ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative General Status
2.2. Preoperative Functional and General Status
2.3. Main Pulmonary Artery Diameter and Normalized Pulmonary Artery Diameter
2.4. Indexes of Inflammatory Status
- -
- Platelets to Lymphocytes Ratio (PLR) and albumin multiplying lymphocytes, known as the Prognostic Nutritional Index (PNI);
- -
- HALP amalgamated index, which is measured as hemoglobin (g/L) × albumin (g/L) × lymphocyte (/L)/platelet (/L);
- -
- Serum Polymorpho-nuclear Neutrophil to Lymphocytes Ratio (NLR);
- -
- Systemic Immune-inflammation Index (SII): serum platelets × neutrophil/lymphocytes;
- -
- Advanced Lung Cancer Inflammation Index (ALI): serum albumin × BMI/NLR; BMI = weight (kg)/height (m)2.
2.5. Peri- and Postoperative Anesthesiologist Management
2.6. Definition of ARDS
- -
- Acute onset within 7 days after surgery with ventilation setting for positive end-expiratory pressure (PEEP) of ≥5 cm H2O and bilateral lung infiltration, detected through chest x-ray: cannot be fully explained by effusion, lobar, lung collapse or nodules;
- -
- Absence of hydrostatic or cardiogenic pulmonary edema;
- -
- Partial pressure of arterial oxygen and fraction of inspired oxygen (PaO2/FiO2) 300 mmHg or less and classified in 3 categories of severity: mild (200 mmHg < PaO2/FiO2 < 300 mmHg), moderate (100 mmHg < PaO2/FiO2 < 200 mmHg) and severe (PaO2/FiO2 < 100 mmHg).
2.7. Statistical Methods
3. Results
3.1. Preoperative and Surgical Treatments
3.2. Postoperative Findings
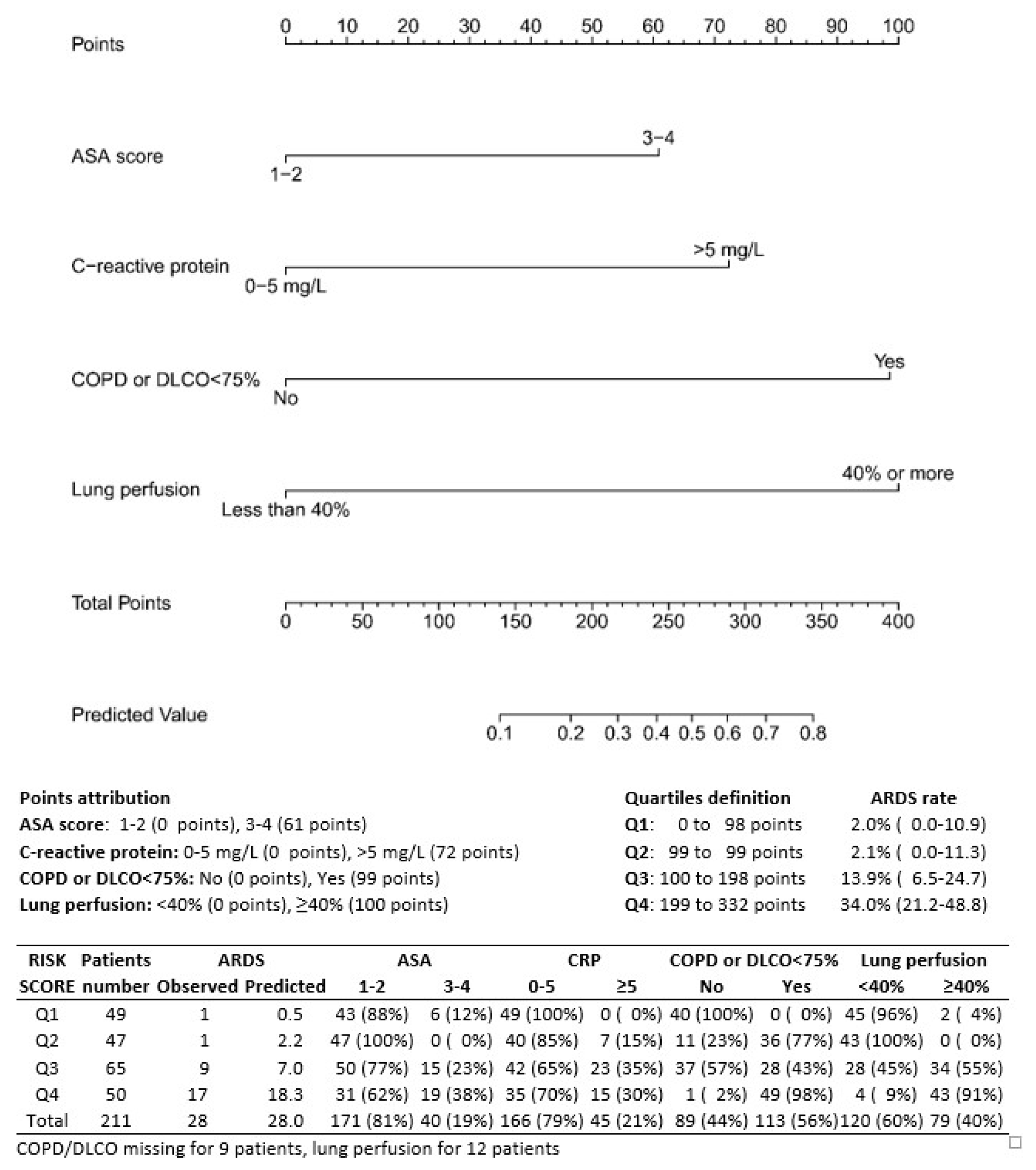
3.3. Predicting Factors of ARDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepulveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.-F. Postoperative Pneumonia after Major Lung Resection. Am. J. Respir. Crit. Care Med. 2006, 173, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Algar, F.J.; Alvarez, A.; Salvatierra, A.; Baamonde, C.; Aranda, J.L.; López-Pujol, F.J. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur. J. Cardio-Thoracic Surg. 2003, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.S.; Burt, B.M.; Sugarbaker, D.J. Management of Complications After Pneumonectomy. Thorac. Surg. Clin. 2015, 25, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, A.; Pardolesi, A.; Maisonneuve, P.; Petrella, F.; Galetta, D.; Gasparri, R.; Spaggiari, L. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin. Thorac Cardiovasc. Surg. 2018, 30, 104–113. [Google Scholar] [CrossRef]
