Simple Summary
Accumulating evidence indicates that hypoxia-inducible factor-1 (HIF-1) plays a pivotal role in tumor biology, particularly in hypoxic environments. Over the past few decades, a number of HIF-1 inhibitors have been identified as potential therapeutic agents for various cancers. However, none of these inhibitors have been successfully translated into clinically available cancer treatments. This review describes the HIF-1 pathway and its roles in tumor proliferation, angiogenesis, and metastasis. In addition, the implications of HIF-1 in the development of drug resistance and cancer-related pain are explored. Finally, the current status of HIF-1 inhibitors in clinical trials and their future perspectives are highlighted, along with their modes of action. This review provides new insights into anticancer drug development targeting HIF-1. HIF-1 inhibitors may be promising combinational therapeutic interventions to improve the efficacy of current cancer treatments and reduce drug resistance and cancer-related pain.
Abstract
Hypoxia-inducible factor-1 (HIF-1) is a key transcription factor that regulates the transcription of many genes that are responsible for the adaptation and survival of tumor cells in hypoxic environments. Over the past few decades, tremendous efforts have been made to comprehensively understand the role of HIF-1 in tumor progression. Based on the pivotal roles of HIF-1 in tumor biology, many HIF-1 inhibitors interrupting expression, stabilization, DNA binding properties, or transcriptional activity have been identified as potential therapeutic agents for various cancers, yet none of these inhibitors have yet been successfully translated into clinically available cancer treatments. In this review, we briefly introduce the regulation of the HIF-1 pathway and summarize its roles in tumor cell proliferation, angiogenesis, and metastasis. In addition, we explore the implications of HIF-1 in the development of drug resistance and cancer-related pain: the most commonly encountered obstacles during conventional anticancer therapies. Finally, the current status of HIF-1 inhibitors in clinical trials and their perspectives are highlighted, along with their modes of action. This review provides new insights into novel anticancer drug development targeting HIF-1. HIF-1 inhibitors may be promising combinational therapeutic interventions to improve the efficacy of current cancer treatments and reduce drug resistance and cancer-related pain.
1. Introduction
Cancer is the second major cause of death worldwide, and its incidence is rapidly increasing [1]. For decades, oncologists have continuously endeavored to improve the efficacy and safety of cancer treatments. Various anticancer interventions, including chemotherapy, radiation, and immunotherapy, have been introduced into clinical situations, yet most of the current conventional cancer treatments are found to exhibit certain limitations mainly due to insufficient efficacy, unwanted side effects on the tumor-bearing host, or the development of resistance to the therapy [2,3]. In an attempt to overcome these obstacles, considerable attention has been paid to identifying and characterizing novel and unique molecular targets involved in the regulation of tumor cell proliferation, angiogenesis, and metastasis. Thus, drugs for this targeted therapy deserve particular attraction due to their specificity toward cancer cells while sparing toxicity to non-target host cells [4,5].
Hypoxia (low oxygen level) is one of the hallmarks of the tumor microenvironment. The hypoxic tumor microenvironment of solid tumors is developed due to transient fluctuations in the blood flow (acute hypoxia) or a deficient oxygen supply (chronic hypoxia) [6]. A large number of target genes involved in the processes of tumor progression are triggered to increase oxygen delivery to the hypoxic environment, decrease oxygen consumption, or activate alternative metabolic pathways [6]. One of the important mechanisms is the activation of hypoxia-inducible factor-1 (HIF-1), as a key transcription factor that regulates the transcription of many genes responsible for the adaptation, survival, and aggressiveness of tumor cells [7,8,9]. HIF-1 is also known to facilitate cancer promotion by regulating natural killer cell-mediated antitumor responses and preventing cancer cells from cytotoxic T-lymphocytes induced in the tumor microenvironment [10]. Thus, HIF-1 may affect tumor cell survival by directly and indirectly influencing cancer progression and immunity [4]. Moreover, the overexpression of HIF-1 is associated with poor prognosis and the development of resistance to chemo/radiotherapy in multiple types of human cancers [11,12,13]. From the outcomes of phase II and III clinical trials of HIF inhibitors in the treatment of several types of cancers [14], it is evident that HIF-1 is a promising target for the development of novel cancer therapy. However, none of these inhibitors have thus far been successfully translated into clinical treatments. Therefore, it is necessary to gain an in-depth understanding of the molecular mechanisms by which HIF-1 regulates tumor biology, as well as the benefits and warrants of the current HIF inhibitors.
In this review, we briefly introduce the regulation of the HIF-1 signaling pathway and summarize its roles in tumor progression. In addition, we explore the implications of HIF-1 in the development of drug resistance and cancer-related pain: the most commonly encountered obstacles during conventional anticancer therapies. Furthermore, the current status of HIF-1 inhibitors in clinical trials and their modes of action and perspectives are highlighted in this review. This review may provide new paradigms for treating cancers with HIF-1 inhibitors, either as a single agent or in combination with other conventional therapeutic agents, to improve therapeutic efficacy and minimize drug resistance and cancer-related pain.
2. Regulation of HIF-1 Activity in Normoxic and Hypoxic Conditions
HIFs are heterodimeric transcription factors belonging to the basic helix-loop-helix Per/ARNT/Sim (PAS) family that induce the transcription of various genes in response to the cellular oxygen tension [15]. HIFs are composed of a hypoxically inducible α subunit and a constitutively expressed β subunit [15,16,17]. There are three isoforms of the α subunit in humans: HIF-1α, HIF-2α, and HIF-3α. Extensive investigations have focused on the functions of the different α subunits in various diseases, such as cancers that frequently overexpress the α subunits [17]. In this review, we focus on HIF-1α as the most well-studied isoform in tumor biology.
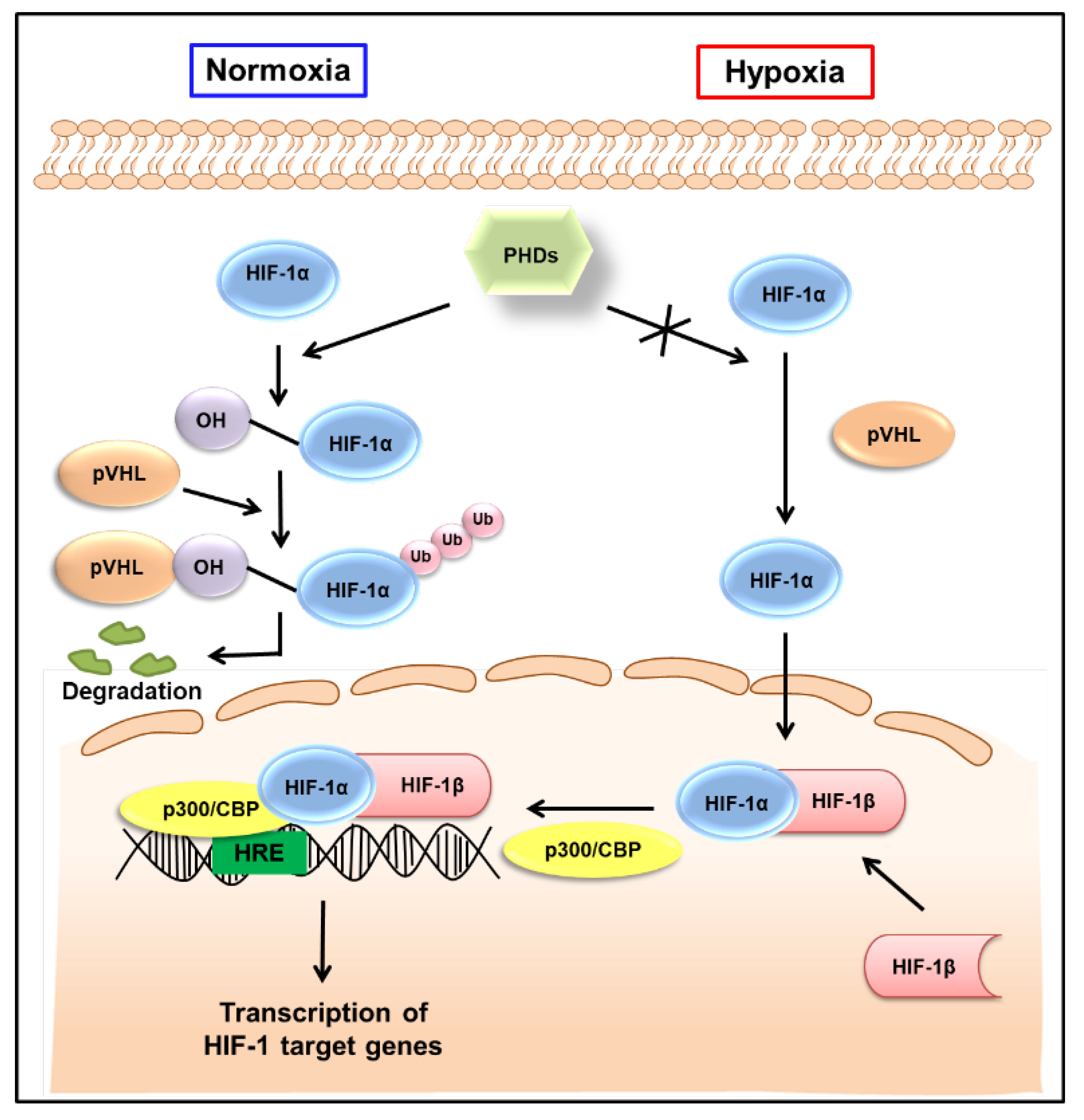
The HIF-1 signaling pathway has been extensively reviewed elsewhere [18,19]. As shown in Figure 1, HIF-1α is hydroxylated by the prolyl hydroxylase activity of the prolyl hydroxylase domain (PHD)-containing proteins in normoxic conditions, allowing von the Hippel−Lindau tumor suppressor protein (pVHL) to interact with HIF-1α, eventually leading to its degradation. By contrast, HIF-1α is stable in hypoxic conditions. It is translocated to the nucleus and, together with HIF-1β, interacts with the transcriptional coactivator p300/cyclic AMP response element-binding protein (CBP) and binds to the hypoxia-responsive elements (HREs) in DNA to induce the transcription of target genes (Figure 1). The HIF-1 target genes are well-known to be involved in tumor progression, promoting tumor cell growth, vascularization, and metastasis [7]. As HIF-1β is constitutively expressed in the nucleus, the transcriptional activity of HIF-1 primarily depends on the expression and activation of HIF-1α [20]. Therefore, HIF-1α has been considered a promising target to interfere with many aspects of tumor progression.
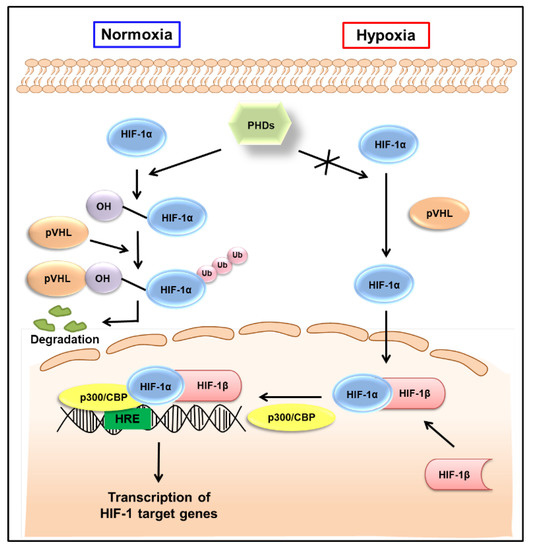
Figure 1.
Regulation of HIF-1 activity in normoxic and hypoxic conditions. Under normoxic conditions, HIF-1α is hydroxylated on prolyl residues by PHDs, allowing pVHL to recognize and interact with HIF-1α, eventually leading to the degradation of HIF-1α. By contrast, HIF-1α is stable in hypoxic conditions. It is translocated to the nucleus, where, together with HIF-1β, it binds to hypoxia-responsive elements (HREs) and exerts its transcriptional activity. HIF-1—hypoxia-inducible factor-1; PHDs—prolyl hydroxylase domains; pVHL—von Hippel−Lindau tumor suppressor protein; Ub—ubiquitination; CBP—cyclic adenosine monophosphate response element binding protein.
3. Roles of HIF-1 in Tumor Progression
HIF-1 has been demonstrated to be involved in the transcriptional activation of essential genes that regulate the critical processes required for tumor survival and progression. In addition, it is evident that HIF-1 plays a key role in innate and adaptive immune responses and inflammation and, thus, is also considered to be a major transcriptional regulator of immunity and inflammation [21]. The molecular mechanism(s) mediating the initiation and progression of tumors by HIF-1-regulated inflammation includes the increased production of pro-inflammatory mediators, such as cytokines and chemokines, and pro-inflammatory transcription factors, such as nuclear factor κB (NF-κB) [22,23]. Due to the complex role of HIF-1 in various inflammatory cells, an extensive description is necessary to characterize the multifaceted link between inflammation and cancer [24,25]. Since HIF-1 has been strongly indicated in a myriad of cancer cell activities during tumor progression, from the fundamental stages of tumor growth and migration to metastasis [26], we focus on how HIF-1 triggers the transcriptional activation of genes that modulate tumor cell proliferation, angiogenesis, and metastasis in this section.
3.1. Role of HIF-1 in Tumor Cell Proliferation and Survival
Increasing evidence suggests that, upon stimulation by growth factors, phosphatidyl inositol-4,5-bisphosphate-3-kinase (PI3K) can upregulate the expression of HIF-1α through the activation of protein kinase B (Akt) and the mammalian target of rapamycin (mTOR), a serine/threonine kinase downstream of Akt, in various human cancers [27]. HIF-1α then induces the expression of various growth factors known to promote cell migration, regeneration, and proliferation, such as transforming growth factor-β (TGF-β), insulin-like growth factor 2 (IGF2), endothelin-1 (END-1), erythropoietin (EPO), and macrophage migration inhibitory factor (MIF) [18,28,29]. In addition, growth factor receptors, such as the epidermal growth factor receptor (EGFR), are also known to be induced by HIF-1α [18,28].
Tumor cells exhibit increased metabolic flexibility and adaptability to sustain their cell growth and survival in the tumor microenvironment characterized by relative nutrient deprivation, hypoxia, and hypovascularity. Unlike normal cells, tumor cells tend to turn their metabolism from an oxygen-dependent tricarboxylic acid (TCA) cycle to glycolysis: an oxygen-independent pathway [30]. Glycolysis is a principal route of ATP synthesis in tumor cells and has been comprehensively reviewed [30]. Increased glycolysis is thought to be a natural response to the hypoxic condition of rapidly migrating cells via the HIF-1 pathway [31]. HIF-1 activation increases many glycolytic enzymes, including hexokinase 2 (HK-2) and phosphofructokinase 1 (PFK1), which appear to be oncogenes required to mediate tumor initiation and growth [32,33]. Another glycolytic enzyme, pyruvate dehydrogenase kinase (PDK), is also augmented by HIF-1. The upregulation of PDK1 decreases acetyl-CoA flux into the TCA cycle, subsequently converting the metabolic program from mitochondrial respiration to glycolysis [34,35]. Moreover, the HIF-1-enhanced induction of lactate dehydrogenase (LDH) mediates the conversion of pyruvate to lactate, accompanied by the recycling of cytosolic NAD+, which is necessary for further glycolysis [36]. In addition to glycolysis, HIF-1 also supports the process of glucose uptake and oxidation by inducing the expression of glucose transporters (GLUT), including GLUT1 and GLUT3 [37], which then increase cellular glucose uptake and oxidation. The intermediary metabolites of the glycolytic pathway supply precursors for the synthesis of glycine, serine, purines, pyrimidines, and phospholipids, necessitating HIF-1-regulated metabolic reprogramming for tumor cell growth and maintenance under stress [28]. These modifications by activated HIF-1 ultimately enhance the ability of tumor cells to upregulate glycolysis, protect cells from oxidative damage, and promote ATP production to sustain tumor cell proliferation, even in the absence of oxygen. These findings illustrate the notable efforts to restrain these processes by inhibiting HIF-1. For example, a novel HIF-1α inhibitor, IDF-11774, blocking HIF-1α accumulation under hypoxia in human colon cancer cells has been found to suppress tumor growth by inhibiting the expression of GLUT1 and PDK1 [38]. Recently, the disubstituted adamantyl derivative LW1564 has been reported to impair tumor metabolism by suppressing HIF-1α accumulation, thereby inhibiting the tumor growth of hepatocellular carcinoma cells in vitro and in vivo [39].
