1. Introduction
Breast cancer is one of the most prevalent and common malignancies among women worldwide and continues to be the second-leading cause of death in women. According to Global Cancer Statistics 2020, 2.3 million women were diagnosed with breast cancer and 685,000 deaths were recorded globally in 2020 [
1]. It is estimated that 287,850 new breast cancer cases with 43,250 cancer deaths are expected to occur in 2022 in the United States alone [
2]. The etiology of breast cancer is complex and still unclear. However, advancement in breast cancer research for the past decade indicated the involvement of five important factors: aging, family history, reproductive factors, estrogen and lifestyle [
3]. Among the various subtypes of breast cancers, triple negative breast cancer (TNBC) is an aggressive subtype with poor prognosis compared to other breast cancer subtypes, accounting for 10−15% of all breast cancer cases. TNBC is heterogeneous in nature with a lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) expression [
4]. Current treatment options for breast cancer include surgery, chemotherapy, radiotherapy, endocrine therapy, and targeted therapy. However, there are limited treatment options for TNBC, as it is insensitive to chemotherapy, endocrine therapy, and targeted therapies, due mainly to a lack of specific receptors [
5]. Drug resistance development over time with standard chemotherapeutic agents and a high relapse rate with such treatments also mitigate progress. To address these issues, the development of novel agents for use in monotherapies, along with combination therapies involving standard chemotherapy drugs and new agents, are also being widely investigated [
6,
7].
Haloperidol, an antipsychotic drug used for the treatment of schizophrenia, psychosis, and bipolar disorders, was reported to show anticancer properties [
8,
9,
10,
11,
12]. Haloperidol is also known to exhibit non-selective interaction with sigma (σ) receptors (σ1 and σ2) [
13] and, as such, it has been speculated that it acts through sigma receptors (σRs). In our search for novel sigma-2 receptor ligands (σ2RLs), we have identified two σ2R selective ligands, SYA013 and SYA014, which are structural modifications of haloperidol. SYA013 is a homopiperazine analog in which piperidin-4-ol of haloperidol is replaced, while SYA014 is an oxime derivative of SYA013 (
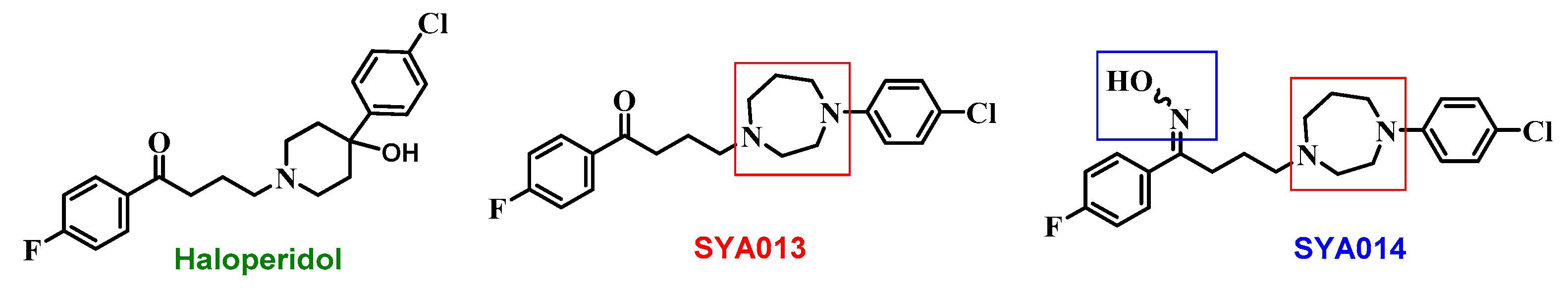
Figure 1). Similar to haloperidol, SYA013 also binds to σRs. However, unlike haloperidol, SYA013 and SYA014 exhibit a 4.3 and 1.5 fold preferential binding affinity towards σ2Rs over σ1Rs, respectively [
14]. Our earlier studies revealed the cytotoxic effects of the compounds SYA013 and SYA014 in solid tumor cell lines such as MDA-MB-468, MDA-MB-231 (TNBC), A549 cells (human alveolar basal epithelial adenocarcinoma), PC-3 (prostate cancer), and MIA PaCa-2 and Panc-1 (pancreatic cancer). These compounds were also evaluated for their effects on the viability of MCF-10A cells (non-tumorigenic breast epithelial cells) in order to evaluate their selective cytotoxicities to proliferative cells [
14]. In addition, we have recently reported on the anticancer effects and mechanisms of the action of SYA013 against TNBC cell lines (MDA-MB-231 and MDA-MB-468) [
15].
