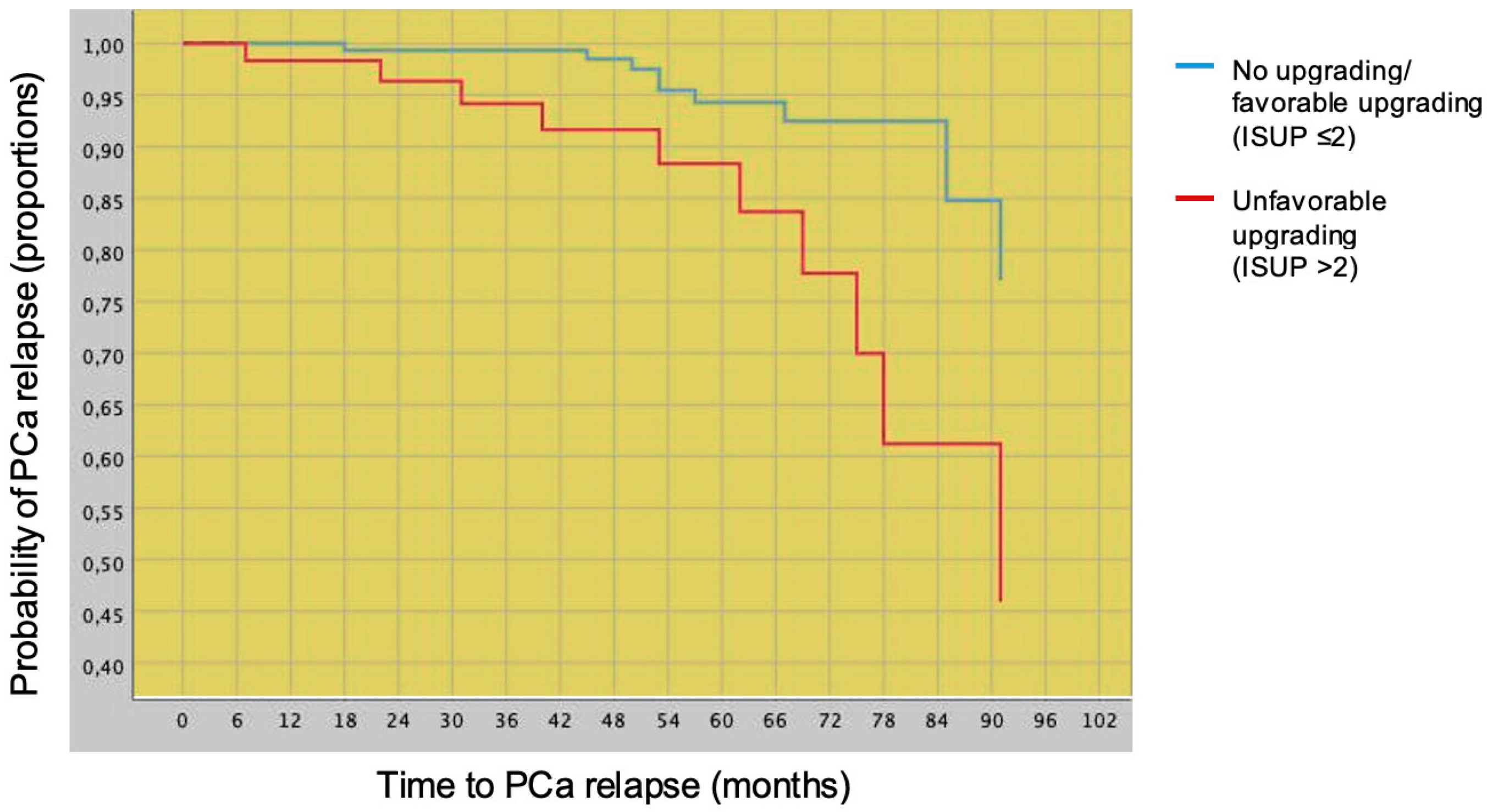
3.2. Prognostic Impact of Favorable vs. Unfavorable Tumor Upgrading on PCa Relapse after Radical Prostatectomy
3.3. Predictors of Unfavorable Tumor Upgrading at Final Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- EAU Prostate Cancer Guidelines 2022. Available online: http://www.uroweb.org (accessed on 22 July 2022).