Cell proliferation requires the precise spatiotemporal regulation of the intracellular pH [40]. In hypoxia, pyruvate is converted to lactate in a reaction catalyzed by LDH, and the accumulation of lactic acid may result in an acidic environment. Tumors adapt to pH changes and control their normal proliferation function by carbonic anhydrases (CAs), which reversely convert carbon dioxide and water to carbonic acid. The activity of CAIX and CAXII are decreased by VHL [41] and are significantly induced in hypoxic conditions [42,43,44], suggesting that their transcription may be regulated by HIF-1. Numerous CA inhibitors are also emerging as promising agents for the treatment of hypoxic tumors by disrupting HIF-1-regulated pH homeostasis [43,44,45]. Thus, regulation of the intracellular pH by HIF-1 also plays an important role in driving tumor proliferation.
A balance between cell proliferation and apoptotic cell death is critical in maintaining biological processes and homeostasis. The dysregulation of this balance has been implicated in multiple types of disease, including cancer [46]. It has been demonstrated that the overexpression of HIF-1α inhibits hypoxia-induced apoptosis in human oral squamous cell carcinoma cell lines via inhibiting cytochrome c release from the mitochondria and reactive oxygen species (ROS) generation. An increased expression of anti-apoptotic Bcl-2 and Bcl-XL, as well as decreased levels of pro-apoptotic Bax and Bak, were also observed [47]. Moreover, cobalt chloride, a chemical inducer of HIF-1, reduced the tert-butyl hydroperoxide-induced apoptotic death of HepG2 human hepatoma cells, as measured by DNA fragmentation [48]. These data suggest that HIF-1 may exert an anti-apoptotic role in various types of cancer cells.
Autophagy is a highly conserved mechanism in which cell contents are transported to lysosomes for breakdown or are utilized to produce macromolecules for energy synthesis [49]. The evidence suggests that HIF-1α-mediated autophagy in tumor cells supports cell survival but not cell death [50]. It was previously reported that HIF-1 was required for hypoxia-induced mitochondrial autophagy (mitophagy), preventing oxidative phosphorylation during the adaptation of tumor cells to hypoxia [51]. The HIF-1-induced autophagy process was then demonstrated as a survival mechanism by inducing the expression of Bcl-2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3) and a similar protein BNIP3L to disrupt the Bcl-2/Beclin-1 or Bcl-XL/Beclin-1 complex [50]. Another possible mechanism of HIF-1-dependent autophagy involves the p27/E2 transcription factor 1 (E2F1) signaling pathway. HIF-1 positively modulates p27, subsequently repressing E2F1 activity to induce autophagy [52]. The HIF-1α-dependent upregulation of autophagy and cell survival has been postulated in prostate cancer [53], pancreatic cancer [54], and colorectal cancer (CRC) [55].
Collectively, HIF-1 plays crucial roles in tumor cell proliferation and survival. HIF-1 stimulates the expression of many growth factors, such as TGF-β, IGF2, END-1, and EPO, as well as growth factor receptors, such as EGFR. In addition, HIF-1 regulates the metabolic pathways of tumor cells to gain energy and nutrients for survival and growth by promoting aerobic glycolysis and autophagy. The regulation of the intracellular pH and apoptosis by HIF-1 may also contribute to tumor cell proliferation and growth. Further investigation of a wide variety of tumor cell types under different hypoxic conditions may consolidate the roles of HIF-1 in tumor cell proliferation and survival.
3.2. Role of HIF-1 in Angiogenesis
Angiogenesis, the physiological process through which new blood vessels form from pre-existing vessels, is also a widely known hallmark of cancers [56]. The sustained expansion of a tumor mass requires new blood vessel formation to rapidly provide proliferative tumor cells with a sufficient supply of oxygen and nutrients [57]. Multiple HIF-1-targeted genes have been shown to modulate angiogenesis [58,59]. Thus, HIF-1 has become an attractive target for cancer therapy [60,61].
HIF-1 activates angiogenesis by stimulating the production of the vascular endothelial growth factor (VEGF) and many other angiogenic factors, such as the placenta-like growth factor (PLGF), platelet-derived growth factor-β (PDGF-β), and angiopoietin (ANG) [62]. For example, HIF-1 binds to HRE within the VEGF promoter, resulting in increased gene transcription. In addition, VEGF mRNA levels are shown to be enhanced by HIF-1 through the regulation of mRNA stability [63]. VEGF then binds to five different receptors: VEGFR-1, VEGFR-2, VEGFR-3, neuropilin-1, and neuropilin-2. VEGFR signaling leads to a cascade of events, including the migration and proliferation of endothelial cells (ECs) and the induction of vascular permeability in tumor vessels [64]. HIF-1α also induces VEGFR-1, -2, and -3 during hypoxia in vascular and lymphatic ECs [65,66].
ANGs are the ligands of the Tie-2 receptor tyrosine kinase. While ANG-1 plays an essential role in embryonic angiogenesis, both ANG-1 and ANG-2 have been demonstrated to be involved in tumor angiogenesis [67,68]. By contrast, ANG-3 was reported to inhibit the tumor angiogenesis and metastasis of Lewis lung carcinoma and TA3 mammary carcinoma cells, probably through the inhibition of ANG-1- and VEGF-induced activation of extracellular signal-regulated protein kinase 1/2 (ERK1/2) and Akt [68]. Using primary human ECs, ANG-4 was demonstrated to be induced by HIF-1 and participate in hypoxia-induced angiogenesis. These findings support the key roles of HIF-1 in the angiogenic processes of different types of tumor cells [69].
Additionally, HIF-1α promotes angiogenesis by regulating the matrix metalloproteinases (MMPs), plasminogen activator inhibitor (PAI), and vascular tone governing nitric oxide synthase (NOS) [70,71]. The MMP-mediated digestion of the extracellular matrix (ECM) initially allowed the proliferative ECs to migrate through the matrix as an essential step for the development of new blood vessels. MMP-2, which correlates with the tumor grade and vascularity in many cancer types, such as human astrocytoma, is shown to be upregulated by HIF-1-dependent pathways in hypoxia [72]. HIF-1 also increased the expression of PAI-1, which plays an important role in the degradation of ECM proteins and the facilitation of cell migration, contributing to angiogenesis [73]. Moreover, in hypoxia, the HIF-1α-induced expression of inducible NOS (iNOS) further increases NO concentration, which contributes to tumor progression by promoting the neovascularization of tumor masses [74]. The relationship between HIF-1α and NO in cancer angiogenesis appears quite complex and poorly understood. Thus, further study is needed for clarification. Taken together, accumulating evidence supports the fact that hypoxia and HIF-1 are the key regulators of blood vessel growth through the upregulation of many pivotal angiogenic factors, particularly VEGF, ANG, MMPs, PAI-1, and NOS.
3.3. Role of HIF-1 in Tumor Cell Metastasis
Metastasis is a highly complex process that accounts for the majority of deaths in cancer patients [75]. Hypoxia-induced HIF-1 affects multiple steps within the metastatic cascade, including epithelial−mesenchymal transition (EMT), intravasation, extravasation, and pre-metastatic niche formation [76].
EMT is considered an early step in the metastasis process, which is defined by the loss of epithelial cell−cell adhesion and the acquisition of mesenchymal characteristics. While E-cadherin deficiency lowers cell adhesion junctions and cell polarity, the mesenchymal proteins reorganize the cytoskeleton to facilitate a motile phenotype of the tumor cells [75]. HIF-1 directly induces the transcription of Snail [77], ZEB1 [78], TWIST [79], and TCF3 [80], whose gene products, in turn, repress E-cadherin expression. HIF-1 also indirectly promotes EMT via other signaling pathways, including chemokines [23], NF-κB [81], TGF-β [82], and Notch [83,84] signaling pathways.
Intravasation is the entry of tumor cells into the circulatory system and is required for cell survival in this transit as circulating tumor cells (CTCs). Before penetrating the surrounding interstitial ECM, EMT tumor cells first have to destroy the integrity of the basement membrane (BM). Tumor cells regulate their surface receptors, such as integrins, to form a connection with BM components and invade through this link or secrete collagen-degrading enzymes, such as MMPs [75]. HIF-1 is shown to activate the expression of the urokinase plasminogen activator surface receptor [85] and hepatocyte growth factor receptor [86] to alter the interactions between integrins and ECM. The HIF-1-dependent pathway is also involved in the upregulation of MMP-2 and MMP-9, which are mediators of BM degradation [87]. Furthermore, HIF-1-induced VEGF can facilitate vascular permeability, thereby increasing the chances of intravasation by tumor cells. To survive in the bloodstream, the CTCs must avoid anoikis: a type of apoptosis caused by a lack of integrin attachment to ECM. HIF-1 has been demonstrated to mediate anoikis resistance by suppressing the α5 integrin [88].
Extravasation occurs in a distant organ where the blood vessels are usually formed and nurtured, posing a difficult barrier for tumor cells to overcome [75]. CTCs should adhere to ECs and disrupt their connection with ECs to extravasate at the metastatic site. Hypoxia-induced HIF-1 is considered a stimulant of the extravasation of breast cancer to lung cancer via the HIF-1-mediated L1 cell adhesion molecule (L1CAM) and angiopoietin-like 4 (ANGPTL4) [89]. While L1CAM increases the adherence of breast cancer cells to EC monolayers via hemophilic or heterophilic interactions, ANGPTL4 blocks EC−EC interactions, facilitating the vascular metastasis of breast cancer cells to the lung parenchyma. In addition, the tumor cell extravasation into host organs is also a result of chemokine interaction within the microenvironment of specific organs. The interaction of EC-secreted stromal cell-derived factor-1, also known as C-X-C motif chemokine 12 (CXCL12), and C-X-C chemokine receptor 4 (CXCR4) by HIF-1 under hypoxic conditions serve as a good illustration of this step [90]. The CXCL12/CXCR4 axis is also reported to participate in the extravasation of metastasizing cancer cells [91,92]. Based on these findings, the HIF-1-stimulated CXCL12/CXCR4 axis seems necessary for the extravasation of organ-specific sites.
Finally, the metastatic site must be primed before cancer cell arrival to present a suitable microenvironment for cell survival and colonization at the new distant organs (the pre-metastatic niche) [75]. During pre-metastatic niche formation, bone marrow-derived cells are recruited to metastatic sites where they form cell clusters before tumor cell arrival, and then they are eventually colonized by metastatic cancer cells [93]. The lysyl oxidase (LOX) family remodels collagen cross-linking at the site of the pre-metastatic niche and is essential for the recruitment of bone marrow-derived cells [93,94]. It is speculated that HIF-1 is a master regulator of breast cancer metastatic niche formation to the lungs through the secretion of multiple members of LOX. As a result, ECM increases the tensile strength for focal adhesion formation, thereby enhancing lung cancer cell colonization [93,95].
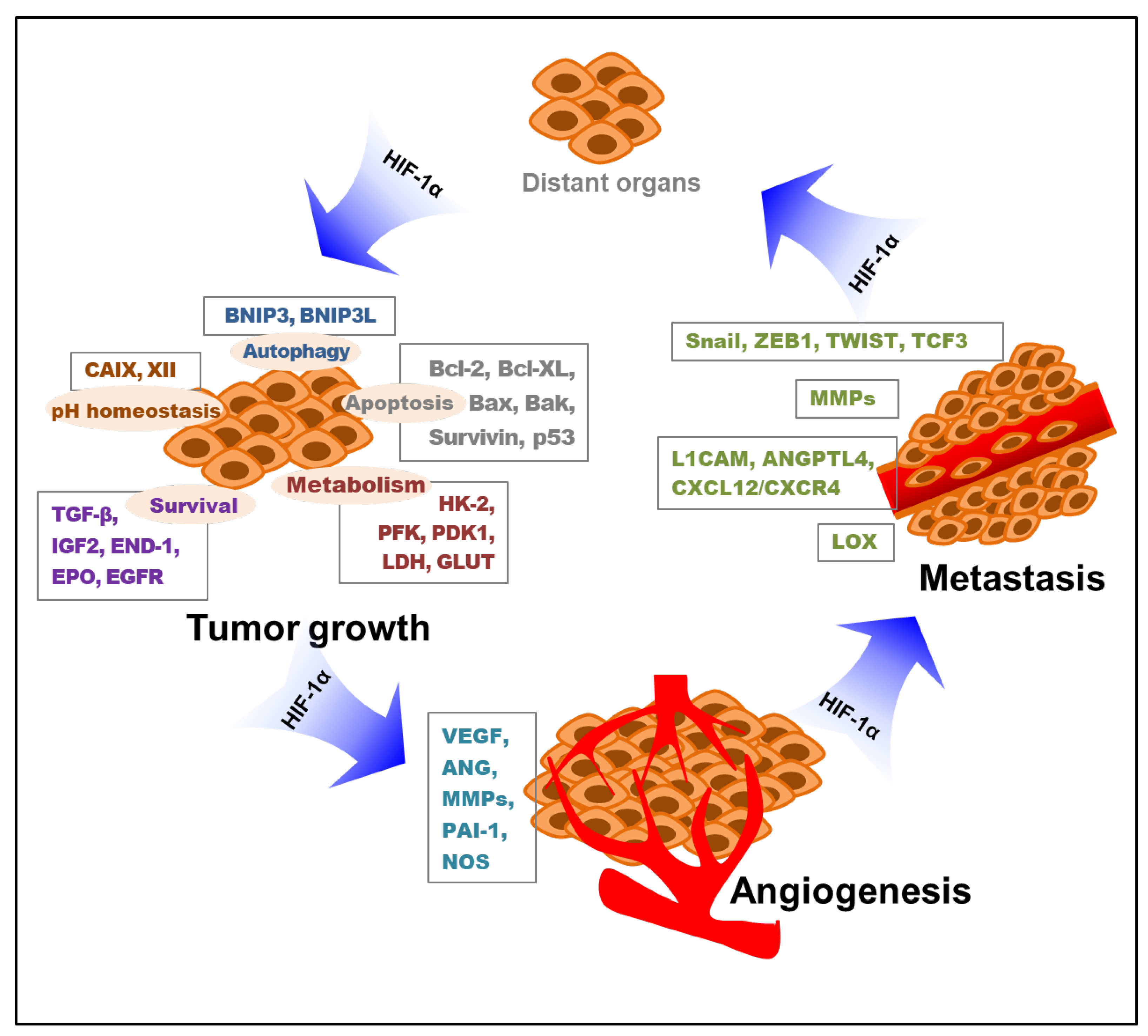
Briefly, hypoxia-induced HIF-1 influences all the important processes of metastasis, from the induction of EMT to their survival in circulation and metastatic colonization. This implies a therapeutic angle whereby patients with metastatic cancer with high levels of HIF-1 may benefit from HIF-1 inhibitors. Collectively, hypoxia-stimulated HIF-1 mediates tumor cell proliferation, survival, angiogenesis, and metastasis. The key roles of HIF-1 in these processes of tumor growth, angiogenesis, and metastasis through the modification of various targeted molecules and pathways are depicted below (Figure 2).