As a continuation of our previous findings, we now explore the anticancer properties of SYA014 against the two TNBC cell lines, MDA-MB-231 and MDA-MB-468, and report its effects on the proliferation, migration, and invasion of TNBC cells. We also attempted to decipher the mechanism/pathways involved in the antiproliferative properties of SYA014 and the role of the σ2R on the antiproliferative effects of SYA014 by blocking the σ2 receptor with RHM-1 (a non-cytotoxic selective σ2R antagonist) [
16]. In addition, we evaluated the combination effect of SYA014 with cisplatin for a possible synergistic anticancer effect on these TNBC (MDA-MB-231 and MDA-MB-468) and MCF10A cell lines. Herein, we present the results of a mechanistic investigation into the anticancer properties of SYA014 on TNBC cell lines, MDA-MB-231 and MDA-MB-468 for possible use in monotherapy or in combination with a standard chemotherapeutic drug, cisplatin.
2. Materials and Methods
2.1. Materials
Human adenocarcinoma breast cancer cells (MDA-MB-231 cells, MDA-MB-468 cells, and MCF-10A) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture media (Dulbecco’s modified eagle medium, high glucose, GlutaMax DMEM), fetal bovine serum (FBS), and antibiotic mixture solution (penicillin−streptomycin-neomycin) were purchased from Life Technologies (Grand Island, NY, USA). Phosphate buffered saline was purchased from Genesee Scientific (San Diego, CA, USA). µ-Slide 8-well (glass bottom) and cell culture inserts were purchased from ibidi (Madison, WI, USA). Caspase assay kits (Caspase Glo 3/7, Caspase Glo 8, Caspase Glo 9) were purchased from Promega Corporation (Madison, WI, USA). ApopNexin Annexin V FITC Apoptosis Kit, cell cycle analysis kit and bioreagents (ethidium bromide and acridine orange) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Apoptosis antibody sampler kit, proapoptosis Bcl-2 family antibody sampler kit, and cytochrome c antibody kit were purchased from Cell Signaling Technology (Danvers, MA, USA). Mini-PROTEAN TGX precast protein gel, Precision Plus protein dual color standards, Immun-Blot PVDF membrane, Laemmli premixed protein sample buffer for SDS-PAGE, tris buffered saline, 10× tris/glycine premixed electrophoresis buffer, blotting-grade blocker nonfat dry milk for western blotting, and clarity western ECL substrate were purchased from Bio-Rad (Hercules, CA, USA). Siramesine and PB28 were purchased from Millipore Sigma (St. Louis, MO, USA). SYA014 was synthesized and characterized in our lab as reported previously [
14]. RHM-1 was synthesized as per the reported procedure elsewhere [
16].
2.2. Cell Culture
MDA-MB-231 and MDA-MB-468 were cultured in Dulbecco’s modified eagle medium high glucose, GlutaMax (DMEM). The media were supplemented with 10% fetal bovine serum (FBS) and PSN (100 U/mL penicillin, 50 µg/mL neomycin). MCF-10 cells were maintained in DMEM/F-12, supplemented with insulin (10 µg/mL), epidermal growth factor (EGF, 20 ng/mL), cholera toxin (100 ng/mL), hydrocortisone (0.5 µg/mL), horse serum (5%), and penicillin/streptomycin (100 U/mL/100 µg/mL). The cells were incubated in an incubator with 5% carbon dioxide (CO2)/95% humidified air at 37 °C. The cells were sub-cultured upon ~80–90% confluency. All the assays were performed with experimental media containing 5% FBS, unless stated otherwise. The σ2RL SYA014 was dissolved in dimethyl sulfoxide (DMSO) and acetone at a 1:9 ratio. Cisplatin was dissolved in 0.9% sodium chloride (normal saline). Stocks of siramesine and PB28 were prepared in dimethyl sulfoxide (DMSO).