- National Comprehensive Cancer Netweork, Prostate Cancer (Version 4.2022). Available online: http://www.nccn.org (accessed on 22 July 2022).
- Caster, J.M.; Falchook, A.D.; Hendrix, L.H.; Chen, R.C. Risk of Pathologic Upgrading or Locally Advanced Disease in Early Prostate Cancer Patients Based on Biopsy Gleason Score and PSA: A Population-Based Study of Modern Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 244–251. [Google Scholar] [CrossRef]
- Flammia, R.S.; Hoeh, B.; Hohenhorst, L.; Sorce, G.; Chierigo, F.; Panunzio, A.; Tian, Z.; Saad, F.; Leonardo, C.; Briganti, A.; et al. Adverse Upgrading and/or Upstaging in Contemporary Low-Risk Prostate Cancer Patients. Int. Urol. Nephrol. 2022, 54, 2521–2528. [Google Scholar] [CrossRef]
- Kovac, E.; Vertosick, E.A.; Sjoberg, D.D.; Vickers, A.J.; Stephenson, A.J. Effects of Pathological Upstaging or Upgrading on Metastasis and Cancer-Specific Mortality in Men with Clinical Low-Risk Prostate Cancer. BJU Int. 2018, 122, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, J.; Wenzel, P.; Salomon, G.; Budäus, L.; Schlomm, T.; Minner, S.; Wittmer, C.; Kraft, S.; Krech, T.; Steurer, S.; et al. Heterogeneity in D’Amico classification-based low-risk prostate cancer: Differences in upgrading and upstaging according to active surveillance eligibility. Urol. Oncol. 2015, 33, 329.e13–329.e19. [Google Scholar] [CrossRef]
- Davaro, F.; Weinstein, D.; Wong, R.; Siddiqui, S.; Hinyard, L.; Hamilton, Z. Increasing rate of pathologic upgrading in low-risk prostate cancer patients in the active surveillance era. Can. J. Urol. 2022, 29, 11059–11066. [Google Scholar]
- Gandaglia, G.; van den Bergh, R.C.N.; Tilki, D.; Fossati, N.; Ost, P.; Surcel, C.I.; Sooriakumaran, P.; Tsaur, I.; Valerio, M.; Kretschmer, A.; et al. How Can We Expand Active Surveillance Criteria in Patients with Low- and Intermediate-Risk Prostate Cancer without Increasing the Risk of Misclassification? Development of a Novel Risk Calculator. BJU Int. 2018, 122, 823–830. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Zhao, Z.; Huang, L.-C.; Penson, D.F.; Koyama, T.; Kaplan, S.H.; Greenfield, S.; Luckenbaugh, A.N.; Klaassen, Z.; Conwill, R.; et al. Association of Treatment Modality, Functional Outcomes, and Baseline Characteristics With Treatment-Related Regret Among Men With Localized Prostate Cancer. JAMA Oncol. 2022, 8, 50–59. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Cavicchioli, F.; Mattevi, D.; de Luyk, N.; Corsi, P.; Sebben, M.; Tafuri, A.; Processali, T.; Cerasuolo, M.; Tamanini, I.; et al. Clinical Factors of Disease Reclassification or Progression in a Contemporary Cohort of Prostate Cancer Patients Elected to Active Surveillance. Urol. Int. 2017, 98, 32–39. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Inverardi, D.; Corsi, P.; Sebben, M.; Cacciamani, G.; Tafuri, A.; Processali, T.; Pirozzi, M.; Mattevi, D.; de Marchi, D.; et al. Prostate-Specific Antigen Levels and Proportion of Biopsy Positive Cores Are Independent Predictors of Upgrading Patterns in Low-Risk Prostate Cancer. Minerva Urol. Nefrol. 2020, 72, 66–71. [Google Scholar] [CrossRef]