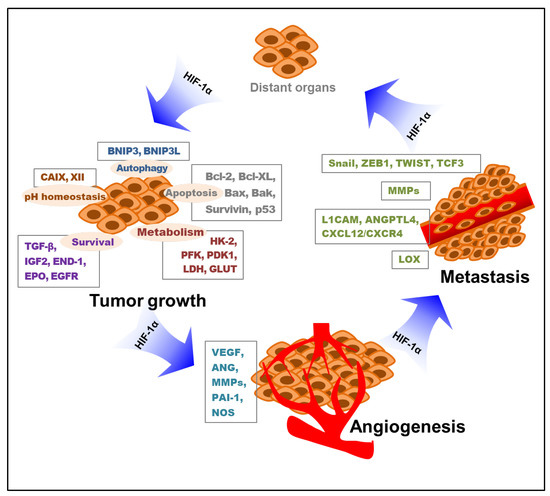
Figure 2.
Roles of HIF-1 in tumor growth, angiogenesis, and metastasis. Various targeted molecules and pathways involved in the HIF-1-mediated tumor progress are indicated. HIF-1—hypoxia-inducible factor-1; BNIP3—Bcl-2/adenovirus E1B 19 kDa interacting protein 3; BNIP3L—Bcl-2/adenovirus E1B 19 kDa interacting protein 3-like; CAIX—carbonic anhydrase IX; CAXII—carbonic anhydrase XII; TGF-β—transforming growth factor-β; IGF2—insulin-like growth factor 2; END-1—endothelin-1; EPO—erythropoietin; EGFR—epidermal growth factor receptor; HK-2—hexokinase 2; PFK—phosphofructokinase; PDK1—pyruvate dehydrogenase kinase 1; LDH—lactate dehydrogenase; GLUT—glucose transporter; VEGF—vascular endothelial growth factor; ANG—angiopoietin; MMP—matrix metalloproteinase; PAI-1—plasminogen activator inhibitor-1; NOS—nitric oxide synthase; L1CAM—L1 cell adhesion molecule; ANGPTL4—angiopoietin-like 4; CXCL12—C-X-C motif chemokine 12; CXCR4—C-X-C chemokine receptor 4; LOX—lysyl oxidase; HIF-1α—hypoxia-inducible factor-1α.
4. Roles of HIF-1 in Anticancer Drug Resistance and Cancer-Related Pain
4.1. Role of HIF-1 in Anticancer Drug Resistance
The overexpression of the HIF-1α protein is associated with the development of resistance to chemo- and radiotherapy in multiple types of human cancers [11,12,96,97,98]. Several molecular mechanisms by which hypoxia and HIF-1 drive tumors to therapeutic resistance are discussed below.
One of the molecular mechanisms clarifying the contribution of HIF-1 in drug resistance is drug efflux which is a major obstacle to effective drug delivery and retention [99]. At least three drug transporters are found to be regulated by HIF-1, including the multidrug resistance 1 protein (MDR1), multidrug resistance-associated protein 1 (MRP1), and breast cancer resistance protein (BCRP). The high expression of the human MDR1 gene and its encoded transporter, P-glycoprotein (P-gp), can reduce the intracellular concentrations of numerous chemotherapeutic agents, such as vinca alkaloids, anthracyclines, paclitaxel, and doxorubicin, by acting as a drug efflux pump [100,101]. Thus, tumor cells that overexpress MDR1/P-gp usually show resistance to chemotherapy. It has been shown that hypoxia-regulated HIF-1α is correlated with the MDR1/P-gp expression in many cancer cell lines, such as breast, colon, and bladder cancer cells [99,102,103]. MRP1 is also identified as the cause of multidrug resistance by transporting unmodified hydrophobic compounds, such as anthracyclines [104]. HIF-1 elevated the transcription of the gene encoding MRP1 (ABCC1) in response to hypoxia, and the inhibition of HIF-1 by siRNA against HIF-1 significantly reversed this effect in colon cancer [105]. Similar to MDR1 or MRP1, BCRP is also known as an ATP-dependent efflux multidrug transporter, pumping chemotherapeutic agents out of the cells [106]. BCRP is associated with the resistance of breast and colon cancers to chemotherapy and is induced by the hypoxic condition in a HIF-1-dependent manner [107,108].
Another mechanism underlying the development of resistance to anticancer therapy may originate from the capacity of hypoxia-induced HIF-1 to repair DNA damage. DNA damage is the central point of cancer treatment, as the majority of anticancer drugs use this basic mode of action to kill tumor cells. However, tumor cells may respond and attempt to overcome this DNA damage initiated by chemo/radiotherapy through various repair mechanisms [109,110]. Chemo/radiotherapy may induce single-stranded DNA breaks (SSBs) or double-stranded DNA breaks (DSBs) to start apoptosis. The repair of SSBs is carried out by poly(ADP-ribose) polymerase 1 (PARP-1) or by the regulation of the XPA and XPD genes: the key enzymes involved in the base excision repair process. To repair DSBs, tumor cells may develop the DNA damage response, which is a complex network that targets various DNA lesions [111]. Increasing evidence shows that most molecules in the DNA repair pathway are regulated by HIF-1α. For example, HIF-1α mediates the overexpression of PARP-1, XPA, and XPD, which subsequently leads to the resistance of cisplatin in non-small cell lung cancer (NSCLC) and testicular germ cell tumors [112,113]. HIF-1α also regulates the activities of several kinases involved in the DSBs repair pathway in radiation-treated mouse mesenchymal stromal cells [114]. Additionally, HIF-1α has been shown to inhibit the formation of both radiotherapy-induced DSBs and SSBs, followed by increased radioresistance in hepatocellular carcinoma [115]. These collective results support the emerging role of HIF-1α in promoting DNA repair and chemo/radioresistance in a range of tumor cells.
Moreover, HIF-1α affects chemo/radiosensitivity via the regulation of genes that are related to tumor metabolism. Numerous genetic alterations appear to be involved in metabolic reprogramming in a HIF-dependent manner. The HIF-1α-mediated PDK3 upregulation significantly inhibited cell apoptosis and increased resistance to either cisplatin or paclitaxel in colon cancer [116]. The inhibition of HIF-1α-induced LDH expression can restore the sensitivity to bortezomib in multiple myeloma (MM) cells [117]. Pyruvate kinase M2 (PKM2) is also a transcriptional target of HIF-1α. Thus, it has been proposed that PKM2 inhibition may be used to sensitize hypoxic tumors to chemo/radiotherapy [118]. The stabilization of HIF-1 also increases the expression of carbonyl reductase 1 (CBR1) in hypoxic hepatocellular carcinoma cells and MCF-7 breast cancer cells. CBR1 is an NADPH-dependent enzyme that catalyzes the biogenic transformation of doxorubicin to its metabolite doxorubicinol, which is much less effective against cancer than doxorubicin. In this way, HIF-1 may induce the metabolic reprogramming of cancer cells and desensitize them to anticancer drugs [119].
Resistance to apoptosis, a common feature of tumor cells, is indicated as a promoter of cancer malignancy [120,121]. There is substantial evidence to suggest that HIF-1α can contribute to abnormalities in the apoptosis machinery, leading to the resistant phenotype of tumor cells in chemo/radiotherapy. HIF-1 may prevent apoptotic cell death by suppressing the intrinsic or extrinsic cell death pathway. HIF-1α is reported to inhibit pro-apoptotic proteins, such as Bax and caspase 3/8, and activate anti-apoptotic proteins, such as c-myc, survivin, STAT3, and transcription factor 4 (TCF4) [122]. The HIF-1-mediated inhibition of p53 activation in response to 5-fluorouracil (5-FU) is also reported, demonstrating the p53-dependent suppression of 5-FU-induced apoptosis by HIF-1 [123]. In addition, HIF-1α regulates the balance between NF-κB-associated pro- and anti-apoptosis, resulting in the resistance of pancreatic cancer cells to gemcitabine [124]. Alternatively, HIF-1 suppresses the extrinsic cell death pathway by blocking the binding of a death ligand to a death receptor on the cell surface, which permits the cells to tolerate higher amounts of chemotherapeutic harm before the activation of cell death pathways. For example, HIF-1 increased the expression of decoy receptors (DcR1 and DcR2), which then competed with DR4 and DR5 receptors for binding to the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), thereby neutralizing TRAIL toxicity [125]. Accordingly, HIF-1α is an important mediator of chemo/radioresistance in solid tumors by inhibiting apoptosis.
As described earlier (Section 3.1), autophagy is an essential pro-survival pathway in hypoxia conditions and has an emerging role in the development of resistance to anticancer therapy. For their survival against chemo/radiotherapy-induced apoptosis, tumor cells degrade cellular components through autophagic processes and reuse the products for metabolic biosynthesis and as energy sources. Numerous studies have demonstrated that the HIF-1α-mediated activation of autophagy causes chemo/radioresistance in tumor cells [126,127]. Autophagy-related proteins (ATGs), such as Beclin-1, and the microtubule-associated protein 1A/1B-light chain 3 (LC3) are critical proteins involved in autophagic regulation and the formation of autophagosome [128,129]. Cisplatin increased the protein levels of ATG5, Beclin-1, and LC3 in lung cancer cells, exhibiting features indicative of autophagy. Hypoxia was shown to robustly augment cisplatin-induced autophagy in a HIF-1-dependent manner, thereby leading to cisplatin resistance by decreasing its sensitivity [130]. Other studies also demonstrated that HIF-1α-induced autophagy could contribute to the cisplatin resistance of ovarian and lung cancer cells via the upregulation of Beclin-1 and LC3 [130,131]. Moreover, suppression of the HIF-1α/BNIP3/Beclin-1 signaling pathway inhibited autophagy and enhanced both gemcitabine-induced apoptosis and gemcitabine sensitivity in bladder cancer cells under hypoxic conditions [132]. Based on these observations, it has been proposed that targeting autophagy may be sufficient to restore lung cancer cell susceptibility to cisplatin [130]. Similarly, hypoxia-induced autophagy is reported to be involved in the radioresistance of human osteosarcoma and lung cancer cells by HIF-1α activation [133,134].
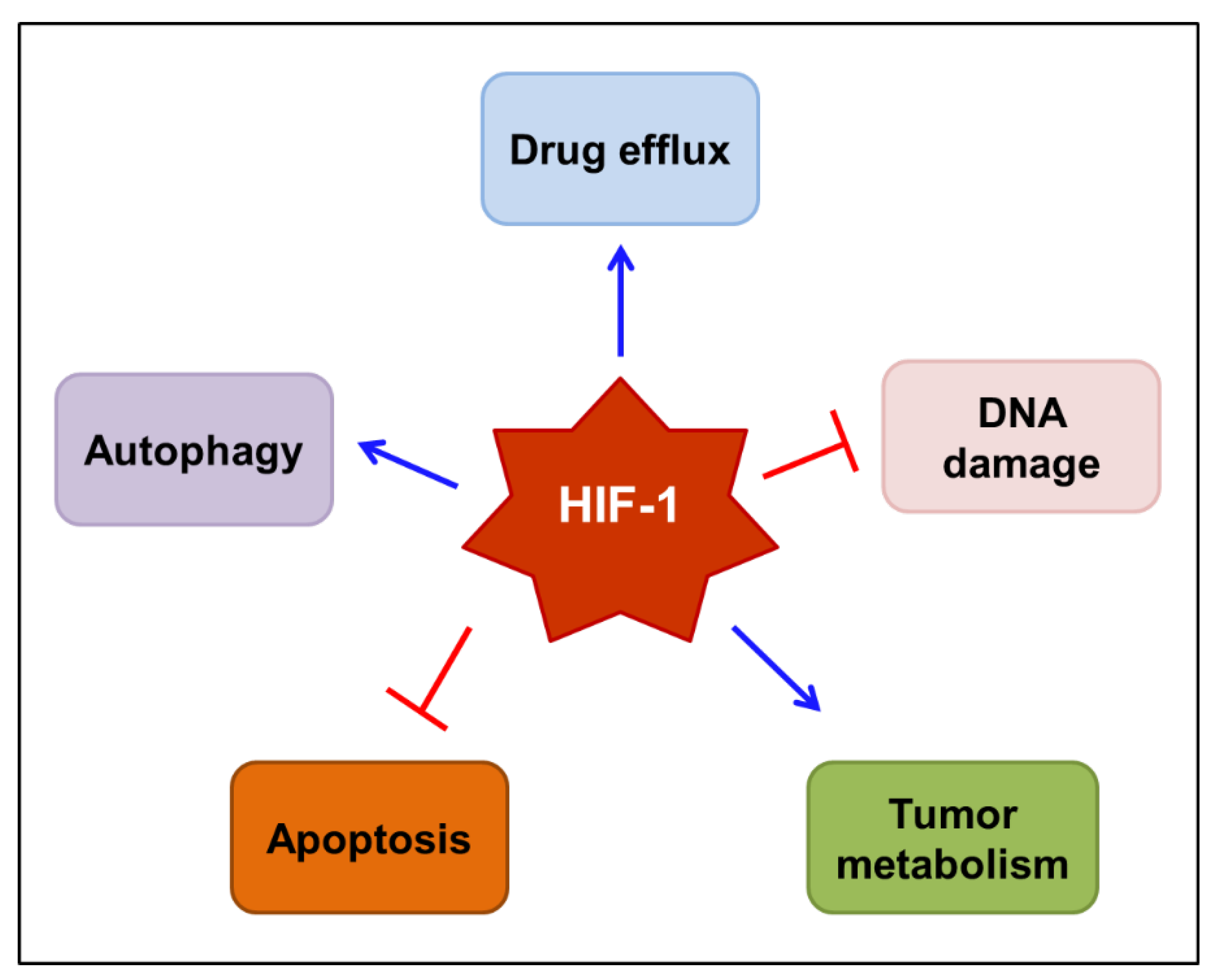
Taken together, HIF-1 has an emerging role in the chemo/radioresistance of tumors. The complex mechanisms by which HIF-1 mediates resistance to anticancer therapies are gradually being elucidated. The mechanisms proposed to underlie HIF-1-mediated drug resistance are outlined in Figure 3. These include the induction of drug efflux transporters, such as MDR1/P-gp, the repairment of DNA damage, the reprogramming of tumor metabolism, the interruption of apoptosis, and the augmentation of autophagy. Therefore, it would be reasonable to use HIF-1 inhibitors to prevent the development of resistance to chemo/radiotherapy.