2.3. Cell Viability and Proliferation Assays
The cells suspended in 100 μL media were seeded at a density of 2 × 10
4/well in 96 well plates and allowed to attach over 24 h in media containing 5% FBS. Cells were treated with SYA014 (0–200 μM) in 1 μL solvent. MDA-MB-231 cells were also treated with cisplatin (0–200 μM). The control cells were treated with the equivalent volume of the respective solvent (1:9 ratio DMSO/acetone for σ2RLs, and normal saline for cisplatin). MDA-MB-231 cells were treated for 24, 48, and 72 h. All other cells (HEK 293, MCF-10, and MDA-MB-468) were treated for 48 h. To determine the effect of viability of SYA014 on spheroids (3D) culture, MDA-MB-231 cells were seeded in 96-Well, U-shaped-bottom Nunclon Sphera plates (Thermo-Scientific, Waltham, MA) at 2.5 × 10
4 cells per well in media supplemented with (5% FBS). The cells were incubated at 37 °C/5% CO
2 and spheroids were formed after 24 h. The cells were then exposed to SYA014 (0–200 μM) and the treatment was repeated after 24 h for a total exposure of 48 h. Resazurin reagent (20 μL of 0.05%) was then added to each well and incubated for 1–2 h, after which the cell viability was determined as previously described [
14,
17]. The fluorescence was measured at 560 nm excitation wavelength and a detection wavelength of 590 nm using the FLx 800 Microplate Fluorescence Reader from BioTek (Winooski, VT. USA). To determine the effect of SYA014 on MDA-MB-231 and MDA-MB-468 cell proliferation, cells were plated at 20,000/well in 24-well plates for 24 h and treated with SYA014 (0–5 μM) for 48 h. Viable cells in triplicate wells were counted using the Countess II Automated Cell Counter (Life Technology Corporation (Grand Island, NY, USA)). The significance of the effect was established using GraphPad Prism version 5.0.
2.4. Colony Formation Assay
MDA-MB-231 and MDA-MB-468 cells were seeded at a density of 1.5 × 10
5/mL in T-25 flasks and incubated overnight. The next day, cells were treated with SYA014 (0–20 μM) for 48 h. After the completion of incubation, cells were trypsinized, counted and re-plated in 6-well plates at a density of 500–1000 cells per well. Cells were maintained in complete growth media for 12 days. After the completion of the incubation period, cells were fixed with a solution of acetic acid in methanol (1:7) and stained with 1% crystal violet solution prepared in methanol. Colonies of MDA-MB-231 and MDA-MB-468 cells consisting of 50 or more cells were counted using the NIH ImageJ [
18],
http://rsb.info.nih.gov/ij/) accessed on 16 May 2018. The plating efficiency (PE) was determined by dividing the number of colonies formed by the number of cells seeded under untreated conditions × 100. The surviving fraction (SF) was calculated by dividing the PE of treated samples by the PE of control × 100 [