- Porcaro, A.B.; Cacciamani, G.E.; Sebben, M.; Tafuri, A.; Processali, T.; Rizzetto, R.; de Luyk, N.; Pirozzi, M.; Amigoni, N.; Corsi, P.; et al. Lymph Nodes Invasion of Marcille’s Fossa Associates with High Metastatic Load in Prostate Cancer Patients Undergoing Extended Pelvic Lymph Node Dissection: The Role of “Marcillectomy”. Urol. Int. 2019, 103, 25–32. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Porcaro, A.B.; Sebben, M.; Tafuri, A.; Rizzetto, R.; de Luyk, N.; Ciocchetta, E.; Processali, T.; Pirozzi, M.; Amigoni, N.; et al. Extended Pelvic Lymphadenectomy for Prostate Cancer: Should the Cloquet’s Nodes Dissection Be Considered Only an Option? Minerva Urol. Nefrol. 2019, 71, 136–145. [Google Scholar] [CrossRef]
- Sebben, M.; Tafuri, A.; Shakir, A.; Pirozzi, M.; Processali, T.; Rizzetto, R.; Amigoni, N.; Tiso, L.; de Michele, M.; Panunzio, A.; et al. The Impact of Extended Pelvic Lymph Node Dissection on the Risk of Hospital Readmission within 180 Days after Robot Assisted Radical Prostatectomy. World J. Urol. 2020, 38, 2799–2809. [Google Scholar] [CrossRef]
- van der Kwast, T.H.; Amin, M.B.; Billis, A.; Epstein, J.I.; Griffiths, D.; Humphrey, P.A.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working Group 2: T2 Substaging and Prostate Cancer Volume. Mod. Pathol. 2011, 24, 16–25. [Google Scholar] [CrossRef]
- Parry, M.G.; Cowling, T.E.; Sujenthiran, A.; Nossiter, J.; Berry, B.; Cathcart, P.; Aggarwal, A.; Payne, H.; van der Meulen, J.; Clarke, N.W.; et al. Risk Stratification for Prostate Cancer Management: Value of the Cambridge Prognostic Group Classification for Assessing Treatment Allocation. BMC Med. 2020, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; Cumberbatch, M.; de Santis, M.; Tilki, D.; Fanti, S.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur. Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef]
- Tilki, D.; Preisser, F.; Graefen, M.; Huland, H.; Pompe, R.S. External Validation of the European Association of Urology Biochemical Recurrence Risk Groups to Predict Metastasis and Mortality After Radical Prostatectomy in a European Cohort. Eur. Urol. 2019, 75, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Imnadze, M.; Sjoberg, D.D.; Vickers, A.J. Adverse Pathologic Features at Radical Prostatectomy: Effect of Preoperative Risk on Oncologic Outcomes. Eur. Urol. 2016, 69, 143–148. [Google Scholar] [CrossRef]
- Nasri, J.; Barthe, F.; Parekh, S.; Ratnani, P.; Pedraza, A.M.; Wagaskar, V.G.; Olivier, J.; Villers, A.; Tewari, A. Nomogram Predicting Adverse Pathology Outcome on Radical Prostatectomy in Low-Risk Prostate Cancer Men. Urology 2022, 166, 189–195. [Google Scholar] [CrossRef]