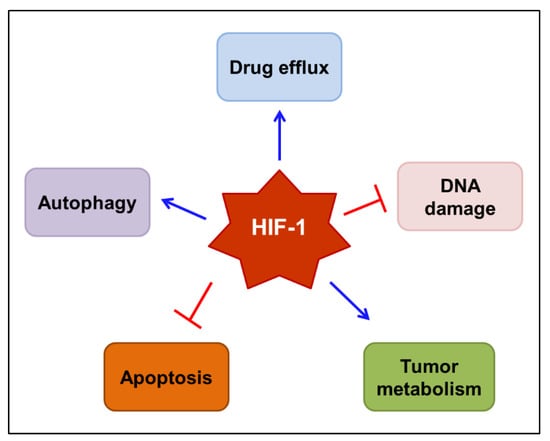
Figure 3.
Proposed mechanisms by which HIF-1 mediates resistance to anticancer therapy. HIF-1 may mediate the resistance to chemo/radiotherapy by inducing drug efflux transporters, such as MDR1/P-gp, repairing DNA damage, reprogramming tumor metabolism, interrupting programmed cell death, including apoptosis, and augmenting autophagy. Blue arrows indicate stimulation, while blocked red arrows indicate inhibition. HIF-1—hypoxia-inducible factor-1; MDR1—multidrug resistance 1 protein; P-gp—P-glycoprotein.
Numerous studies have demonstrated the possibility of preventing the development of resistance to chemo/radiotherapy using HIF inhibitors in combination with various anticancer drugs or radiation. For example, LW6, a well-designated HIF-1α inhibitor, was discovered as a potent inhibitor of BCRP, thereby preventing resistance to doxorubicin [135]. Similarly, LC478 inhibited the induction of P-gp, which resulted in an increased docetaxel absorption in a rat model of colorectal adenocarcinoma [136]. EZN-2208 (PEG-SN38) was found to overcome the ATP-binding cassette superfamily G member 2 (ABCG2)-mediated topotecan resistance by increasing the DNA damage repair in breast cancer 1 (BRCA1)-deficient mouse mammary tumors [137,138], while chetomin blocked the reprogramming of tumor metabolism in human malignant glioma cells to prevent hypoxic radioresistance [139]. Some HIF-1 inhibitors, such as PX-478, PMX290, and FK228, were shown to enhance the antitumor effects of chemo/radiotherapy by inducing apoptosis in pancreatic ductal adenocarcinoma (PDAC) and various other cancers [140,141,142,143,144,145,146,147,148,149,150,151]. Both drug efflux and apoptosis mechanisms are involved in the prevention of resistance to chemotherapy by other HIF-1 inhibitors, including YC-1, RAD001 (everolimus), SCH66336 (lonafarnib), and echinomycin [152,153,154,155,156,157]. Selected HIF-1 inhibitors demonstrated an ability to prevent chemo/radiotherapy resistance in various types of cancer or cancer cell lines and are summarized in Table 1.

Table 1.
Selected HIF-1 inhibitors were demonstrated to prevent chemo/radiotherapy resistance in various types of cancer or cancer cell lines.
4.2. Role of HIF-1 in Cancer-Related Pain
Cancer and anticancer therapies can often lead to physical and psychological burdens in patients. One of the serious burdens is cancer-related pain, affecting approximately 40% of patients with cancer [159]. Patients can experience pain in every stage of cancer until the end of life. It may interfere with their treatment process, lead to treatment refusal, and impact their survival [160]. Peripheral neuropathic cancer pain (PNCP) is one of the most complex conditions among cancer-related pain syndromes. PNCP syndromes can be either acute or chronic [161]. Acute PNCP is most frequently associated with cancer diagnostic or therapeutic interventions. Tissues, especially nerves, are directly injured by diagnostic approaches, resulting in pain. Chemo/radiotherapy induces acute PNCP at the beginning of treatment or as a side effect [161]. Chronic PNCP results from treatment complications or malignancy itself [161]. Currently, the molecular mechanisms by which chemo/radiotherapy induces PNCP to remain elusive. However, the pain level was shown to be closely related to the growth of the tumor and its microenvironment and the fact that hypoxic nerves are more vulnerable than normal nerves. In the long term, chronic hypoxia leads to fibrosis in perineural tissues and causes PNCP [162]. Therefore, the activation of HIF-1 may be involved in cancer-related pain, especially PNCP.
Bortezomib is a well-known anticancer drug that causes peripheral neuropathic pain as an unwanted side effect. Bortezomib has been demonstrated to induce aerobic glycolysis in sensory neurons, leading to the augmented extrusion of metabolites that sensitize primary afferents and causes pain [163,164]. Boyette-Davis et al. [163] found that the treatment of mice with bortezomib stabilized the expression of HIF-1α. Moreover, the bortezomib-induced neuropathic pain was abolished by the knockdown of HIF-1α expression, disruption of HIF-1α translation, or its binding to HREs. These results establish that the stabilization of HIF-1α expression is closely associated with the initiation of PNCP [165]. Strikingly, the phase I/II trial involving 72 patients with refractory MM, no patients developed severe peripheral neuropathy when using tanespimycin (17-allylamino-17-demethoxygeldanamycin: 17-AAG) in combination with bortezomib, indicating that HIF-1 inhibitors in combination with conventional anticancer therapy could reduce the therapy-induced pain and enhance the treatment efficacy [166].
A possible correlation between cancer-related pain and the expression levels of HIF-1 and VEGF was examined in patients with liver cancer. Before and after an intervention, such as treatment with analgesics, hepatic surgeries, or chemo/radiotherapy, the mRNA expression levels of HIF-1 and VEGF in the liver cancer group with pain were significantly higher than those in the group without pain. Furthermore, the HIF-1 and VEGF mRNA expression in the group with pain markedly increased after the intervention. A visual analog scale used to evaluate the pain level was positively correlated with the HIF-1 and VEGF expression in the group of liver cancer patients with pain before and after the intervention, suggesting that chemo/radiotherapy may result in pain via the activation of the HIF-1 pathway [167]. In an in vivo study using a metastatic bone cancer pain model, the downregulation of annexin A3, a highly expressed protein during bone cancer pain, alleviated pain by inhibiting the HIF-1/VEGF signaling pathway [168]. Altogether, increased HIF-1 and VEGF levels were closely correlated with therapeutic failure, possibly due to increased pain occurrence and pain levels. Thus, targeting the HIF-1 and VEGF pathways may provide a potential benefit for alleviating cancer-related pain.
Notably, the tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) has been reported to play an important role in the development of hypersensitive pain in tumor-affected areas [169]. The disruption of GM-CSF signaling by the downregulation of its receptor reduced the hypersensitive pain evoked by bone cancer in vivo [170]. GM-CSF and other hematopoietic factors are also shown to be expressed in the sensory nerves of peripheral tissues in pancreatic cancer [171]. The overexpression of GM-CSF induced nerve cell migration and was significantly correlated with pain in patients with PDAC. The expression levels of HIF-1α and GM-CSF in PANC-1 pancreatic cancer cells were increased when cultured in the hypoxic condition compared with those in the normoxic condition. Moreover, when HIF-1α was overexpressed in PANC-1 cells and MIA PaCa2 cells (human pancreatic carcinoma cells), GM-CSF mRNA and protein expression levels were also markedly increased, indicating that the hypoxia microenvironment could regulate the expression of GM-CSF through the overexpression of HIF-1α [172]. HIF-1α directly activates the transcription and expression of GM-CSF and mediates tumor−nerve interactions in PDAC. Therefore, agents targeting the HIF-1α/GM-CSF signaling might inhibit tumor−nerve interactions and help to alleviate the pain in PDAC patients [172].
Although HIF-1α is protective in terms of acute heat and cold pain in the early phase, the ongoing activation of injured neurons may promote the development of chronic neuropathic pain via the upregulation of HIF-1 target genes, including GCH1 encoding GTP cyclohydrolase 1 (GTPCH) and its product tetrahydrobiopterin (BH4), which further drives NO production. Therefore, the therapeutic interruption of HIF-1/GTPCH/BH4 activation may reduce neuropathic pain. Due to the dual roles of HIF-1 in pain regulation, the drugs targeting HIF-1 may cause side effects in terms of heat and cold pain sensitivity. However, in patients with neuropathy associated with cancer treatment, HIF-1 inhibitors may provide a combination of tumor growth inhibition and pain reduction [173].
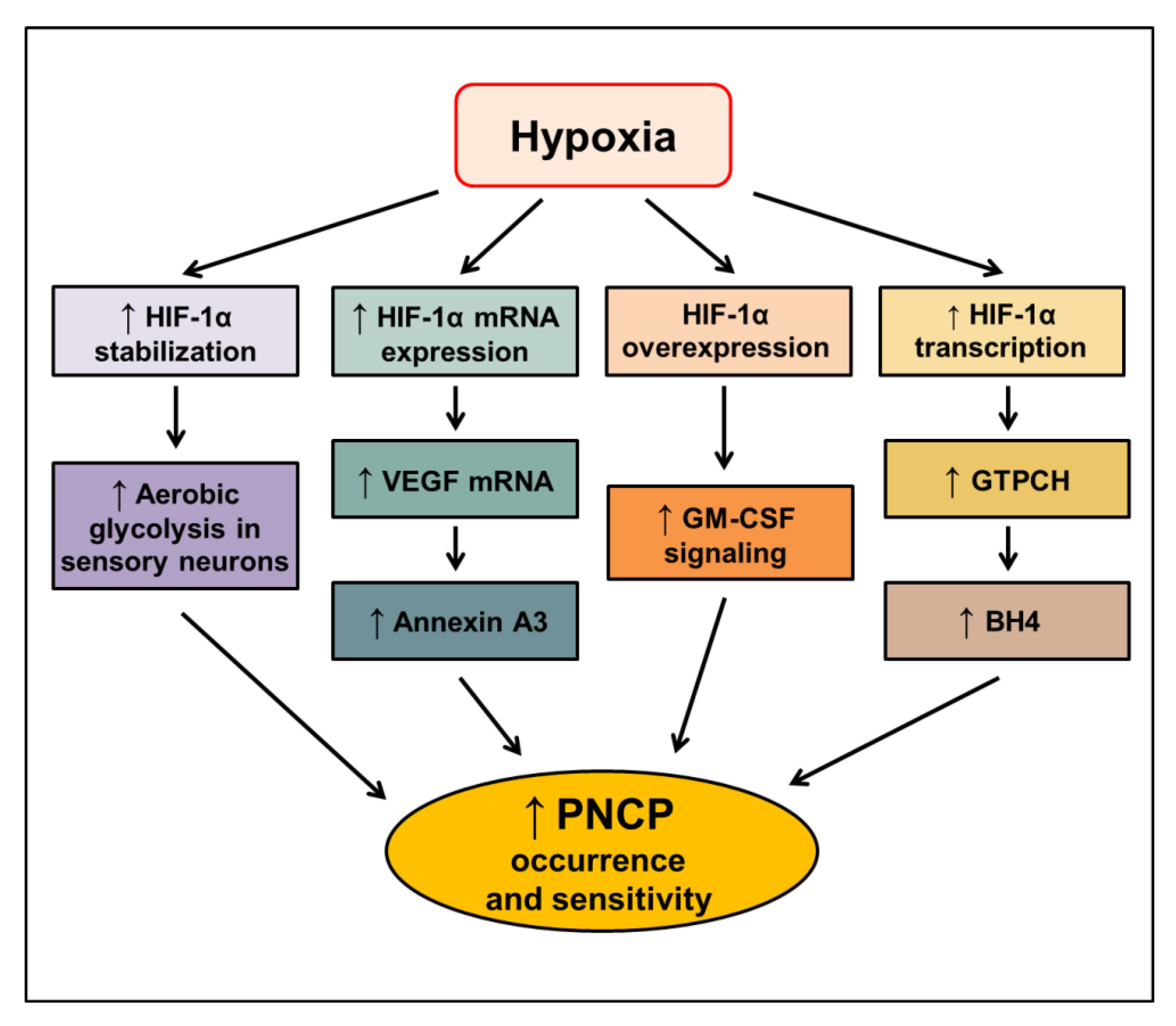
Altogether, the activation of HIF-1 is closely associated with cancer-related pain, especially PNCP. The proposed signaling pathways mediating the increased occurrence and sensitivity of PNCP through HIF-1α in hypoxic conditions are elucidated (Figure 4). However, the molecular mechanisms by which HIF-1 regulates cancer-related pain remain poorly understood. Further studies should address the role of HIF-1 in cancer pain to provide advantages for targeting HIF-1 as a novel anticancer approach.
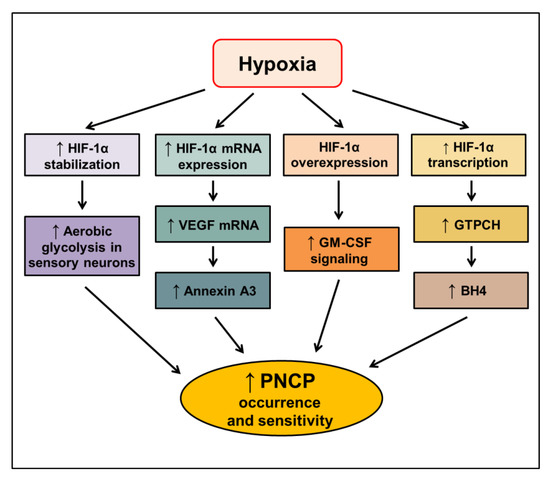
Figure 4.
Proposed signaling pathways inducing peripheral neuropathic cancer pain (PNCP) through HIF-1α in hypoxic conditions. HIF—hypoxia-inducible factor-1, VEGF—vascular endothelial growth factor; GM-CSF—granulocyte-macrophage colony-stimulating factor; GTPCH—GTP cyclohydrolase 1; BH4—tetrahydrobiopterin. The upward arrow indicates an increase in the respective signal.