19].
2.5. Spheroid Morphology Experiments
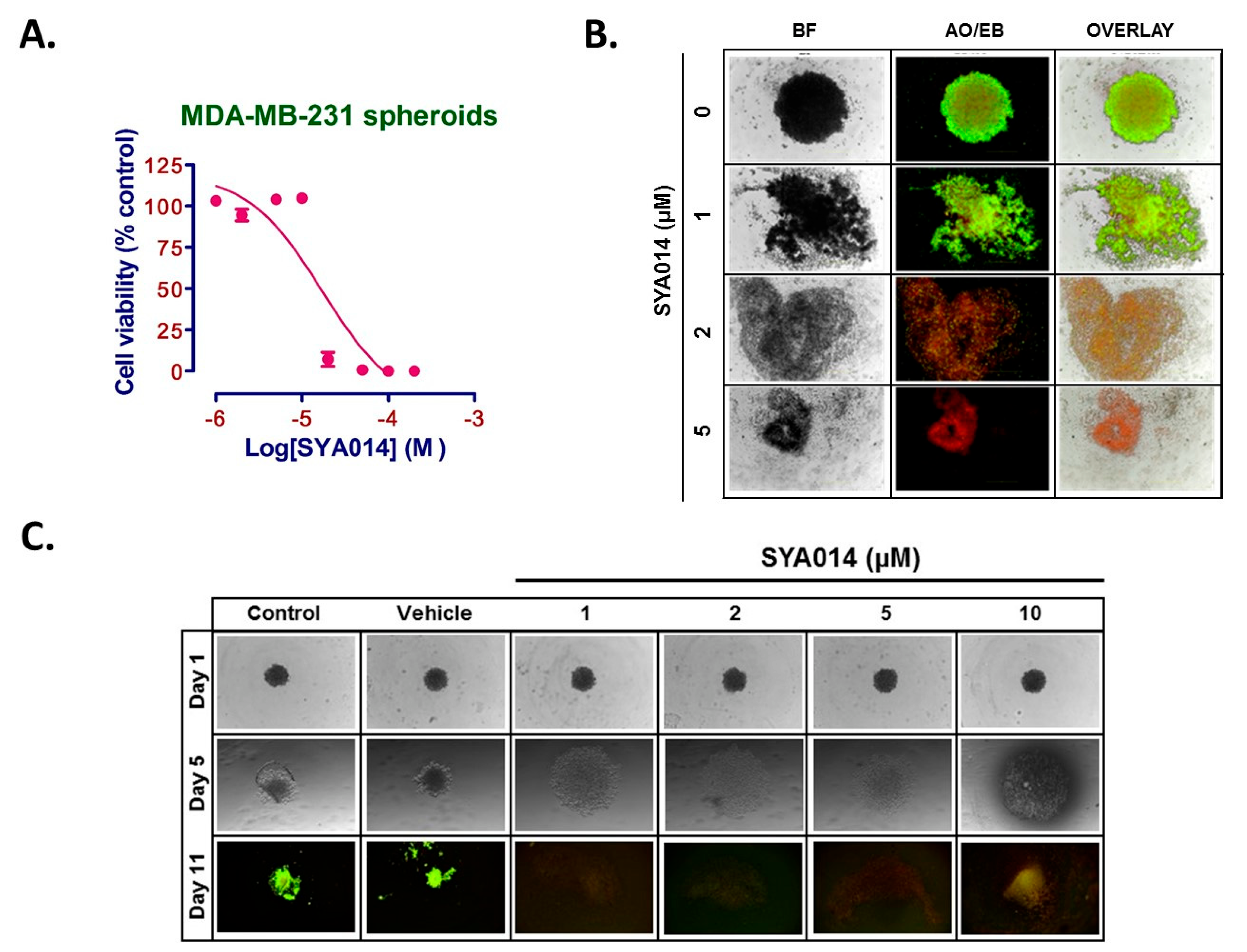
To evaluate the effect of SYA014 on MDA-MB-231 pre-formed spheroids, MDA-MB-231 cells were seeded at 5 × 103 cells/well in a 96-Well, U-shaped-bottom Nunclon Sphera plate and incubated at 37 °C/5% CO2. Cells were incubated for 3 days for the formation of spheroids and then the pre-formed spheroids were treated with SYA014 (0–10 μM) for 48 h. Next, staining of the spheroids was performed with acridine orange/ethidium bromide (AO/EB) solution (5 μg/mL). Stained spheroids were subjected to fluorescence microscopy using Nikon Ti Eclipse Microscope and the images were captured at 4x magnification.
MDA-MB-231 cells were plated in 96-Well, U-shaped-bottom Nunclon Sphera plate at the density of 2 × 104 cells/mL. Cells were then treated with varying concentrations of SYA014 (0–10 μM) and incubated at 37 °C/5% CO2 for a period of 24 h. The drug treatment was repeated after 24 h and the cells incubated for another 24 h. The effect of SYA014 on the inhibition of the formation of spheroids was evaluated after a total of 48 h exposure to SYA014. The size and morphology of the spheroids was continuously monitored for 11 days. Brightfield images were captured using a Nikon Ti Eclipse Microscope to monitor the progress of the inhibitory effect. A total of 50% of the media in each well was replaced with fresh media, and bright field images were obtained every day until 11th day. On the 11th day, the spheroids were stained with acridine orange/ethidium bromide (AO/EB) solution (5 μg/mL). Stained spheroids were examined by fluorescence microscopy using a Nikon Ti Eclipse Microscope, with the images captured at 4x magnification.
2.6. Cell Migration Assay
The potential of SYA014 to inhibit the migration and invasion of MDA-MB-231 cells was determined using the wound-healing/scratch technique. Ibidi culture inserts in ibidi µ-slide 8-well glass bottom chamber slides were used to generate scratch/wound with two confluent monolayers of cells separated by the cell-free zone. MDA-MB-231 cells were seeded at a density of 5 × 10
4 cells in ibidi cell culture inserts, incubated to confluence, and serum-starved for 24 h. Cells were treated with SYA014 (0–10 μM) for 24 h and wound closure was monitored using a Nikon Eclipse Ti 100 inverted microscope (Nikon Instruments Inc., Melville, NY, USA). Wound closure images were captured at 0, 6, 12, and 24 h post-treatment and the images were analyzed as previously described [
20]. The effect of SYA014 on the migration of MDA-MB-231 cells was calculated by counting the number of migrated cells to the cell-free area. Data were analyzed using GraphPad Prism version 5.0.