- Butler, S.S.; Mahal, B.A.; Lamba, N.; Mossanen, M.; Martin, N.E.; Mouw, K.W.; Nguyen, P.L.; Muralidhar, V. Use and Early Mortality Outcomes of Active Surveillance in Patients with Intermediate-Risk Prostate Cancer. Cancer 2019, 125, 3164–3171. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Bianchi, A.; Gallina, S.; Serafin, E.; Mazzucato, G.; Vidiri, S.; D’Aietti, D.; Rizzetto, R.; Tafuri, A.; Cerrato, C.; et al. Advanced Age Portends Poorer Prognosis after Radical Prostatectomy: A Single Center Experience. Aging Clin. Exp. Res. 2022, 34, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, A.; Iwata, A.; Shakir, A.; Iwata, T.; Gupta, C.; Sali, A.; Sugano, D.; Mahdi, A.S.; Cacciamani, G.E.; Kaneko, M.; et al. Systematic Biopsy of the Prostate Can Be Omitted in Men with PI-RADSTM 5 and Prostate Specific Antigen Density Greater than 15. J. Urol. 2021, 206, 289–297. [Google Scholar] [CrossRef] [PubMed]

| Overall Cohort n = 237 (100%) | No PCa Relapse n = 217 (91.6%) | PCa Relapse n = 20 (8.4%) | Univariable Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | ||||
| Clinical factors | |||||
| Age (years) | 65 (60–69) | 65 (59–69) | 66 (61–70) | 1.03 (0.96–1.12) | 0.411 |
| BMI (kg/m2) | 26.0 (24.0–28.0) | 26.1 (23.9–28.1) | 25.5 (24.2–26.8) | 0.99 (0.84–1.15) | 0.867 |
| PV (mL) | 40.0 (30.0–50.0) | 40.0 (30.5–50.0) | 39.5 (26.2–51.5) | 0.99 (0.97–1.02) | 0.995 |
| PSA (ng/mL) | 5.8 (4.5–7.6) | 5.7 (4.5–7.5) | 6.5 (4.1–9.0) | 1.15 (0.92–1.14) | 0.208 |
| PSAD < 0.15 (ng/mL/cc) | 128 (54.0) | 120 (55.3) | 8 (40.0) | Ref. | |
| PSAD ≥ 0.15 (ng/mL/cc) | 109 (46.0) | 97 (44.7) | 12 (60.0) | 1.80 (0.74–4.42) | 0.198 |
| Biopsy core taken (n) | 14 (12–15) | 14 (12–15) | 15 (12–16) | ||
| BPC (%) | 25.0 (14.0–35.5) | 25.0 (14.0–35.0) | 29.0 (16.2–36.0) | 1.01 (0.98–1.03) | 0.543 |
| cT1 | 215 (90.7) | 197 (90.8) | 18 (90.0) | Ref. | |
| cT2a | 22 (9.3) | 20 (9.2) | 2 (10.0) | 1.88 (0.43–8.17) | 0.401 |
| Pathological factors | |||||
| ISUP 1 | 79 (33.3) | 76 (35.0) | 3 (15.0) | Ref. | |
| ISUP 2 | 98 (41.4) | 91 (41.9) | 7 (35.0) | 2.52 (0.65–9.76) | 0.182 |
| ISUP > 2 | 60 (25.3) | 50 (23.1) | 10 (50.0) | 5.81 (1.59–21.16) | 0.008 |
| PW (g) | 52.0 (43.0–66.0) | 52.0 (43.0–65.5) | 54.5 (41.0–72.2) | 1.00 (0.98–1.03) | 0.949 |
| TL (%) | 10.0 (6.2–20.0) | 10.0 (5.0–20.0) | 15.0 (10.0–20.0) | 0.99 (0.95–1.03) | 0.749 |
| pT2 | 219 (92.4) | 202 (93.1) | 17 (85.0) | Ref. | |
| EPE (ECE or SVI) | 18 (7.6) | 15 (6.9) | 3 (15.0) | 2.17 (0.64–7.42) | 0.216 |
| Negative surgical margins | 186 (78.5) | 176 (81.1) | 10 (50.0) | Ref. | |
| Positive surgical margins | 51 (21.5) | 41 (18.9) | 10 (50.0) | 3.67 (1.51–8.92) | 0.004 |
| Endpoint | HR (95% CI) | p-Value |
|---|---|---|
| Hazard ratio adjusted for clinical factors (*) | 3.067 (1.168–8.053) | 0.023 |
| Hazard ratio adjusted for surgical margins status (*) | 2.937 (1.209–7.132) | 0.017 |
| Hazard ratio adjusted for tumor load and unfavorable tumor stage (*) | 3.935 (1.444–10.723) | 0.007 |
| No Upgrading (ISUP 1) or Favorable Tumor Upgrading (ISUP 2) n = 177 (74.7%) | Unfavorable Tumor Upgrading (ISUP > 2) n = 60 (25.3%) | Univariable Analysis | Multivariable Analysis (*) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Clinical factors | Clinical model | |||||