- Janet-Vendroux, A.; Loi, M.; Bobbio, A.; Lococo, F.; Lupo, A.; Ledinot, P.; Magdeleinat, P.; Roche, N.; Damotte, D.; Regnard, J.-F.; et al. Which is the Role of Pneumonectomy in the Era of Parenchymal-Sparing Procedures? Early/Long-Term Survival and Functional Results of a Single-Center Experience. Lung 2015, 193, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, A.; Bertolaccini, L.; Sedda, G.; Prisciandaro, E.; Loi, M.; Iacono, G.L.; Spaggiari, L. Pneumonectomy and broncho-pleural fistula: Predicting factors and stratification of the risk. Updat. Surg. 2022, 74, 1471–1478. [Google Scholar] [CrossRef]
- Dulu, A.; Pastores, S.M.; Park, B.; Riedel, E.; Rusch, V.; Halpern, N.A. Prevalence and Mortality of Acute Lung Injury and ARDS After Lung Resection. Chest 2006, 130, 73–78. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Riviello, E.D.; Kiviri, W.; Twagirumugabe, T.; Mueller, A.; Banner-Goodspeed, V.M.; Officer, L.; Novack, V.; Mutumwinka, M.; Talmor, D.S.; Fowler, R.A. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am. J. Respir. Crit. Care Med. 2016, 193, 52–59. [Google Scholar] [CrossRef]
- Tang, S.S.; Redmond, K.; Griffiths, M.; Ladas, G.; Goldstraw, P.; Dusmet, M. The mortality from acute respiratory distress syndrome after pulmonary resection is reducing: A 10-year single institutional experience. Eur. J. Cardio-Thoracic Surg. 2008, 34, 898–902. [Google Scholar] [CrossRef]
- Hamaji, M.; Keegan, M.T.; Cassivi, S.D.; Shen, K.R.; Wigle, D.A.; Allen, M.S.; Iii, F.C.N.; Deschamps, C.; Nichols, F.C. Outcomes in patients requiring mechanical ventilation following pneumonectomy. Eur. J. Cardio-Thoracic Surg. 2014, 46, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, C.A.; Williams, E.A.; Evans, T.W.; Pastorino, U.; Goldstraw, P. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann. Thorac. Surg. 2000, 69, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Parola, A.; Papalia, E.; Filosso, P.L.; Mancuso, M.; Oliaro, A.; Dato, G.M.A.; Maggi, G. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur. J. Cardio-Thoracic Surg. 2001, 20, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Park, B.J.; Wilton, A.; Seshan, V.E.; Bains, M.S.; Downey, R.J.; Flores, R.M.; Rizk, N.; Rusch, V.; Amar, D. Incidence and Risk Factors for Lung Injury After Lung Cancer Resection. Ann. Thorac. Surg. 2007, 84, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Yoon, J.W.; Suh, G.Y.; Kim, J.; Kim, K.; Yang, M.; Kim, H.; Kwon, O.J.; Shims, Y.M. Risk Factors for Post-pneumonectomy Acute Lung Injury/Acute Respiratory Distress Syndrome in Primary Lung Cancer Patients. Anaesth. Intensiv. Care 2009, 37, 14–19. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, S.W.; Park, S.-I.; Kim, Y.H.; Kim, D.K. Risk factor analysis for postoperative acute respiratory distress syndrome and early mortality after pneumonectomy: The predictive value of preoperative lung perfusion distribution. J. Thorac. Cardiovasc. Surg. 2010, 140, 26–31. [Google Scholar] [CrossRef]
- Licker, M.; de Perrot, M.; Spiliopoulos, A.; Robert, J.; Diaper, J.; Chevalley, C.; Tschopp, J.-M. Risk Factors for Acute Lung Injury After Thoracic Surgery for Lung Cancer. Obstet. Anesthesia Dig. 2003, 97, 1558–1565. [Google Scholar] [CrossRef]
- Blanc, K.; Zaimi, R.; Dechartres, A.; Lefebvre, A.; Janet-Vendroux, A.; Hamelin-Canny, E.; Roche, N.; Alifano, M.; Rabbat, A. Early acute respiratory distress syndrome after pneumonectomy: Presentation, management, and short- and long-term outcomes. J. Thorac. Cardiovasc. Surg. 2018, 156, 1706–1714.e5. [Google Scholar] [CrossRef]
- Mazzella, A.; Iacono, G.L.; Alifano, M. Postpneumonectomy respiratory failure and acute respiratory distress syndrome: Risk factors and outcome. Shanghai Chest 2021, 5, 8. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Ni, Y.; Yu, Y.; Dai, R.; Shi, G. Diffusing capacity in chronic obstructive pulmonary disease assessment: A meta-analysis. Chronic Respir. Dis. 2021, 18, 14799731211056340. [Google Scholar] [CrossRef] [PubMed]
- Peretti, M.; Hervochon, R.; Loi, M.; Blanc, K.; Roche, N.; Alifano, M. Predictors of post-pneumonectomy respiratory failure and ARDS: Usefulness of normalized pulmonary artery diameter. Intensiv. Care Med. 2018, 44, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Daffrè, E.; Prieto, M.; Huang, H.; Janet-Vendroux, A.; Blanc, K.; N’Guyen, Y.-L.; Fournel, L.; Alifano, M. Normalized Pulmonary Artery Diameter Predicts Occurrence of Postpneumonectomy Respiratory Failure, ARDS, and Mortality. Cancers 2020, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Fournel, L.; Charrier, T.; Huriet, M.; Iaffaldano, A.; Lupo, A.; Damotte, D.; Arrondeau, J.; Alifano, M. Prognostic impact of inflammation in malignant pleural mesothelioma: A large-scale analysis of consecutive patients. Lung Cancer 2022, 166, 221–227. [Google Scholar] [CrossRef]