2.7. Cell Invasion Assay
The effect of SYA014 on cell invasion (2D) was determined using Matrigel-coated 24-well plates with 8 µm pore inserts. The 24-well plate with Matrigel invasion inserts (Corning, Bedford, MA) was rehydrated with serum-free DMEM with glutamate for 2 h at 37 °C/5% CO
2. MDA-MB-231 cells (2 × 10
5 cells/mL) suspended in 500 μL media containing 0.1% FBS SYA014 (0–10 μM) were added in the upper chamber of the inserts while 750 μL media with 10% FBS was placed into the lower chambers as a chemo-attractant. The plates were then incubated at 37 °C/5% CO
2 for 22 h to allow the cells to invade from the upper chamber to the lower chamber of inserts through the Matrigel. The invading cells were rinsed with phosphate buffered saline (PBS), fixed with 4% formaldehyde in PBS, permeabilized with 100% methanol, and stained with 2% crystal violet for 1 h. The Olympus IX70 microscope was used to image the invading cells using the 4x objective. Invaded cells were counted using Imagej [
18],
http://rsb.info.nih.gov/ij/ accessed on 16 May 2018, and quantified using GraphPad Prism version 5.0.
The ability of SYA014 to inhibit invasion of cells from a formed spheroid in 3D format was determined using a protocol for this assay that was adapted as described by Vinci et al. [
21]. Briefly, MDA-MB-231 cells were plated in 96-Well, U-shaped-bottom Nunclon Sphera plate (Thermo-Scientific, Waltham, MA, USA) at a density of 5 × 10
4 cells/mL in complete growth media. Following the formation of compact spheroids, 850 μL of BD Matrigel (Corning, Bedford, MA, USA) was pipetted into the 10 pre-chilled micro-centrifuge tubes. SYA014 (3.4 μL–34 μL) were added to each tube to yield a final concentration of 0–20 μM of SYA014 in the mixture. The mixture was mixed gently and 100 μL of growth media from each well containing the spheroid was carefully replaced with 100 μL of Matrigel/SYA014 mixture. The plate was incubated for 1 h to embed the spheroids while solidifying the Matrigel, after which an additional 100 μL of Matrigel containing SYA014 was added to the corresponding wells (0–20 μM). The bright field images of Matrigel-embedded spheroids were taken at 0 h and after 72 h using a Nikon Ti Eclipse Microscope at 4x magnification. The area of invasion was quantified using NIS Element software, and the data analyzed using GraphPad Prism version 5.0.
2.8. Apoptosis Assays
The effect of SYA014 on the induction of cell death was determined using three different assays. We employed the modified ethidium bromide/acridine orange (EB/AO) staining method [
22] and the Annexin V/propidium iodide staining method followed by flow cytometry analysis and caspase activation analysis using Caspase-Glo
® assay system.
2.8.1. Ethidium Bromide/Acridine Orange (EB/AO) Staining
MDA-MB-231 and MDA-MB-468 cells were seeded at a density of 5 × 104 cells/mL into a 96-well plate and incubated overnight at 37 °C in 5% CO2/95% humidified air. The next day, cells were treated with SYA014 (0.1–20 μM) and incubated. After 24 h of incubation with SYA014, the drug treatment was repeated and incubated for another 24 h. After 48 h of the first treatment, 10 μL of EB/AO solution (EB and AO solutions were prepared at 100 μg/mL for each separately in DPBS, and 1 part of EB and 1 part of AO from each of the 100 μg/mL solutions were mixed to prepare the EB/AO solution) was added directly into each well containing media and incubated for 10 min in the dark. The stained cells were observed under a Nikon Eclipse Ti 100 inverted fluorescent microscope (Nikon Instruments, Inc., Melville, NY, USA). Changes in the morphology of the nuclei were observed and images were captured for the control and treated cells.