5. Current Clinical Status of HIF-1 Inhibitors as Potential Anticancer Therapy
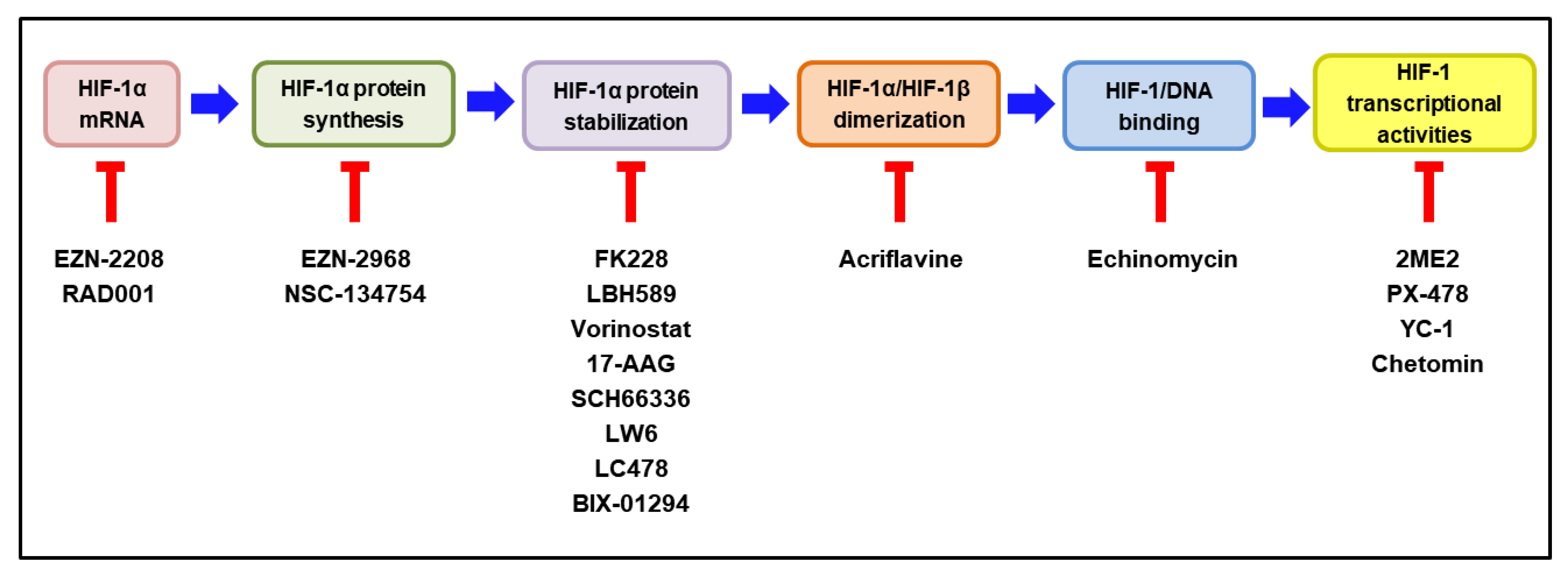
According to the extensive involvement of HIF-1 in tumor progression, many HIF-1 inhibitors have been developed and evaluated as potential anticancer drugs in pre-clinical and clinical studies [14,174,175]. HIF-1 inhibitors can affect the expression or function of HIF-1 through a wide range of molecular mechanisms, including the inhibition of HIF-1α mRNA, HIF-1α protein synthesis, HIF-1α stabilization, HIF-1α/HIF-1β dimerization, HIF-1/DNA binding, and HIF-1 transcriptional activities [176,177]. Intriguingly, MO-460, an analog of a benzofuran-based natural product (R)-(-)-moracin-O, has been reported to inhibit HIF-1α accumulation by the previously unidentified mechanism. It was demonstrated to interact with the heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2B1), thereby inhibiting the initiation of HIF-1α translation [178]. The molecular mechanisms of the representative HIF-1 inhibitors are delineated in Figure 5. Some of these HIF-1 inhibitors have been evaluated for their efficacy and safety in clinical trials. The current clinical status of these inhibitors, either as a monotherapy or in combination with other anticancer therapies, is discussed below.
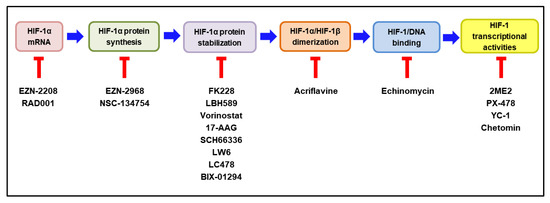
Figure 5.
Molecular mechanisms of HIF-1 inhibitors. The steps required to activate HIF-1 are illustrated in colored boxes, and the representative HIF-1 inhibitors acting at each step are listed under the respective box. HIF—hypoxia-inducible factor-1. The blocked red arrows indicate inhibition.
5.1. HIF-1 Inhibitor Decreasing HIF-1α mRNA Expression
EZN-2208
EZN-2208, a polyethylene glycol (PEG)-coated SN-38 (PEG-SN38), which is an active metabolite of irinotecan, inhibits the HIF-1 pathway through the suppression of HIF-1α at the mRNA level [151]. By this mechanism, it was shown that to reduce the expression of MMP2, VEGF1, GLUT1, GLUT3, and TGFβ1, is to subsequently inhibit the angiogenesis of tumor cells [151]. The clinical tolerability and antitumor activity of EZN-2208, either as a monotherapy or in combination with bevacizumab, has been demonstrated in a phase I study in patients with refractory solid tumors [179,180]. In a randomized phase II study, patients with metastatic CRC who previously received 5-FU, oxaliplatin, and irinotecan were assigned with EZN-2208 monotherapy for patients with Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant tumors. Overall, the response rate and progression-free survivals were 0% and 1.8 months, respectively. The overall response rate and progression-free survivals were 10.7% and 4.9 months, respectively, in patients with KRAS-wild type tumors who received EZN-2208 plus cetuximab, whereas the corresponding values were 14.3% and 3.7 months in the patients who received irinotecan plus cetuximab [181]. These results demonstrate that EZN-2208, either as a monotherapy or in combination with cetuximab, was well-tolerated in patients with refractory CRC and that the overall survival and progression-free survival of patients with KRAS-wild type tumors were similar when treated with cetuximab plus irinotecan or EZN-2208.
5.2. HIF-1 Inhibitor Decreasing HIF-1α Protein Synthesis
EZN-2968
EZN-2968 is a locked nucleic acid oligonucleotide that inhibits HIF-1α protein synthesis by blocking the translation of HIF-1α mRNA. The inhibition of HIF-1α by EZN-2968 reduced tumor growth by inhibiting cell proliferation through a delayed progression of the S-phase, which might be caused by the shift to a mitochondrial oxidative metabolism [182]. In a phase I study involving 10 patients with refractory solid tumors, one patient with a duodenal neuroendocrine tumor showed prolonged stabilization of the disease for 24 weeks. Reduced levels of the HIF-1α protein and the mRNA of some target genes were observed in two patients [183]. Although the trial has been closed prematurely, it provides preliminary proof that the modulation of HIF-1α synthesis and its target genes may exhibit potential anticancer activity.
5.3. HIF-1 Inhibitors Decreasing HIF-1α Stabilization
Drugs that decrease HIF-1α stabilization by inducing its degradation include histone deacetylase inhibitors, such as FK228, LBH589, and vorinostat; heat shock protein 90 inhibitors include 17-AAG and farnesyl transferase inhibitors, such as SCH66336 (Figure 5).
5.3.1. FK228 (Romidepsin)
Romidepsin was shown to inhibit hypoxia-responsive angiogenesis factors, such as VEGF and the basic fibroblast growth factor, through the suppression of HIF-1α stabilization [184,185]. Romidepsin (8 mg/m2) plus erlotinib was well tolerated, showed disease control, and exhibited beneficial effects in a phase I study involving 17 patients with advanced NSCLC [186]. However, a phase II trial of single romidepsin treatment showed limited activity in the squamous cell carcinoma of the head and neck (SCCHN) [187].
5.3.2. LBH589 (Panobinostat)
Panobinostat caused apoptosis mediated by chromatin fragmentation, the activation of caspases-3 and 7, and PARP via HIF-1α destabilization in cisplatin-resistant lung cancer cells [188]. In a phase I study, the combination of carfilzomib with panobinostat proved a safe and effective treatment option for patients with relapsed/refractory MM [189,190]. To compare the clinical tolerability of panobinostat in combination with other anticancer drugs, 768 patients with refractory MM were enrolled in a randomized, double-blind phase III trial. Patients received 21-day cycles of a placebo or panobinostat (20 mg, oral), both in combination with bortezomib (1.3 mg/m2, intraperitoneal) and dexamethasone (20 mg, oral). The median follow-up was 6.47 months in the panobinostat group and 5.59 months in the placebo group. The median progression-free survival was significantly longer in the panobinostat group than in the placebo group (11.99 months vs. 8.08 months) [191]. These results suggest that panobinostat could be a useful addition to conventional anticancer therapies for patients with refractory MM.
5.3.3. Vorinostat
Vorinostat was the first histone deacetylase inhibitor approved by the United States Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma. Vorinostat potently inhibited HIF-1α stabilization via the acetylation of its associated chaperone heat shock protein 90 (Hsp90) and subsequently suppressed the downstream molecules, including EPO, GLUT 1, and VEGF [192]. The combination of vorinostat with temozolomide and radiation therapy had acceptable tolerability in newly diagnosed glioblastoma in phase I and phase II trials involving 15 and 107 patients, respectively [193]. In another phase II study involving 32 patients with advanced melanoma, 16 had stable disease, while 14 had progressive disease as their best response. Two patients with cutaneous melanoma that scored stable disease had early unconfirmed partial responses with subsequent progression. Patients with stable disease or partial response (n = 18) had a median progression-free survival of 5 months [194]. However, a double-blind, randomized, placebo-controlled phase III trial reported that vorinostat did not improve overall survival and was not recommended for patients with advanced malignant pleural mesothelioma [195].
5.3.4. 17-AAG (Tanespimycin)
Tanespimycin was found to inhibit Hsp90-implicated HIF-1α signaling, including HIF-1α destabilization and VEGF secretion, consequently suppressing angiogenesis and tumor growth [196]. In a phase II study assessing the efficacy of 17-AAG and its toxicity profile in patients with metastatic, hormone-refractory prostate cancer, 17-AAG did not show any anticancer activity regarding the prostate-specific antigen (PSA) response. Due to an insufficient PSA response and severe toxicity, enrollment was stopped at the end of the first stage of the study design [197].
However, tanespimycin in combination with bortezomib was well tolerated in thephase I/II study. Among 67 efficacy-evaluable patients with refractory MM, there were two complete responses (3%) and eight partial responses (12%), with an overall objective response rate of 27%, including eight minimal responses (12%) [166]. Although the evaluation of 17-AAG as a single agent is not warranted, 17-AAG might have promising efficacy in combination with other anticancer drugs.
5.3.5. SCH66336 (Lonafarnib)
It has been revealed that the antiangiogenic activities of SCH66336 are mediated by the disrupted connection of HIF-1α and Hsp90 and destabilization of HIF-1α, leading to a decreased expression of HIF-1α [198]. SCH66336 has shown marked antitumor activities as a monotherapy in a phase Ib study of SCCHN (https://clinicaltrials.gov/ct2/show/NCT00038584 accessed on 30 October 2018). Then, a phase II study was conducted to examine its efficacy and safety in patients with recurrent, refractory SCCHN. However, due to no objective responses observed in the first 15 patients, further evaluation of SCH66336 in refractory SCCHN was not planned [199].
In another phase II trial of SCH66336, patients with metastatic CRC refractory to 5-FU and irinotecan received SCH66336 as a twice-daily oral administration. Although three patients showed stable disease for several months, no objective responses were observed. The administration of SCH66336 was accompanied by gastrointestinal toxicity. The future development of this drug is not recommended as a monotherapy for this disease [200].
However, the combination treatment of SCH66336 with paclitaxel was well-tolerated, with minimal toxicity in patients with taxane-refractory/resistant metastatic NSCLC. The evaluation of this combination therapy in additional clinical trials is warranted [155].
5.4. HIF-1 Inhibitor Decreasing HIF-1α/HIF-1β Dimerization
Acriflavine
Acriflavine binds to the PAS-B domain of HIF-1α, blocking its interaction with HIF-1β, which leads to the suppression of tumor growth and tumor vascularization [201]. Moreover, acriflavine also enhances the antitumor activity of sunitinib in a breast cancer model [202]. However, there is no study investigating its efficacy in clinical conditions.
5.5. HIF-1 Inhibitor Decreasing HIF-1α/DNA Binding
Echinomycin
Echinomycin, a small-molecule inhibitor of HIF-1/DNA binding, did not show significant antitumor activity in phase II studies in patients with ovarian cancer, breast cancer, renal cell carcinoma, or CRC [203,204,205,206].
5.6. HIF-1 Inhibitor Decreasing HIF-1α Transcriptional Activity
2-Methoxyestradiol (2ME2, Panzem)
2ME2, a natural metabolite of estradiol inhibiting transcriptional activities of HIF-1α, showed strong antiangiogenic and antiapoptotic effects on cancer cells [207]. Sixty MM patients enrolled in the phase II clinical study were treated with 2ME2, but no partial responses have been observed so far. The lack of objective responses to the 2ME2 treatment may be due to the inadequate dosage of the drug, as indicated by the low plasma level [208]. Another phase II trial of 2ME2 in 50 patients with metastatic prostate cancer was also terminated after the enrollment of 21 patients due to the futility and lack of objective response to the treatment [209].
There were no objective responses in a phase II trial of 2ME2 in patients with advanced, platinum-resistant ovarian cancer, but 2ME2 was still well-tolerated, and seven patients had stable disease as the best response. Of those, two patients had stable disease for greater than 12 months. The rate of clinical benefit was 31.3%. Fairly stable plasma levels of 2ME2, ranging within the predicted therapeutic window, were observed [210].
In another phase II clinical trial, 2ME2 was used in combination with bevacizumab for the treatment of 31 patients with a locally metastatic carcinoid tumor. Among them, 21 patients showed a reduction in tumor size, and the median progression-free survival was 11.3 months, supporting the concept that this regimen had some degree of antitumor activity when used in combination with other anticancer agents [211].
In summary, the antitumor activities of HIF-1 inhibitors have been well-demonstrated in many preclinical studies [177]. Despite the limited efficacy of various HIF-1 inhibitors in clinical trials, vorinostat has been reported to exert clinically recognizable benefits in the treatment of melanoma [194]. However, many HIF-1 inhibitors have failed to show efficacy in clinical trials. To improve the therapeutic efficacy of HIF inhibitors and reduce drug resistance and cancer-related pain, the development of combination therapies may be necessary. In fact, several HIF-1 inhibitors administered in combination with the other conventional therapeutic agents for the treatment of refractory cancers suggested better outcomes. Further preclinical and clinical studies are warranted to elucidate the promising HIF-1 inhibitors for combination therapies. The current status of clinical trials evaluating HIF-1α inhibitors as potential anticancer therapies is summarized in Table 2.

Table 2.
Current clinical status evaluating HIF-1α inhibitors as potential anticancer therapies.
6. Conclusions and Future Perspectives
The roles of hypoxia in biological hallmarks of cancer involve complicated signaling centered on its main mediator, HIF-1. Evidence has shown that HIF-1 significantly impacts cancer progression, from tumor cell proliferation to angiogenesis and tumor metastasis. Clinical trials indicate that some HIF-1 inhibitors have potential either as a monotherapy or in combination with other conventional anticancer therapies. Further studies prove that hypoxia drives not only tumor biology but also the tumor microenvironment that contributes to the development of resistance to cancer treatment. We also provided a comprehensive view of the underlying molecular mechanisms by which HIF-1 regulates chemo/radioresistance. Furthermore, a novel insight into the correlation between cancer-related pain and HIF-1 activation was addressed in this review. Several HIF-1 inhibitors are found to enhance the therapeutic efficacy of anticancer drugs, overcome resistance to anticancer drugs, and prevent cancer-related pain. Each therapy has advantages and limitations, and each cancer type has different characteristics. Thus, the identification of hypoxic markers and genomic analyses are urgently required to allow HIF-1 inhibitors to be tailored to specific cancer types and individual patients. A more detailed understanding of HIF-1 regulation in tumor progression would improve cancer care.