- Tønnesen, H.; Petersen, K.R.; Højgaard, L.; Stokholm, K.H.; Nielsen, H.J.; Knigge, U.P.; Kehlet, H. Postoperative morbidity among alcohol abusers. Ugeskr Laeger 1994, 156, 287–290. [Google Scholar]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Pastorino, U.; Morelli, D.; Leuzzi, G.; Gisabella, M.; Suatoni, P.; Taverna, F.; Bertocchi, E.; Boeri, M.; Sozzi, G.; Cantarutti, A.; et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur. J. Cancer 2017, 79, 90–97. [Google Scholar] [CrossRef]
- Nagata, M.; Ito, H.; Matsuzaki, T.; Furumoto, H.; Isaka, T.; Nishii, T.; Yokose, T.; Nakayama, H. Body mass index, C-reactive protein and survival in smokers undergoing lobectomy for lung cancer†. Eur. J. Cardiothorac. Surg. 2017, 51, 1164–1170. [Google Scholar] [CrossRef][Green Version]
- Alifano, M.; Falcoz, P.E.; Seegers, V.; Roche, N.; Schussler, O.; Younes, M.; Antonacci, F.; Forgez, P.; Dechartres, A.; Massard, G.; et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 142, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Chau, E.H.L.; Slinger, P. Perioperative Fluid Management for Pulmonary Resection Surgery and Esophagectomy. Semin. Cardiothorac. Vasc. Anesthesia 2013, 18, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Langmuir, V.K.; Singer, A.H.; Skinner, D.B. Hemodynamic pulmonary edema in dog lungs after contralateral pneumonectomy and mediastinal lymphatic interruption. Lung 1984, 162, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, R.A.; Normandin, D.; Landtwing, D.; Peters, R.M. Postpneumonectomy pulmonary edema. J. Thorac. Cardiovasc. Surg. 1984, 87, 359–365. [Google Scholar] [CrossRef]
- Evans, R.G.; Naidu, B. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 498–504. [Google Scholar] [CrossRef]
| Patient Characteristics | Total | NO ARDS | YES ARDS | |
|---|---|---|---|---|
| Patients (%) | Patients (%) | Patients (%) | p-Value | |
| Total | 211 (100.0) | 183 (100.0) | 28 (100.0) | |
| Age | ||||
| <60 | 61 (28.9) | 57 (31.1) | 4 (14.3) | |
| 60–64 | 35 (16.6) | 30 (16.4) | 5 (17.9) | |
| 65–69 | 60 (28.4) | 51 (27.9) | 9 (32.1) | |
| 70–74 | 36 (17.1) | 31 (16.9) | 5 (17.9) | |
| 75+ | 19 (9.0) | 14 (7.7) | 5 (17.9) | 0.047 |
| Sex | ||||
| Men | 149 (70.6) | 130 (71.0) | 19 (67.9) | |
| Women | 62 (29.4) | 53 (29.0) | 9 (32.1) | 0.82 |
| BMI | ||||
| Underweight | 8 (3.8) | 8 (4.4) | 0 (0.0) | |
| Normal | 100 (47.4) | 83 (45.4) | 17 (60.7) | |