2.8.2. Annexin V/Propidium Iodide (AV/PI) Staining and Flow Cytometry Analysis
Next, we performed Annexin V/Propidium iodide (AV/PI) staining and flow cytometry analysis to determine the cell death mechanism induced by SYA014. Annexin V is a phospholipid-binding protein with a high affinity for phosphatidylserines, which are normally located in the inner part of the cell membrane but translocate to the surface during early apoptosis due to loss of membrane integrity. The propidium iodide, on the other hand, can enter the cells only when the cell membrane is compromised as seen in late apoptosis as well as in necrosis. MDA-MB-231 and MDA-MB-468 cells (5 × 105 cells/well) suspended in media supplemented with 5% FBS were plated in 6-well culture plates (Costar Corning, NY, USA) and incubated at 37 °C in 5% CO2/95% humidified air and allowed to attach overnight. The next day, the cells were treated with SYA014 (0–10 μM) continuously for 48 h. The mode of cell death was determined as per the manufacturer’s protocol using the ApopNexin fluorescein isothiocyanate (FITC) apoptosis detection kit (EMD Millipore, Temecula, CA, USA). Briefly, after the completion of incubation, the cells were washed with DPBS and harvested with accutase. The cells were pelleted using a centrifuge at 500× g for 5 min. The cell pellets were washed with 1X DPBS (2 × 5 mL) on ice and re-suspended in 1X binding buffer. Annexin V FITC and PI were added to the cell suspensions and incubated for 10 min at room temperature in the dark. Flow cytometry analysis was performed using the FACS Calibur with Cell Quest Pro software (BD Biosciences, Franklin Lakes, New Jersey, USA).
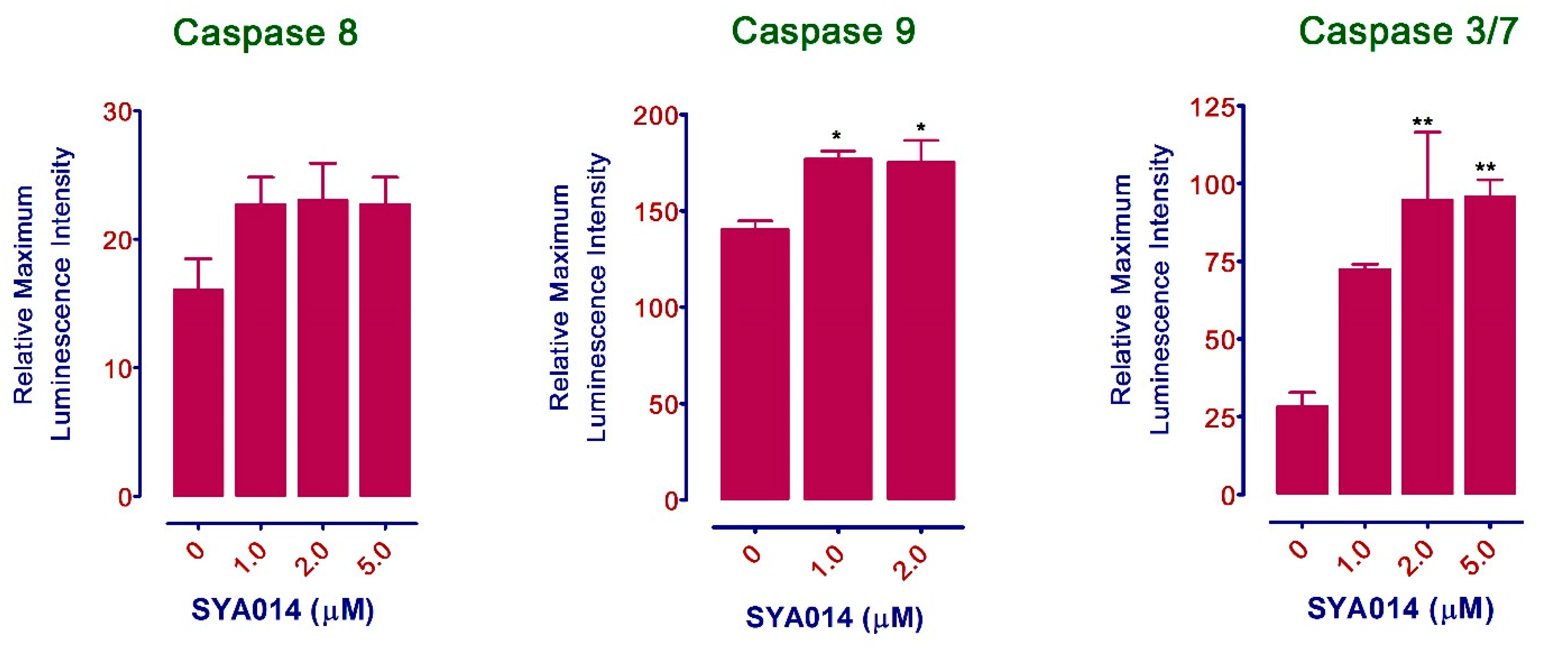
2.8.3. Caspase Activation Assay