| Age (years) | 65 (58–68) | 67 (63–71) | 1.08 (1.02–1.13) | 0.006 | 1.07 (1.02–1.13) | 0.009 |
| BMI (kg/m2) | 26.2 (24.2–28.0) | 25.4 (23.7–27.9) | 0.99 (0.90–1.10) | 0.857 | ||
| PSA (ng/mL) | 5.6 (4.5–7.1) | 6.5 (4.5–8.7) | 1.18 (1.02–1.36) | 0.028 | ||
| PV (mL) | 40.0 (30.5–52.0) | 40.0 (30.0–47.0) | 0.99 (0.97–1.01) | 0.145 | ||
| BPC (%) | 24 (14–33) | 29 (15.5–42.7) | 1.011 (0.994–1.028) | 0.202 | ||
| PSAD < 0.15 (ng/mL/cc) | 105 (59.3) | 23 (38.3) | Ref. | Ref. | ||
| PSAD ≥ 0.15 (ng/mL/cc) | 72 (40.7) | 37 (61.7) | 2.35 (1.29–4.27) | 0.005 | 2.32 (1.26–4.27) | 0.007 |
| cT1c | 165 (93.2) | 50 (83.3) | Ref. | |||
| cT2a | 12 (6.8) | 10 (16.7) | 2.75 (1.12–6.74) | 0.027 | ||
| Pathological factors | Pathological model | |||||
| PW (g) | 58.0 (44.0–69.3) | 50.5 (42.0–60.0) | 0.99 (0.98–1.01) | 0.432 | ||
| TL (%) | 10.0 (5.0–20.0) | 15.0 (11.2–24.2) | 1.03 (1.01–1.06) | 0.011 | 1.03 (1.00–1.05) | 0.031 |
| pT2 | 172 (97.2) | 47 (78.3) | Ref. | Ref. | ||
| EPE (ECE or SVI) | 5 (2.8) | 13 (21.7) | 9.52 (3.23–28.04) | <0.001 | 8.54 (2.88–25.32) | <0.001 |
| Negative surgical margins | 148 (83.6) | 38 (63.3) | Ref. | |||
| Positive surgical margins | 29 (16.4) | 22 (36.7) | 2.96 (1.60–5.71) | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcaro, A.B.; Panunzio, A.; Bianchi, A.; Sebben, M.; Gallina, S.; De Michele, M.; Orlando, R.; Serafin, E.; Mazzucato, G.; Vidiri, S.; et al. Prognostic Impact and Clinical Implications of Unfavorable Upgrading in Low-Risk Prostate Cancer after Robot-Assisted Radical Prostatectomy: Results of a Single Tertiary Referral Center. Cancers 2022, 14, 6055. https://doi.org/10.3390/cancers14246055
Porcaro AB, Panunzio A, Bianchi A, Sebben M, Gallina S, De Michele M, Orlando R, Serafin E, Mazzucato G, Vidiri S, et al. Prognostic Impact and Clinical Implications of Unfavorable Upgrading in Low-Risk Prostate Cancer after Robot-Assisted Radical Prostatectomy: Results of a Single Tertiary Referral Center. Cancers. 2022; 14(24):6055. https://doi.org/10.3390/cancers14246055
Chicago/Turabian StylePorcaro, Antonio Benito, Andrea Panunzio, Alberto Bianchi, Marco Sebben, Sebastian Gallina, Mario De Michele, Rossella Orlando, Emanuele Serafin, Giovanni Mazzucato, Stefano Vidiri, and et al. 2022. "Prognostic Impact and Clinical Implications of Unfavorable Upgrading in Low-Risk Prostate Cancer after Robot-Assisted Radical Prostatectomy: Results of a Single Tertiary Referral Center" Cancers 14, no. 24: 6055. https://doi.org/10.3390/cancers14246055
APA StylePorcaro, A. B., Panunzio, A., Bianchi, A., Sebben, M., Gallina, S., De Michele, M., Orlando, R., Serafin, E., Mazzucato, G., Vidiri, S., D’Aietti, D., Princiotta, A., Montanaro, F., Marafioti Patuzzo, G., De Marco, V., Brunelli, M., Pagliarulo, V., Cerruto, M. A., Tafuri, A., & Antonelli, A. (2022). Prognostic Impact and Clinical Implications of Unfavorable Upgrading in Low-Risk Prostate Cancer after Robot-Assisted Radical Prostatectomy: Results of a Single Tertiary Referral Center. Cancers, 14(24), 6055. https://doi.org/10.3390/cancers14246055