| Overweight | 85 (40.3) | 75 (41.0) | 10 (35.7) | |
| Obese | 18 (8.5) | 17 (9.3) | 1 (3.6) | 0.30 |
| Area body surface | ||||
| Q1 | 70 (33.2) | 60 (32.8) | 10 (35.7) | |
| Q2 | 71 (33.6) | 61 (33.3) | 10 (35.7) | |
| Q3 | 70 (33.2) | 62 (33.9) | 8 (28.6) | 0.90 |
| Comorbidities | ||||
| Cardiac | 62 (29.4) | 51 (27.9) | 11 (39.3) | 0.27 |
| Hypertension | 110 (52.1) | 92 (50.3) | 18 (64.3) | 0.22 |
| Pulmonary | 15 (7.1) | 13 (7.1) | 2 (7.1) | 1.00 |
| COPD | 61 (28.9) | 49 (26.8) | 13 (46.4) | 0.04 |
| Cancer | 23 (10.9) | 18 (9.8) | 5 (17.9) | 0.20 |
| Diabetes | 20 (9.5) | 16 (8.7) | 4 (14.3) | 0.31 |
| Smoking | ||||
| Non-smoker | 66 (31.3) | 57 (31.1) | 9 (32.1) | |
| Current smoker | 40 (19.0) | 32 (17.5) | 8 (28.6) | |
| Ex-smoker | 102 (48.3) | 92 (50.3) | 10 (35.7) | 0.26 |
| Alcohol | ||||
| No | 207 (98.1) | 180 (98.4) | 27 (96.4) | |
| Yes | 4 (1.9) | 3 (1.6) | 1 (3.6) | 0.44 |
| Presurgical treatment | ||||
| No | 101 (47.9) | 90 (49.2) | 11 (39.3) | |
| Yes | 110 (52.1) | 93 (50.8) | 17 (60.7) | 0.42 |
| Patient Characteristics | Total | NO ARDS | YES ARDS | |
|---|---|---|---|---|
| Patients (%) | Patients (%) | Patients (%) | p-Value | |
| LUNG FUNCTION | ||||
| FEV1 PPO (missing for 10) | ||||
| Median (range) | 52 (25–131) | 52 (27–131) | 48 (25–96) | 0.04 |
| <50% | 100 (49.8) | 81 (47.1) | 19 (67.9) | |
| ≥50% | 101 (50.2) | 91 (52.9) | 9 (32.1) | 0.04 |
| DLCO/VA (missing for 19 patients) | ||||
| Median (range) | 51 (17–122) | 52 (17–122) | 48 (27–102) | 0.30 |
| <50% | 96 (50.0) | 80 (48.5) | 16 (59.3) | |
| ≥50% | 96 (50.0) | 85 (51.5) | 11 (40.7) | 0.41 |
| DLCO PPO (missing for 13) | ||||
| Reduction (<75%) | 75 (37.9) | 60 (35.1) | 15 (55.6) | |
| Normal (>75%) | 123 (62.1) | 111 (64.9) | 12 (44.4) | 0.05 |
| COPD or DLCO < 75% (missing for 9) | ||||
| No | 89 (44.1) | 85 (48.9) | 4 (14.3) | |
| Yes | 113 (55.9) | 89 (51.1) | 24 (85.7) | 0.0008 |
| Lung perfusion (missing for 12) | ||||
| Median (range) | 37 (2–54) | 36 (3–51) | 43 (2–54) | 0.01 |
| <40% | 120 (60.3) | 112 (64.7) | 8 (30.8) | |
| ≥40% | 79 (39.7) | 61 (35.3) | 18 (69.2) | 0.002 |
| Vo2MAX | ||||
| Median (range) | 19.7 (11.8–32.7) | 19.9 (11.8–32.7) | 17.0 (12.7–21.2) | 0.10 |
| Vo2MAX (missing for 158) | ||||
| T1 (<18) | 17 (48.6) | 13 (44.8) | 4 (66.7) | |
| T2 (18.0–21.7) | 18 (51.4) | 16 (55.2) | 2 (33.3) | |
| T3 (>21.7) | 18 (51.4) | 18 (62.1) | 0 (0.0) | 0.03 |
| VESSELS (missing for 6) | ||||