The effect of SYA014 on the activation of Caspases in MDA-MB-231 cells was evaluated using the Caspase-Glo 3/7 and caspase-Glo 8 and 9 Assay Kits (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, MDA-MB-231 cells were plated in white-walled 96-well plates at a density of 1.5 × 104 cells/well (for caspase 3/7) and 3 × 104 cells/well (for caspase 8 and 9) in 100 μL media with 5% FBS. The cells were incubated at 37 °C in 5% CO2/95% humidified air overnight. The next day, cells were treated with SYA014 (0–5 μM) continuously for 48 h. After the completion of the incubation, the multiplexing reagent (prepared by mixing Caspase-Glo® Buffer and the lyophilized substrate) was added to each well at 100 μL/well while protecting it from light. The cells were incubated at room temperature for 1 h. The luminescence readings were measured using a luminometer. The relative maximum luminescence intensity was quantified using the GraphPad Prism version 5.0.
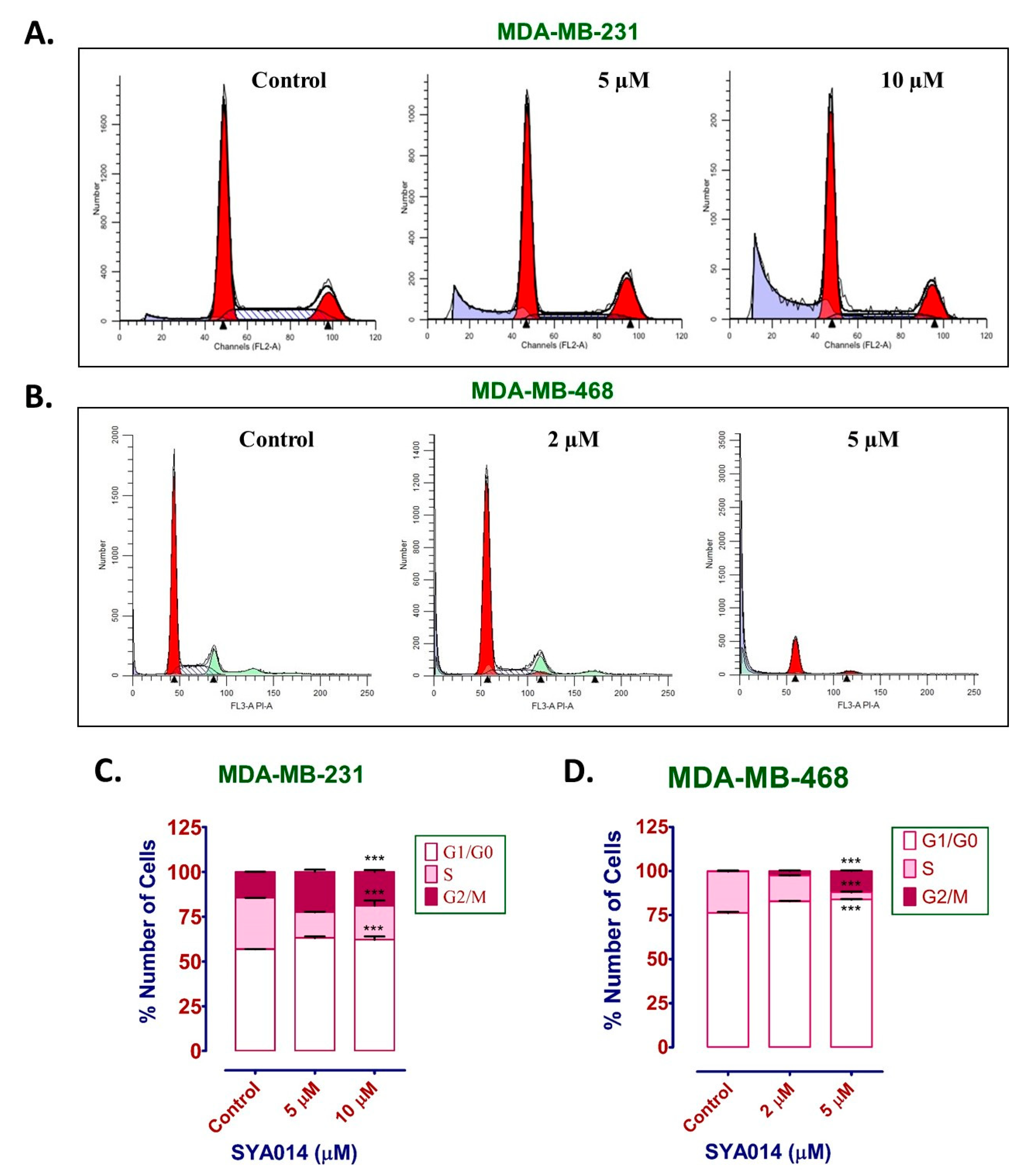
2.9. Cell Cycle Analysis
The effect of SYA014 on the cell cycle was determined in MDA-MB-231 cells using the propidium iodide staining protocol. Briefly, MDA-MB-231 and MDA-MB-468 cells were plated at a density of 2 × 10
5 cells per well in 6-well plates in media supplemented with 10% FBS. Cells were incubated at 37 °C in 5% CO
2/95% humidified air overnight for attachment. The next day, media was replaced with fresh media containing 5% FBS and then cells were treated with SYA014 (0–10 μM) and incubated for 24 h. SYA014 treatment was repeated after 24 h and incubated for another 24 h. After 48 h of incubation from the first treatment, cells were harvested by scraping, washed with PBS, and centrifuged at 300×
g for 5 min. The cell pellets were re-suspended in 100 μL PBS and passed through a 28 gauge 5/8 inch needle to keep it as a single cell suspension. Cells were fixed with the dropwise addition of cold 70% ethanol while vortexing, and cells were stored at −20 °C overnight. The next day, cells were washed with PBS (2 times), and centrifuged at 500×
g for 5 min. Modified Vindelov’s reagent (ribonuclease A and propidium iodide in PBS) [
23] was added to the cells and the phase distribution of cells was determined using a Becton Dickinson FACSort flow cytometer with CellQuest software (Mansfield, MA, USA). The percentage of cells in each phase was determined in the gated population of singlet cells and plotted using the GraphPad Prism version 5.0.