Author Contributions
Conceptualization, K.L. and J.C.; methodology, B.P.B. and P.L.N.; software, P.L.N.; validation, K.L. and J.C.; formal analysis, B.P.B.; investigation, B.P.B. and P.L.N.; resources, P.L.N.; data curation, B.P.B.; writing—original draft preparation, B.P.B. and P.L.N.; writing—review and editing, J.C.; visualization, B.P.B. and P.L.N.; supervision, J.C.; project administration, K.L. and J.C; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF–2018R1A5A2023127).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Licona, A.; Pérez-Añorve, I.X.; Flores-Fortis, M.; Moral-Hernández, O.D.; González-de la Rosa, C.H.; Suárez-Sánchez, R.; Chávez-Saldaña, M.; Aréchaga-Ocampo, E. Deciphering the epigenetic network in cancer radioresistance. Radiother. Oncol. 2021, 159, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cho, J.; Lee, K. Tumour Regression via Integrative Regulation of Neurological, Inflammatory, and Hypoxic Tumour Microenvironment. Biomol. Ther. 2020, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Padma, V.V. An overview of targeted cancer therapy. BioMedicine 2015, 5, 19. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Kumar, V.; Gabrilovich, D.I. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014, 143, 512–519. [Google Scholar] [CrossRef]
- Akanji, M.A.; Rotimi, D.; Adeyemi, O.S. Hypoxia-Inducible Factors as an Alternative Source of Treatment Strategy for Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 8547846. [Google Scholar] [CrossRef]
- Chouaib, S.; Noman, M.Z.; Kosmatopoulos, K.; Curran, M.A. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017, 36, 439–445. [Google Scholar] [CrossRef]
- Liu, L.; Ning, X.; Sun, L.; Zhang, H.; Shi, Y.; Guo, C.; Han, S.; Liu, J.; Sun, S.; Han, Z.; et al. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008, 99, 121–128. [Google Scholar] [CrossRef]
- Iijima, M.; Gombodorj, N.; Tachibana, Y.; Tachibana, K.; Yokobori, T.; Honma, K.; Nakano, T.; Asao, T.; Kuwahara, R.; Aoyama, K.; et al. Development of single nanometer-sized ultrafine oxygen bubbles to overcome the hypoxia-induced resistance to radiation therapy via the suppression of hypoxia-inducible factor1α. Int. J. Oncol. 2018, 52, 679–686. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022, 80, 379–390. [Google Scholar] [CrossRef]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Carver, L.A.; Hogenesch, J.B.; Bradfield, C.A. Tissue specific expression of the rat Ah-receptor and ARNT mRNAs. Nucleic Acids Res. 1994, 22, 3038–3044. [Google Scholar] [CrossRef]
- Graham, A.M.; Presnell, J.S. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE 2017, 12, e0179545. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Activation of the HIF pathway in cancer. Curr. Opin. Genet. Dev. 2001, 11, 293–299. [Google Scholar] [CrossRef]
- Schönberger, T.; Fandrey, J.; Prost-Fingerle, K. Ways into Understanding HIF Inhibition. Cancers 2021, 13, 159. [Google Scholar] [CrossRef]
- Kallio, P.J.; Pongratz, I.; Gradin, K.; McGuire, J.; Poellinger, L. Activation of hypoxia-inducible factor 1α: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl. Acad. Sci. USA 1997, 94, 5667–5672. [Google Scholar] [CrossRef]
- Scholz, C.C.; Taylor, C.T. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharmacol. 2013, 13, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers 2020, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Grimshaw, M.J. Endothelins and hypoxia-inducible factor in cancer. Endocr.-Relat. Cancer 2007, 14, 233–244. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard, W.A., 3rd; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 2012, 337, 975–980. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Naik, R.; Ban, H.S.; Jang, K.; Kim, I.; Xu, X.; Harmalkar, D.; Shin, S.A.; Kim, M.; Kim, B.K.; Park, J.; et al. Methyl 3-(3-(4-(2,4,4-Trimethylpentan-2-yl)phenoxy)-propanamido)benzoate as a Novel and Dual Malate Dehydrogenase (MDH) 1/2 Inhibitor Targeting Cancer Metabolism. J. Med. Chem. 2017, 60, 8631–8646. [Google Scholar] [CrossRef]
- Ren, B.F.; Deng, L.F.; Wang, J.; Zhu, Y.P.; Wei, L.; Zhou, Q. Hypoxia regulation of facilitated glucose transporter-1 and glucose transporter-3 in mouse chondrocytes mediated by HIF-1α. Jt. Bone Spine 2008, 75, 176–181. [Google Scholar] [CrossRef]
- Won, M.; Ban, H.S.; Lee, K.; Lee, H.; Kim, B.-K.; Kim, H.; Naik, R.; Park, S.-K.; Park, J.-T.; Kim, I.; et al. Abstract 1164: A novel hypoxia-inducible factor-1 inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cancer Res. 2017, 77, 1164. [Google Scholar] [CrossRef]
- Kim, I.; Kim, M.; Park, M.K.; Naik, R.; Park, J.H.; Kim, B.-K.; Choi, Y.; Chang, K.Y.; Won, M.; Ban, H.S.; et al. The disubstituted adamantyl derivative LW1564 inhibits the growth of cancer cells by targeting mitochondrial respiration and reducing hypoxia-inducible factor (HIF)-1α accumulation. Exp. Mol. Med. 2020, 52, 1845–1856. [Google Scholar] [CrossRef]
- Flinck, M.; Kramer, S.H.; Pedersen, S.F. Roles of pH in control of cell proliferation. Acta Physiol. 2018, 223, e13068. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Kuzmin, I.; Wei, M.H.; Pack, S.; Geil, L.; Johnson, B.E.; Stanbridge, E.J.; Lerman, M.I. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc. Natl. Acad. Sci. USA 1998, 95, 12596–12601. [Google Scholar] [CrossRef] [PubMed]
- Silagi, E.S.; Schoepflin, Z.R.; Seifert, E.L.; Merceron, C.; Schipani, E.; Shapiro, I.M.; Risbud, M.V. Bicarbonate Recycling by HIF-1-Dependent Carbonic Anhydrase Isoforms 9 and 12 Is Critical in Maintaining Intracellular pH and Viability of Nucleus Pulposus Cells. J. Bone Miner. Res. 2018, 33, 338–355. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Chia, S.; Bedard, P.L.; Chu, Q.; Lyle, M.; Tang, L.; Singh, M.; Zhang, Z.; Supuran, C.T.; Renouf, D.J.; et al. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients with Advanced Solid Tumors. Am. J. Clin. Oncol. 2020, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs 2018, 27, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Elkamhawy, A.; Choi, Y.H.; Lee, C.H.; Lee, K.; Cho, J. Suppression of Tumor Growth and Cell Migration by Indole-Based Benzenesulfonamides and Their Synergistic Effects in Combination with Doxorubicin. Int. J. Mol. Sci. 2022, 23, 9903. [Google Scholar] [CrossRef]
- Hipfner, D.R.; Cohen, S.M. Connecting proliferation and apoptosis in development and disease. Nat. Rev. Mol. Cell Biol. 2004, 5, 805–815. [Google Scholar] [CrossRef]
- Sasabe, E.; Tatemoto, Y.; Li, D.; Yamamoto, T.; Osaki, T. Mechanism of HIF-1α-dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005, 96, 394–402. [Google Scholar] [CrossRef]
- Piret, J.P.; Mottet, D.; Raes, M.; Michiels, C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann. N. Y. Acad. Sci. 2002, 973, 443–447. [Google Scholar] [CrossRef]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef]
- Wang, P.; Long, M.; Zhang, S.; Cheng, Z.; Zhao, X.; He, F.; Liu, H.; Ming, L. Hypoxia inducible factor-1α regulates autophagy via the p27-E2F1 signaling pathway. Mol. Med. Rep. 2017, 16, 2107–2112. [Google Scholar] [CrossRef]
- Farrall, A.L.; Whitelaw, M.L. The HIF1α-inducible pro-cell death gene BNIP3 is a novel target of SIM2s repression through cross-talk on the hypoxia response element. Oncogene 2009, 28, 3671–3680. [Google Scholar] [CrossRef]
- Ma, J.; Weng, L.; Jia, Y.; Liu, B.; Wu, S.; Xue, L.; Yin, X.; Mao, A.; Wang, Z.; Shang, M. PTBP3 promotes malignancy and hypoxia-induced chemoresistance in pancreatic cancer cells by ATG12 up-regulation. J. Cell. Mol. Med. 2020, 24, 2917–2930. [Google Scholar] [CrossRef]
- Qureshi-Baig, K.; Kuhn, D.; Viry, E.; Pozdeev, V.I.; Schmitz, M.; Rodriguez, F.; Ullmann, P.; Koncina, E.; Nurmik, M.; Frasquilho, S.; et al. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy 2020, 16, 1436–1452. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Gruber, M.; Simon, M.C. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr. Opin. Hematol. 2006, 13, 169–174. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Kim, B.; Lee, K.; Jung, H.; Bhattarai, D.; Kwon, H.J. HIF-1α suppressing small molecule, LW6, inhibits cancer cell growth by binding to calcineurin b homologous protein 1. Biochem. Biophys. Res. Commun. 2015, 458, 14–20. [Google Scholar] [CrossRef]
- Naik, R.; Han, S.; Lee, K. Chemical biology approach for the development of hypoxia inducible factor (HIF) inhibitor LW6 as a potential anticancer agent. Arch. Pharm. Res. 2015, 38, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.D.; Hackett, S.F.; Hirota, K.; Oshima, Y.; Cai, Z.; Berg-Dixon, S.; Rowan, A.; Yan, Z.; Campochiaro, P.A.; Semenza, G.L. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ. Res. 2003, 93, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Condorelli, F.; Park, J.; Ferrara, N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 1997, 272, 23659–23667. [Google Scholar] [CrossRef]
- Nilsson, I.; Shibuya, M.; Wennstrom, S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp. Cell Res. 2004, 299, 476–485. [Google Scholar] [CrossRef]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.J.; Yu, Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 2004, 64, 6119–6126. [Google Scholar] [CrossRef]
- Yamakawa, M.; Liu, L.X.; Date, T.; Belanger, A.J.; Vincent, K.A.; Akita, G.Y.; Kuriyama, T.; Cheng, S.H.; Gregory, R.J.; Jiang, C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ. Res. 2003, 93, 664–673. [Google Scholar] [CrossRef]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 2005, 7, 134–153. [Google Scholar] [CrossRef]
- Rigiracciolo, D.C.; Scarpelli, A.; Lappano, R.; Pisano, A.; Santolla, M.F.; De Marco, P.; Cirillo, F.; Cappello, A.R.; Dolce, V.; Belfiore, A.; et al. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget 2015, 6, 34158–34177. [Google Scholar] [CrossRef]
- Lolmede, K.; Durand de Saint Front, V.; Galitzky, J.; Lafontan, M.; Bouloumie, A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. 2003, 27, 1187–1195. [Google Scholar] [CrossRef]
- Kietzmann, T.; Samoylenko, A.; Roth, U.; Jungermann, K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood 2003, 101, 907–914. [Google Scholar] [CrossRef]
- Jung, F.; Palmer, L.A.; Zhou, N.; Johns, R.A. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ. Res. 2000, 86, 319–325. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Chang, J.; Erler, J. Hypoxia-mediated metastasis. Adv. Exp. Med. Biol. 2014, 772, 55–81. [Google Scholar] [CrossRef]
- Imai, T.; Horiuchi, A.; Wang, C.; Oka, K.; Ohira, S.; Nikaido, T.; Konishi, I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am. J. Pathol. 2003, 163, 1437–1447. [Google Scholar] [CrossRef]
- Hugo, H.J.; Pereira, L.; Suryadinata, R.; Drabsch, Y.; Gonda, T.J.; Gunasinghe, N.P.; Pinto, C.; Soo, E.T.; van Denderen, B.J.; Hill, P.; et al. Direct repression of MYB by ZEB1 suppresses proliferation and epithelial gene expression during epithelial-to-mesenchymal transition of breast cancer cells. Breast Cancer Res. 2013, 15, R113. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Zagzag, D.; Nagasawa, H.; Rainey, K.; Okuyama, H.; Baek, J.H.; Semenza, G.L. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006, 66, 2725–2731. [Google Scholar] [CrossRef]
- Giannoni, E.; Bianchini, F.; Calorini, L.; Chiarugi, P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid. Redox Signal. 2011, 14, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.; Leav, I.; Pursell, B.; Bae, D.; Yang, X.; Taglienti, C.A.; Gouvin, L.M.; Sharma, V.M.; Mercurio, A.M. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: Implications for Gleason grading. Cancer Cell 2010, 17, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Sahlgren, C.; Gustafsson, M.V.; Jin, S.; Poellinger, L.; Lendahl, U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 6392–6397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; De Marco, M.A.; Graziani, I.; Gazdar, A.F.; Strack, P.R.; Miele, L.; Bocchetta, M. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007, 67, 7954–7959. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Forsdike, J.; Fitzgerald, C.J.; Macdonald-Goodfellow, S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int. J. Cancer 1999, 80, 617–623. [Google Scholar] [CrossRef]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Qian, Y.; Zhang, H.; Guo, S.; Sunagawa, M.; Hisamitsu, T.; Liu, Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α pathway. Mol. Immunol. 2013, 53, 227–236. [Google Scholar] [CrossRef]
- Rohwer, N.; Welzel, M.; Daskalow, K.; Pfander, D.; Wiedenmann, B.; Detjen, K.; Cramer, T. Hypoxia-inducible factor 1α mediates anoikis resistance via suppression of α5 integrin. Cancer Res. 2008, 68, 10113–10120. [Google Scholar] [CrossRef]
- Zhang, H.; Wong, C.C.; Wei, H.; Gilkes, D.M.; Korangath, P.; Chaturvedi, P.; Schito, L.; Chen, J.; Krishnamachary, B.; Winnard, P.T., Jr.; et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 2012, 31, 1757–1770. [Google Scholar] [CrossRef]
- Jin, F.; Brockmeier, U.; Otterbach, F.; Metzen, E. New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: Hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol. Cancer Res. 2012, 10, 1021–1031. [Google Scholar] [CrossRef]
- Wendel, C.; Hemping-Bovenkerk, A.; Krasnyanska, J.; Mees, S.T.; Kochetkova, M.; Stoeppeler, S.; Haier, J. CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS ONE 2012, 7, e30046. [Google Scholar] [CrossRef]