| Aorta diameter | ||||
| ≤median (32.1) | 102 (49.8) | 90 (50.8) | 12 (42.9) | |
| >median (32.1) | 103 (50.2) | 87 (49.2) | 16 (57.1) | 0.54 |
| Pulmonary artery diameter | ||||
| ≤median (26.0) | 103 (50.2) | 89 (50.3) | 14 (50.0) | |
| >median (26.0) | 102 (49.8) | 88 (49.7) | 14 (50.0) | 1.00 |
| Normalized PAD | ||||
| ≤median (9.80) | 103 (50.2) | 90 (50.8) | 13 (46.4) | |
| >median (9.80) | 102 (49.8) | 87 (49.2) | 15 (53.6) | 0.84 |
| PAD/AoD ratio | ||||
| ≤median (0.8125) | 103 (50.2) | 89 (50.3) | 14 (50.0) | |
| >median (0.8125) | 102 (49.8) | 88 (49.7) | 14 (50.0) | 1.00 |
| BLOOD PARAMETERS | ||||
| White blood cells | ||||
| Low (<3.9) | 2 (0.9) | 2 (1.1) | 0 (0.0) | |
| Normal (3.9–10.2) | 153 (72.5) | 134 (73.2) | 19 (67.9) | |
| High (>10.2) | 56 (26.5) | 47 (25.7) | 9 (32.1) | 0.62 |
| C-reactive protein | ||||
| Normal (≤5.0) | 166 (78.7) | 150 (82.0) | 16 (57.1) | |
| High (>5.0) | 45 (21.3) | 33 (18.0) | 12 (42.9) | 0.006 |
| Hemoglobin | ||||
| Low (<13.5) | 140 (66.4) | 119 (65.0) | 21 (75.0) | |
| Normal (13.5–17.2) | 69 (32.7) | 62 (33.9) | 7 (25.0) | |
| High (17.2) | 2 (0.9) | 2 (1.1) | 0 (0.0) | 0.54 |
| Lymphocytes | ||||
| Low (<1.1) | 24 (11.4) | 21 (11.5) | 3 (10.7) | |
| Normal (1.1–4.5) | 183 (86.7) | 158 (86.3) | 25 (89.3) | |
| High (>4.5) | 4 (1.9) | 4 (2.2) | 0 (0.0) | 1.00 |
| Neutrophils | ||||
| Low (<1.5) | 2 (0.9) | 2 (1.1) | 0 (0.0) | |
| Normal (1.5–7.7) | 167 (79.1) | 146 (79.8) | 21 (75.0) | |
| High (>7.7) | 42 (19.9) | 35 (19.1) | 7 (25.0) | 0.59 |
| Platelets | ||||
| Low (<140) | 5 (2.4) | 4 (2.2) | 1 (3.6) | |
| Normal (140–450) | 186 (88.2) | 164 (89.6) | 22 (78.6) | |
| High (>450) | 20 (9.5) | 15 (8.2) | 5 (17.9) | 0.17 |
| Albumin | ||||
| Low (<3.4) | 26 (12.4) | 20 (11.0) | 6 (21.4) | |
| Normal (3.4–5.4) | 184 (87.6) | 162 (89.0) | 22 (78.6) | 0.13 |
| INFLAMMATORY SCORES | ||||
| HALP score | ||||
| <0.35 | 106 (50.2) | 89 (48.6) | 17 (60.7) | |
| ≥0.35 | 105 (49.8) | 94 (51.4) | 11 (39.3) | 0.31 |
| NLR score | ||||
| <3.0 | 114 (54.0) | 101 (55.2) | 13 (46.4) | |
| ≥3.0 | 97 (46.0) | 82 (44.8) | 15 (53.6) | 0.42 |
| PLR score | ||||
| <150 | 106 (50.2) | 94 (51.4) | 12 (42.9) | |
| ≥150 | 105 (49.8) | 89 (48.6) | 16 (57.1) | 0.42 |
| ALI index | ||||
| <35 | 107 (50.7) | 89 (48.6) | 18 (64.3) | |
| ≥35 | 104 (49.3) | 94 (51.4) | 10 (35.7) | 0.16 |
| SII score | ||||
| <750 | 107 (50.7) | 95 (51.9) | 12 (42.9) | |