- Gassmann, P.; Haier, J.; Schluter, K.; Domikowsky, B.; Wendel, C.; Wiesner, U.; Kubitza, R.; Engers, R.; Schneider, S.W.; Homey, B.; et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia 2009, 11, 651–661. [Google Scholar] [CrossRef]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.T.; Giaccia, A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef]
- Sion, A.M.; Figg, W.D. Lysyl oxidase (LOX) and hypoxia-induced metastases. Cancer Biol. Ther. 2006, 5, 909–911. [Google Scholar] [CrossRef]
- Wong, C.C.-L.; Gilkes, D.M.; Zhang, H.; Chen, J.; Wei, H.; Chaturvedi, P.; Fraley, S.I.; Wong, C.-M.; Khoo, U.-S.; Ng, I.O.-L.; et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl. Acad. Sci. USA 2011, 108, 16369–16374. [Google Scholar] [CrossRef]
- Fang, Y.; Sullivan, R.; Graham, C.H. Confluence-dependent resistance to doxorubicin in human MDA-MB-231 breast carcinoma cells requires hypoxia-inducible factor-1 activity. Exp. Cell Res. 2007, 313, 867–877. [Google Scholar] [CrossRef]
- Huang, L.; Ao, Q.; Zhang, Q.; Yang, X.; Xing, H.; Li, F.; Chen, G.; Zhou, J.; Wang, S.; Xu, G.; et al. Hypoxia induced paclitaxel resistance in human ovarian cancers via hypoxia-inducible factor 1α. J. Cancer Res. Clin. Oncol. 2010, 136, 447–456. [Google Scholar] [CrossRef]
- Jia, X.; Hong, Q.; Lei, L.; Li, D.; Li, J.; Mo, M.; Wang, Y.; Shao, Z.; Shen, Z.; Cheng, J.; et al. Basal and therapy-driven hypoxia-inducible factor-1α confers resistance to endocrine therapy in estrogen receptor-positive breast cancer. Oncotarget 2015, 6, 8648–8662. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Creemers, S.G.; van Koetsveld, P.M.; De Herder, W.W.; Dogan, F.; Franssen, G.J.H.; Feelders, R.A.; Hofland, L.J. MDR1 inhibition increases sensitivity to doxorubicin and etoposide in adrenocortical cancer. Endocr.-Relat. Cancer 2019, 26, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Belisario, D.C.; Polimeni, M.; Annaratone, L.; Riganti, C.; Allia, E.; Ghigo, D.; Bosia, A.; Sapino, A. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: A potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer 2012, 12, 4. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, Z.; Liang, L.; Cheng, Y.; Zhou, J.; Li, J.; Xu, Y. HIF-1α/MDR1 pathway confers chemoresistance to cisplatin in bladder cancer. Oncol. Rep. 2016, 35, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Deeley, R.G.; Cole, S.P.C. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006, 580, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, S.; Han, J.; Zheng, L.; Yang, Z.; Zhao, L. Hypoxia-inducible factor-1α induces multidrug resistance protein in colon cancer. OncoTargets Ther. 2015, 8, 1941–1948. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Pinzón-Daza, M.L.; Cuellar-Saenz, Y.; Nualart, F.; Ondo-Mendez, A.; Del Riesgo, L.; Castillo-Rivera, F.; Garzón, R. Oxidative Stress Promotes Doxorubicin-Induced Pgp and BCRP Expression in Colon Cancer Cells Under Hypoxic Conditions. J. Cell. Biochem. 2017, 118, 1868–1878. [Google Scholar] [CrossRef]
- Bergandi, L.; Canosa, S.; Pittatore, G.; Silvagno, F.; Doublier, S.; Gennarelli, G.; Benedetto, C.; Revelli, A. Human recombinant FSH induces chemoresistance in human breast cancer cells via HIF-1α activation. Biol. Reprod. 2019, 100, 1521–1535. [Google Scholar] [CrossRef]
- Yang, S.F.; Chang, C.W.; Wei, R.J.; Shiue, Y.L.; Wang, S.N.; Yeh, Y.T. Involvement of DNA damage response pathways in hepatocellular carcinoma. BioMed Res. Int. 2014, 2014, 153867. [Google Scholar] [CrossRef]
- Stover, E.H.; Konstantinopoulos, P.A.; Matulonis, U.A.; Swisher, E.M. Biomarkers of Response and Resistance to DNA Repair Targeted Therapies. Clin. Cancer Res. 2016, 22, 5651–5660. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Rezaeian, A.H.; Wang, Y.H.; Lin, H.K. DNA damage players are linked to HIF-1α/hypoxia signaling. Cell Cycle 2017, 16, 725–726. [Google Scholar] [CrossRef][Green Version]
- Shenoy, N.; Dronca, R.; Quevedo, F.; Boorjian, S.A.; Cheville, J.; Costello, B.; Kohli, M.; Witzig, T.; Pagliaro, L. Low hypoxia inducible factor-1α (HIF-1α) expression in testicular germ cell tumors—A major reason for enhanced chemosensitivity? Chin. J. Cancer Res. 2017, 29, 374–378. [Google Scholar] [CrossRef][Green Version]
- Sugrue, T.; Lowndes, N.F.; Ceredig, R. Hypoxia enhances the radioresistance of mouse mesenchymal stromal cells. Stem Cells 2014, 32, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Xu, S.; Xiao, L.; Chen, G.; Zhang, W.; Li, J. Radioprotective effect on HepG2 cells of low concentrations of cobalt chloride: Induction of hypoxia-inducible factor-1 alpha and clearance of reactive oxygen species. J. Radiat. Res. 2013, 54, 203–209. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, S.C.; Chien, C.W.; Lin, S.C.; Lee, C.T.; Lin, B.W.; Lee, J.C.; Tsai, S.J. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am. J. Pathol. 2011, 179, 1405–1414. [Google Scholar] [CrossRef]
- Maiso, P.; Huynh, D.; Moschetta, M.; Sacco, A.; Aljawai, Y.; Mishima, Y.; Asara, J.M.; Roccaro, A.M.; Kimmelman, A.C.; Ghobrial, I.M. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res. 2015, 75, 2071–2082. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef]
- Mitani, T.; Ito, Y.; Harada, N.; Nakano, Y.; Inui, H.; Ashida, H.; Yamaji, R. Resveratrol reduces the hypoxia-induced resistance to doxorubicin in breast cancer cells. J. Nutr. Sci. Vitaminol. 2014, 60, 122–128. [Google Scholar] [CrossRef]
- Hickman, J.A. Apoptosis and chemotherapy resistance. Eur. J. Cancer 1996, 32, 921–926. [Google Scholar] [CrossRef]
- Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tan, B.B.; Li, Y.; Fan, L.Q.; Yang, P.G.; Tian, Y. Enhancement of Drug Sensitivity by Knockdown of HIF-1α in Gastric Carcinoma Cells. Oncol. Res. 2016, 23, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Dame, C.; Haugstetter, A.; Wiedenmann, B.; Detjen, K.; Schmitt, C.A.; Cramer, T. Hypoxia-inducible factor 1α determines gastric cancer chemosensitivity via modulation of p53 and NF-κB. PLoS ONE 2010, 5, e12038. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Fidler, I.J. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin. Cancer Res. 2004, 10, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.T.; Wu, C.W.; Lin, W.W. Hypoxia-induced decoy receptor 2 gene expression is regulated via a hypoxia-inducible factor 1α-mediated mechanism. Biochem. Biophys. Res. Commun. 2010, 391, 1274–1279. [Google Scholar] [CrossRef]
- Minassian, L.M.; Cotechini, T.; Huitema, E.; Graham, C.H. Hypoxia-Induced Resistance to Chemotherapy in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 123–139. [Google Scholar] [CrossRef]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updat. 2011, 14, 191–201. [Google Scholar] [CrossRef]
- Chen, C.; Lu, L.; Yan, S.; Yi, H.; Yao, H.; Wu, D.; He, G.; Tao, X.; Deng, X. Autophagy and doxorubicin resistance in cancer. Anti-Cancer Drugs 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef]
- Wu, H.M.; Jiang, Z.F.; Ding, P.S.; Shao, L.J.; Liu, R.Y. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci. Rep. 2015, 5, 12291. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jia, P.; Wang, H.; Jiang, G.; Wang, T. HIF-1α-induced autophagy contributes to cisplatin resistance in ovarian cancer cells. Pharmazie 2018, 73, 533–536. [Google Scholar] [CrossRef]
- Yang, X.; Yin, H.; Zhang, Y.; Li, X.; Tong, H.; Zeng, Y.; Wang, Q.; He, W. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1α activation. Int. J. Oncol. 2018, 53, 215–224. [Google Scholar] [CrossRef]
- Feng, H.; Wang, J.; Chen, W.; Shan, B.; Guo, Y.; Xu, J.; Wang, L.; Guo, P.; Zhang, Y. Hypoxia-induced autophagy as an additional mechanism in human osteosarcoma radioresistance. J. Bone Oncol. 2016, 5, 67–73. [Google Scholar] [CrossRef]
- Zou, Y.M.; Hu, G.Y.; Zhao, X.Q.; Lu, T.; Zhu, F.; Yu, S.Y.; Xiong, H. Hypoxia-induced autophagy contributes to radioresistance via c-Jun-mediated Beclin1 expression in lung cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 761–767. [Google Scholar] [CrossRef]
- Song, J.G.; Lee, Y.S.; Park, J.A.; Lee, E.H.; Lim, S.J.; Yang, S.J.; Zhao, M.; Lee, K.; Han, H.K. Discovery of LW6 as a new potent inhibitor of breast cancer resistance protein. Cancer Chemother. Pharmacol. 2016, 78, 735–744. [Google Scholar] [CrossRef]
- Han, S.; Kim, E.-S.; You, B.; Chae, H.-S.; Liu, Q.; Chin, Y.-W.; Ahn, H.-C.; Chung, S.-J.; Lee, K.; Choi, Y.H. Effect of treatment period with LC478, a disubstituted adamantayl derivative, on P-glycoprotein inhibition: Its application to increase docetaxel absorption in rats. Xenobiotica 2020, 50, 863–874. [Google Scholar] [CrossRef]
- Zander, S.A.; Sol, W.; Greenberger, L.; Zhang, Y.; van Tellingen, O.; Jonkers, J.; Borst, P.; Rottenberg, S. EZN-2208 (PEG-SN38) overcomes ABCG2-mediated topotecan resistance in BRCA1-deficient mouse mammary tumors. PLoS ONE 2012, 7, e45248. [Google Scholar] [CrossRef]
- Onnis, B.; Rapisarda, A.; Melillo, G. Development of HIF-1 inhibitors for cancer therapy. J. Cell. Mol. Med. 2009, 13, 2780–2786. [Google Scholar] [CrossRef]
- Kessler, J.; Hahnel, A.; Wichmann, H.; Rot, S.; Kappler, M.; Bache, M.; Vordermark, D. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: Effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer 2010, 10, 605. [Google Scholar] [CrossRef]
- Zhao, T.; Ren, H.; Jia, L.; Chen, J.; Xin, W.; Yan, F.; Li, J.; Wang, X.; Gao, S.; Qian, D.; et al. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget 2015, 6, 2250–2262. [Google Scholar] [CrossRef]
- Schwartz, D.L.; Bankson, J.A.; Lemos, R., Jr.; Lai, S.Y.; Thittai, A.K.; He, Y.; Hostetter, G.; Demeure, M.J.; Von Hoff, D.D.; Powis, G. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol. Cancer Ther. 2010, 9, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, R.; Molteni, R.; Gariboldi, M.B.; Marras, E.; Perletti, G.; Monti, E. Effect of HIF-1 modulation on the response of two- and three-dimensional cultures of human colon cancer cells to 5-fluorouracil. Eur. J. Cancer 2009, 45, 890–898. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Bao, S.; Wang, M.; Yun, Y.; Zhu, R. FK228 augmented temozolomide sensitivity in human glioma cells by blocking PI3K/AKT/mTOR signal pathways. Biomed. Pharmacother. 2016, 84, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Namgung, Y.; Kim, S.Y.; Kim, I. Down-regulation of Survivin by BIX-01294 Pretreatment Overcomes Resistance of Hepatocellular Carcinoma Cells to TRAIL. Anticancer Res. 2019, 39, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Lin, H.; Moh, J.S.; Chen, K.D.; Wang, I.W.; Ou, Y.C.; You, Y.S.; Lung, C.C. Low-dose LBH589 increases the sensitivity of cisplatin to cisplatin-resistant ovarian cancer cells. Taiwan J. Obstet. Gynecol. 2011, 50, 165–171. [Google Scholar] [CrossRef][Green Version]
- Maiso, P.; Carvajal-Vergara, X.; Ocio, E.M.; Lopez-Perez, R.; Mateo, G.; Gutierrez, N.; Atadja, P.; Pandiella, A.; San Miguel, J.F. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006, 66, 5781–5789. [Google Scholar] [CrossRef]
- Zang, H.; Qian, G.; Zong, D.; Fan, S.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.Y. Overcoming acquired resistance of epidermal growth factor receptor-mutant non-small cell lung cancer cells to osimertinib by combining osimertinib with the histone deacetylase inhibitor panobinostat (LBH589). Cancer 2020, 126, 2024–2033. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K.; Zhu, Q.; Shen, Q.; Zhang, Q.; Yu, J.; Chen, Y.; Lu, W. Synthesis and biological evaluation of paclitaxel and vorinostat co-prodrugs for overcoming drug resistance in cancer therapy in vitro. Bioorg. Med. Chem. 2019, 27, 1405–1413. [Google Scholar] [CrossRef]
- Lautz, T.B.; Jie, C.; Clark, S.; Naiditch, J.A.; Jafari, N.; Qiu, Y.Y.; Zheng, X.; Chu, F.; Madonna, M.B. The effect of vorinostat on the development of resistance to doxorubicin in neuroblastoma. PLoS ONE 2012, 7, e40816. [Google Scholar] [CrossRef]
- Barbone, D.; Cheung, P.; Battula, S.; Busacca, S.; Gray, S.G.; Longley, D.B.; Bueno, R.; Sugarbaker, D.J.; Fennell, D.A.; Broaddus, V.C. Vorinostat eliminates multicellular resistance of mesothelioma 3D spheroids via restoration of Noxa expression. PLoS ONE 2012, 7, e52753. [Google Scholar] [CrossRef]
- Adamski, J.; Price, A.; Dive, C.; Makin, G. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS ONE 2013, 8, e65304. [Google Scholar] [CrossRef]
- Lee, M.R.; Lin, C.; Lu, C.C.; Kuo, S.C.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Lee, F.Y.; Yang, J.S.; Tsai, F.J. YC-1 induces G0/G1 phase arrest and mitochondria-dependent apoptosis in cisplatin-resistant human oral cancer CAR cells. BioMedicine 2017, 7, 12. [Google Scholar] [CrossRef]
- Hu, H.; Miao, X.K.; Li, J.Y.; Zhang, X.W.; Xu, J.J.; Zhang, J.Y.; Zhou, T.X.; Hu, M.N.; Yang, W.L.; Mou, L.Y. YC-1 potentiates the antitumor activity of gefitinib by inhibiting HIF-1α and promoting the endocytic trafficking and degradation of EGFR in gefitinib-resistant non-small-cell lung cancer cells. Eur. J. Pharmacol. 2020, 874, 172961. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.-A.; Liu, Q.-H.; Kong, Q.-Y.; Liu, L.; Cui, T.; Wu, Y.-P. RAD001 can reverse drug resistance of SGC7901/DDP cells. Tumour Biol. 2014, 35, 9171–9177. [Google Scholar] [CrossRef]
- Kim, E.S.; Kies, M.S.; Fossella, F.V.; Glisson, B.S.; Zaknoen, S.; Statkevich, P.; Munden, R.F.; Summey, C.; Pisters, K.M.; Papadimitrakopoulou, V.; et al. Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer 2005, 104, 561–569. [Google Scholar] [CrossRef]
- Smalley, K.S.; Eisen, T.G. Farnesyl transferase inhibitor SCH66336 is cytostatic, pro-apoptotic and enhances chemosensitivity to cisplatin in melanoma cells. Int. J. Cancer 2003, 105, 165–175. [Google Scholar] [CrossRef]
- Wang, E.J.; Johnson, W.W. The farnesyl protein transferase inhibitor lonafarnib (SCH66336) is an inhibitor of multidrug resistance proteins 1 and 2. Chemotherapy 2003, 49, 303–308. [Google Scholar] [CrossRef]
- Shevrin, D.H.; Lad, T.E.; Guinan, P.; Kilton, L.J.; Greenburg, A.; Johnson, P.; Blough, R.R.; Hoyer, H. Phase II trial of echinomycin in advanced hormone-resistant prostate cancer. An Illinois Cancer Council study. Investig. New Drugs 1994, 12, 65–66. [Google Scholar] [CrossRef]
- Evenepoel, M.; Haenen, V.; De Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Dams, L.; Van Dijck, S.; Van der Gucht, E.; De Groef, A. Pain Prevalence During Cancer Treatment: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2022, 63, e317–e335. [Google Scholar] [CrossRef]
- Neufeld, N.J.; Elnahal, S.M.; Alvarez, R.H. Cancer pain: A review of epidemiology, clinical quality and value impact. Future Oncol. 2017, 13, 833–841. [Google Scholar] [CrossRef]
- Esin, E.; Yalcin, S. Neuropathic cancer pain: What we are dealing with? How to manage it? OncoTargets Ther. 2014, 7, 599–618. [Google Scholar] [CrossRef]
- Howard, P. Hypoxia, almitrine, and peripheral neuropathy. Thorax 1989, 44, 247–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyette-Davis, J.A.; Walters, E.T.; Dougherty, P.M. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015, 5, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ludman, T.; Melemedjian, O.K. Bortezomib-induced aerobic glycolysis contributes to chemotherapy-induced painful peripheral neuropathy. Mol. Pain 2019, 15, 1744806919837429. [Google Scholar] [CrossRef] [PubMed]
- Ludman, T.; Melemedjian, O.K. Bortezomib and metformin opposingly regulate the expression of hypoxia-inducible factor alpha and the consequent development of chemotherapy-induced painful peripheral neuropathy. Mol. Pain 2019, 15, 1744806919850043. [Google Scholar] [CrossRef]
- Richardson, P.G.; Chanan-Khan, A.A.; Lonial, S.; Krishnan, A.Y.; Carroll, M.P.; Alsina, M.; Albitar, M.; Berman, D.; Messina, M.; Anderson, K.C. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: Results of a phase 1/2 study. Br. J. Haematol. 2011, 153, 729–740. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, G.-Y.; Guo, Y.-R.; Liang, D.-Q.; Yuan, Y.; Wang, H.-L. Correlation between liver cancer pain and the HIF-1 and VEGF expression levels. Oncol. Lett. 2017, 13, 77–80. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Deng, M.; Huang, J.; Wu, J.; Li, Z.; Xing, M.; Wang, J.; Guo, Q.; Zou, W. Microglial annexin A3 downregulation alleviates bone cancer-induced pain through inhibiting the Hif-1α/vascular endothelial growth factor signaling pathway. Pain 2020, 161, 2750–2762. [Google Scholar] [CrossRef]
- Stosser, S.; Schweizerhof, M.; Kuner, R. Hematopoietic colony-stimulating factors: New players in tumor-nerve interactions. J. Mol. Med. 2011, 89, 321–329. [Google Scholar] [CrossRef]
- Schweizerhof, M.; Stosser, S.; Kurejova, M.; Njoo, C.; Gangadharan, V.; Agarwal, N.; Schmelz, M.; Bali, K.K.; Michalski, C.W.; Brugger, S.; et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 2009, 15, 802–807. [Google Scholar] [CrossRef]
- Mroczko, B.; Szmitkowski, M.; Wereszczynska-Siemiatkowska, U.; Jurkowska, G. Hematopoietic cytokines in the sera of patients with pancreatic cancer. Clin. Chem. Lab. Med. 2005, 43, 146–150. [Google Scholar] [CrossRef]
- Wang, H.; Jia, R.; Zhao, T.; Li, X.; Lang, M.; Lan, C.; Wang, H.; Li, Z.; Zhou, B.; Wu, L.; et al. HIF-1α mediates tumor-nerve interactions through the up-regulation of GM-CSF in pancreatic ductal adenocarcinoma. Cancer Lett. 2019, 453, 10–20. [Google Scholar] [CrossRef]
- Kanngiesser, M.; Mair, N.; Lim, H.-Y.; Zschiebsch, K.; Blees, J.; Häußler, A.; Brüne, B.; Ferreiros Bouzas, N.; Kress, M.; Tegeder, I. Hypoxia-Inducible Factor 1 Regulates Heat and Cold Pain Sensitivity and Persistence. Antioxid. Redox Signal. 2014, 20, 2555–2571. [Google Scholar] [CrossRef]
- Qiu, G.Z.; Jin, M.Z.; Dai, J.X.; Sun, W.; Feng, J.H.; Jin, W.L. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 669–686. [Google Scholar] [CrossRef]
- Garje, R.; An, J.J.; Sanchez, K.; Greco, A.; Stolwijk, J.; Devor, E.; Rustum, Y.; Zakharia, Y. Current Landscape and the Potential Role of Hypoxia-Inducible Factors and Selenium in Clear Cell Renal Cell Carcinoma Treatment. Int. J. Mol. Sci. 2018, 19, 3834. [Google Scholar] [CrossRef]
- Bhattarai, D.; Xu, X.; Lee, K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): A “structure-activity relationship” perspective. Med. Res. Rev. 2018, 38, 1404–1442. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Soung, N.-K.; Kim, H.-M.; Asami, Y.; Kim, D.H.; Cho, Y.; Naik, R.; Jang, Y.; Jang, K.; Han, H.J.; Ganipisetti, S.R.; et al. Mechanism of the natural product moracin-O derived MO-460 and its targeting protein hnRNPA2B1 on HIF-1α inhibition. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Jeong, W.; Park, S.R.; Rapisarda, A.; Fer, N.; Kinders, R.J.; Chen, A.; Melillo, G.; Turkbey, B.; Steinberg, S.M.; Choyke, P.; et al. Weekly EZN-2208 (PEGylated SN-38) in combination with bevacizumab in patients with refractory solid tumors. Investig. New Drugs 2014, 32, 340–346. [Google Scholar] [CrossRef]
- Norris, R.E.; Shusterman, S.; Gore, L.; Muscal, J.A.; Macy, M.E.; Fox, E.; Berkowitz, N.; Buchbinder, A.; Bagatell, R. Phase 1 evaluation of EZN-2208, a polyethylene glycol conjugate of SN38, in children adolescents and young adults with relapsed or refractory solid tumors. Pediatr. Blood Cancer 2014, 61, 1792–1797. [Google Scholar] [CrossRef]
- Garrett, C.R.; Bekaii-Saab, T.S.; Ryan, T.; Fisher, G.A.; Clive, S.; Kavan, P.; Shacham-Shmueli, E.; Buchbinder, A.; Goldberg, R.M. Randomized phase 2 study of pegylated SN-38 (EZN-2208) or irinotecan plus cetuximab in patients with advanced colorectal cancer. Cancer 2013, 119, 4223–4230. [Google Scholar] [CrossRef] [PubMed]
- Borsi, E.; Perrone, G.; Terragna, C.; Martello, M.; Dico, A.F.; Solaini, G.; Baracca, A.; Sgarbi, G.; Pasquinelli, G.; Valente, S.; et al. Hypoxia inducible factor-1 alpha as a therapeutic target in multiple myeloma. Oncotarget 2014, 5, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Rapisarda, A.; Park, S.R.; Kinders, R.J.; Chen, A.; Melillo, G.; Turkbey, B.; Steinberg, S.M.; Choyke, P.; Doroshow, J.H.; et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Mie Lee, Y.; Kim, S.H.; Kim, H.S.; Jin Son, M.; Nakajima, H.; Jeong Kwon, H.; Kim, K.W. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1α activity. Biochem. Biophys. Res. Commun. 2003, 300, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sasakawa, Y.; Naoe, Y.; Noto, T.; Inoue, T.; Sasakawa, T.; Matsuo, M.; Manda, T.; Mutoh, S. Antitumor efficacy of FK228, a novel histone deacetylase inhibitor, depends on the effect on expression of angiogenesis factors. Biochem. Pharmacol. 2003, 66, 897–906. [Google Scholar] [CrossRef]
- Gerber, D.E.; Boothman, D.A.; Fattah, F.J.; Dong, Y.; Zhu, H.; Skelton, R.A.; Priddy, L.L.; Vo, P.; Dowell, J.E.; Sarode, V.; et al. Phase 1 study of romidepsin plus erlotinib in advanced non-small cell lung cancer. Lung Cancer 2015, 90, 534–541. [Google Scholar] [CrossRef]
- Haigentz, M., Jr.; Kim, M.; Sarta, C.; Lin, J.; Keresztes, R.S.; Culliney, B.; Gaba, A.G.; Smith, R.V.; Shapiro, G.I.; Chirieac, L.R.; et al. Phase II trial of the histone deacetylase inhibitor romidepsin in patients with recurrent/metastatic head and neck cancer. Oral Oncol. 2012, 48, 1281–1288. [Google Scholar] [CrossRef]
- Fischer, C.; Leithner, K.; Wohlkoenig, C.; Quehenberger, F.; Bertsch, A.; Olschewski, A.; Olschewski, H.; Hrzenjak, A. Panobinostat reduces hypoxia-induced cisplatin resistance of non-small cell lung carcinoma cells via HIF-1α destabilization. Mol. Cancer 2015, 14, 4. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Mina, R.; Jakubowiak, A.J.; Zimmerman, T.L.; Wolf, J.J.; Lewis, C.; Gleason, C.; Sharp, C.; Martin, T.; Heffner, L.T.; et al. Combining carfilzomib and panobinostat to treat relapsed/refractory multiple myeloma: Results of a Multiple Myeloma Research Consortium Phase I Study. Blood Cancer J. 2019, 9, 3. [Google Scholar] [CrossRef]
- Wood, P.J.; Strong, R.; McArthur, G.A.; Michael, M.; Algar, E.; Muscat, A.; Rigby, L.; Ferguson, M.; Ashley, D.M. A phase I study of panobinostat in pediatric patients with refractory solid tumors, including CNS tumors. Cancer Chemother. Pharmacol. 2018, 82, 493–503. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Feldman, M.J.; Wang, H.; Pang, Y.; Maggio, D.M.; Zhu, D.; Nesvick, C.L.; Dmitriev, P.; Bullova, P.; et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget 2017, 8, 56110–56125. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F., Jr.; Nebozhyn, M.; et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro-Oncology 2018, 20, 546–556. [Google Scholar] [CrossRef]
- Haas, N.B.; Quirt, I.; Hotte, S.; McWhirter, E.; Polintan, R.; Litwin, S.; Adams, P.D.; McBryan, T.; Wang, L.; Martin, L.P.; et al. Phase II trial of vorinostat in advanced melanoma. Investig. New Drugs 2014, 32, 526–534. [Google Scholar] [CrossRef]
- Krug, L.M.; Kindler, H.L.; Calvert, H.; Manegold, C.; Tsao, A.S.; Fennell, D.; Ohman, R.; Plummer, R.; Eberhardt, W.E.; Fukuoka, K.; et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015, 16, 447–456. [Google Scholar] [CrossRef]
- Lang, S.A.; Klein, D.; Moser, C.; Gaumann, A.; Glockzin, G.; Dahlke, M.H.; Dietmaier, W.; Bolder, U.; Schlitt, H.J.; Geissler, E.K.; et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol. Cancer Ther. 2007, 6, 1123–1132. [Google Scholar] [CrossRef]
- Heath, E.I.; Hillman, D.W.; Vaishampayan, U.; Sheng, S.; Sarkar, F.; Harper, F.; Gaskins, M.; Pitot, H.C.; Tan, W.; Ivy, S.P.; et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 2008, 14, 7940–7946. [Google Scholar] [CrossRef]
- Han, J.Y.; Oh, S.H.; Morgillo, F.; Myers, J.N.; Kim, E.; Hong, W.K.; Lee, H.Y. Hypoxia-inducible factor 1α and antiangiogenic activity of farnesyltransferase inhibitor SCH66336 in human aerodigestive tract cancer. J. Natl. Cancer Inst. 2005, 97, 1272–1286. [Google Scholar] [CrossRef]
- Hanrahan, E.O.; Kies, M.S.; Glisson, B.S.; Khuri, F.R.; Feng, L.; Tran, H.T.; Ginsberg, L.E.; Truong, M.T.; Hong, W.K.; Kim, E.S. A phase II study of Lonafarnib (SCH66336) in patients with chemorefractory, advanced squamous cell carcinoma of the head and neck. Am. J. Clin. Oncol. 2009, 32, 274–279. [Google Scholar] [CrossRef]
- Sharma, S.; Kemeny, N.; Kelsen, D.P.; Ilson, D.; O’Reilly, E.; Zaknoen, S.; Baum, C.; Statkevich, P.; Hollywood, E.; Zhu, Y.; et al. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann. Oncol. 2002, 13, 1067–1071. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, H.; Qian, D.Z.; Rey, S.; Liu, J.O.; Semenza, G.L. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA 2009, 106, 17910–17915. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; He, S.; Shen, G.; Wang, Y. HIF-1 Dimerization Inhibitor Acriflavine Enhances Antitumor Activity of Sunitinib in Breast Cancer Model. Oncol. Res. 2014, 22, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Goodman, P.J.; Fleming, T.R.; Taylor, S.A.; Macdonald, J.S. Phase II trial of echinomycin in advanced colorectal cancer. A Southwest Oncology Group study. Investig. New Drugs 1991, 9, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.E.; Wolf, M.K.; Crawford, E.D.; Taylor, S.; Blumenstein, B.; Flanigan, R.; Meyers, F.J.; Hynes, H.E.; Barlogie, B.; Eisenberger, M. Phase II trial of echinomycin for the treatment of advanced renal cell carcinoma. A Southwest Oncology Group study. Investig. New Drugs 1993, 11, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Muss, H.B.; Blessing, J.A.; Baker, V.V.; Barnhill, D.R.; Adelson, M.D. Echinomycin (NSC 526417) in advanced ovarian cancer. A phase II trial of the Gynecologic Oncology Group. Am. J. Clin. Oncol. 1990, 13, 299–301. [Google Scholar] [CrossRef]
- Schilsky, R.L.; Faraggi, D.; Korzun, A.; Vogelzang, N.; Ellerton, J.; Wood, W.; Henderson, I.C. Phase II study of echinomycin in patients with advanced breast cancer: A report of Cancer and Leukemia Group B protocol 8641. Investig. New Drugs 1991, 9, 269–272. [Google Scholar] [CrossRef]
- Schumacher, G.; Neuhaus, P. 2-Methoxyestradiol—A new compound for cancer treatment. Dtsch. Med. Wochenschr. 2006, 131, 825–830. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Richardson, P.G.; Lacy, M.Q.; Dispenzieri, A.; Greipp, P.R.; Witzig, T.E.; Schlossman, R.; Sidor, C.F.; Anderson, K.C.; Gertz, M.A. Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin. Cancer Res. 2007, 13, 6162–6167. [Google Scholar] [CrossRef]
- Harrison, M.R.; Hahn, N.M.; Pili, R.; Oh, W.K.; Hammers, H.; Sweeney, C.; Kim, K.; Perlman, S.; Arnott, J.; Sidor, C.; et al. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal(R) dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Investig. New Drugs 2011, 29, 1465–1474. [Google Scholar] [CrossRef]
- Matei, D.; Schilder, J.; Sutton, G.; Perkins, S.; Breen, T.; Quon, C.; Sidor, C. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: A Hoosier Oncology Group trial. Gynecol. Oncol. 2009, 115, 90–96. [Google Scholar] [CrossRef]
- Kulke, M.H.; Chan, J.A.; Meyerhardt, J.A.; Zhu, A.X.; Abrams, T.A.; Blaszkowsky, L.S.; Regan, E.; Sidor, C.; Fuchs, C.S. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother. Pharmacol. 2011, 68, 293–300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).