| ≥750 | 104 (49.3) | 88 (48.1) | 16 (57.1) | 0.42 |
| Patient Characteristics | Total | NO ARDS | YES ARDS | |
|---|---|---|---|---|
| Patients (%) | Patients (%) | Patients (%) | p-Value | |
| SURGERY | ||||
| Duration of surgery | ||||
| <180 min | 117 (55.7) | 107 (58.5) | 10 (37.0) | |
| ≥180 min | 93 (44.3) | 76 (41.5) | 17 (63.0) | 0.04 |
| Side | ||||
| Right | 107 (50.7) | 89 (48.6) | 18 (64.3) | |
| Left | 104 (49.3) | 94 (51.4) | 10 (35.7) | 0.16 |
| Histology | ||||
| Adenocarcinoma | 96 (45.5) | 84 (45.9) | 12 (42.9) | |
| Squamous or adenosquamous | 88 (41.7) | 72 (39.3) | 16 (57.1) | |
| Other | 27 (12.8) | 27 (14.8) | 0 (0.0) | 0.03 |
| Charlson Comorbidity Index (CCI) | ||||
| 1–3 | 78 (37.0) | 74 (40.4) | 4 (14.3) | |
| 4 | 69 (32.7) | 60 (32.8) | 9 (32.1) | |
| 5–13 | 64 (30.3) | 49 (26.8) | 15 (53.6) | 0.002 |
| ASA | ||||
| 1–2 | 171 (81.0) | 155 (84.7) | 16 (57.1) | |
| 3–5 | 40 (19.0) | 28 (15.3) | 12 (42.9) | 0.002 |
| ANESTH/VENTILATION | ||||
| PEEP | ||||
| Median (range) | 5 (0-12) | 5 (0-12) | 5 (3-10) | 0.82 |
| Postop NIV | ||||
| No | 182 (86.3) | 178 (97.3) | 4 (14.3) | |
| Yes | 29 (13.7) | 5 (2.7) | 24 (85.7) | <0.0001 |
| Intraop ECMO | ||||
| No | 203 (96.2) | 178 (97.3) | 25 (89.3) | |
| Yes | 8 (3.8) | 5 (2.7) | 3 (10.7) | 0.07 |
| MVE | ||||
| Median (range) | 8 (0.4–15) | 8 (3–15) | 8 (0.4–10) | 0.95 |
| Fluid balance (missing for 23) | ||||
| Median (range) | 1100 (0–7700) | 1036 (0–7700) | 1468 (149–4900) | 0.03 |
| ≤500 | 28 (14.9) | 26 (15.9) | 2 (8.3) | |
| >500–1000 | 54 (28.7) | 49 (29.9) | 5 (20.8) | |
| >1000–1500 | 55 (29.3) | 49 (29.9) | 6 (25.0) | |
| >1500 | 51 (27.1) | 40 (24.4) | 11 (45.8) | 0.04 |
| Corticosteroids (missing for 6) | ||||
| No | 205 (97.2) | 178 (97.3) | 27 (96.4) | |
| Yes | 6 (2.8) | 5 (2.7) | 1 (3.6) | 0.34 |
| Blood transfusion (PRE) | ||||
| No | 187 (89.0) | 161 (88.5) | 26 (92.9) | |
| Yes | 23 (11.0) | 21 (11.5) | 2 (7.1) | 0.75 |
| Blood transfusion (POST) | ||||
| No | 162 (77.1) | 143 (78.6) | 19 (67.9) | |
| Yes | 48 (22.9) | 39 (21.4) | 9 (32.1) | 0.23 |
| HOSPITALIZATION | ||||
| ICU | ||||
| Median (range) | 1 (0–89) | 1 (0–66) | 1 (0–89) | 0.0001 |
| Hospital stay | ||||
| Median (range) | 8 (0–180) | 8 (0–180) | 15 (7–180) | <0.0001 |
| 0–7 days | 83 (40.9) | 80 (44.2) | 3 (13.6) | |
| 7–14 days | 87 (42.9) | 80 (44.2) | 7 (31.8) | |
| >14 days | 33 (16.3) | 21 (11.6) | 12 (54.5) | <0.0001 |
| Patient Characteristics | Univariate | Multivariable Model 1 | Multivariable Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| ASA score | ||||||
| 1–2 | 1.00 | 1.00 | 1.00 | |||
| 3–5 | 4.15 (1.78–9.71) | 0.001 | 2.91 (1.08–7.80) | 0.03 | 2.49 (0.88–7.11) | 0.08 |
| DLCO < 75% predicted | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 5.73 (1.91–17.2) | 0.002 | 5.62 (1.72–18.4) | 0.004 | 6.57 (1.84–23.4) | 0.004 |
| C-reactive protein | ||||||
| Normal (≤5.0) | 1.00 | 1.00 | 1.00 | |||
| High (>5.0) | 3.41 (1.48–7.88) | 0.004 | 3.55 (1.32–9.54) | 0.01 | 3.85 (1.36–10.9) | 0.01 |
| Perfusion | ||||||
| <40% | 1.00 | 1.00 | 1.00 | |||
| ≥40% | 4.13 (1.70–10.1) | 0.002 | 5.77 (2.13–15.6) | 0.0006 | 8.32 (2.70–25.7) | 0.0002 |
| Intraoperative fluids | ||||||
| per 500 mL increase | 1.22 (1.01–1.46) | 0.04 | 1.53 (1.11–2.11) | 0.009 | ||
| Number of Risk Factors * | Patients N (%) | ARDS N (%) | ICU | Hospital Stay |
|---|---|---|---|---|
| 0–1 risk factors | 126 (59.7) | 3 (2.4) | 1.2 ± 5.9; 1 [0–66] | 10.5 ± 16.3; 8 [8–180] |
| 2–3 risk factors | 80 (37.9) | 20 (25.0) | 2.4 ± 10.4; 1 [0–89] | 15.2 ± 21.9; 9 [5–180] |
| 4 risk factors | 5 (2.4) | 5 (100) | 4.2 ± 6.1; 2 [1–15] | 11.0 ± 4.6; 11 [7–15] |
| p-Value | <0.0001 † | 0.001 ‡ | <0.001 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzella, A.; Mohamed, S.; Maisonneuve, P.; Borri, A.; Casiraghi, M.; Bertolaccini, L.; Petrella, F.; Lo Iacono, G.; Spaggiari, L. ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer. Cancers 2022, 14, 6048. https://doi.org/10.3390/cancers14246048
Mazzella A, Mohamed S, Maisonneuve P, Borri A, Casiraghi M, Bertolaccini L, Petrella F, Lo Iacono G, Spaggiari L. ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer. Cancers. 2022; 14(24):6048. https://doi.org/10.3390/cancers14246048
Chicago/Turabian StyleMazzella, Antonio, Shehab Mohamed, Patrick Maisonneuve, Alessandro Borri, Monica Casiraghi, Luca Bertolaccini, Francesco Petrella, Giorgio Lo Iacono, and Lorenzo Spaggiari. 2022. "ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer" Cancers 14, no. 24: 6048. https://doi.org/10.3390/cancers14246048
APA StyleMazzella, A., Mohamed, S., Maisonneuve, P., Borri, A., Casiraghi, M., Bertolaccini, L., Petrella, F., Lo Iacono, G., & Spaggiari, L. (2022). ARDS after Pneumonectomy: How to Prevent It? Development of a Nomogram to Predict the Risk of ARDS after Pneumonectomy for Lung Cancer. Cancers, 14(24), 6048. https://doi.org/10.3390/cancers14246048