2.10. Western Blot Analysis
The effect of SYA014 on the expression of other apoptotic, anti-apoptotic (survival), death receptor, cell cycle, and angiogenesis proteins in MDA-MB-231 cells after treatment was evaluated by western blot electrophoresis. Cells were plated in 6-well tissue culture plates at the density of 2 × 105 cells/well in media supplemented with 10% FBS and allowed to adhere to the plates overnight at 37 °C in 5% CO2/95% humidified air. Prior to treatment, the complete media was replaced with 5% supplemented media and treated with SYA014 (0–10 μM). The treatment was repeated after 24 h for a total time of exposure of 48 h. Briefly, the cells were washed with PBS, and lysed with radioimmunoprecipitation assay (RIPA) buffer supplemented with 1X protease inhibitors cocktail (Sigma, St. Louis, MO, USA). The protein concentration in the lysates was evaluated using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Lysates containing 50 μg of protein were mixed with Laemmli sample buffer, and placed in a boiling water bath for 5 min. Aliquots of each sample were resolved using a 10–20% gradient on an SDS-PAGE gel. Resolved proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% fat-free milk (Sigma, St. Louis, MO, USA) for 1 h at room temperature, then immunoblotted using primary antibodies against different protein groups including angiogenesis, death receptors, apoptosis, and cell cycle purchased from Cell Signaling Technology (Danvers, MA, USA) and incubated overnight at 4 °C. The next day, membranes were incubated with horseradish peroxidase-linked anti-rabbit IgG secondary antibodies from Santa-Cruz Biotechnology (Santa Cruz, CA, USA) for 90 min. Enhanced chemiluminescent (ECL) substrate was used for detection of horseradish peroxidase (HRP) activity from antibodies, and the chemiluminescent images were obtained using a ChemiDoc imaging system (Bio-Rad, Hercules, CA, USA).
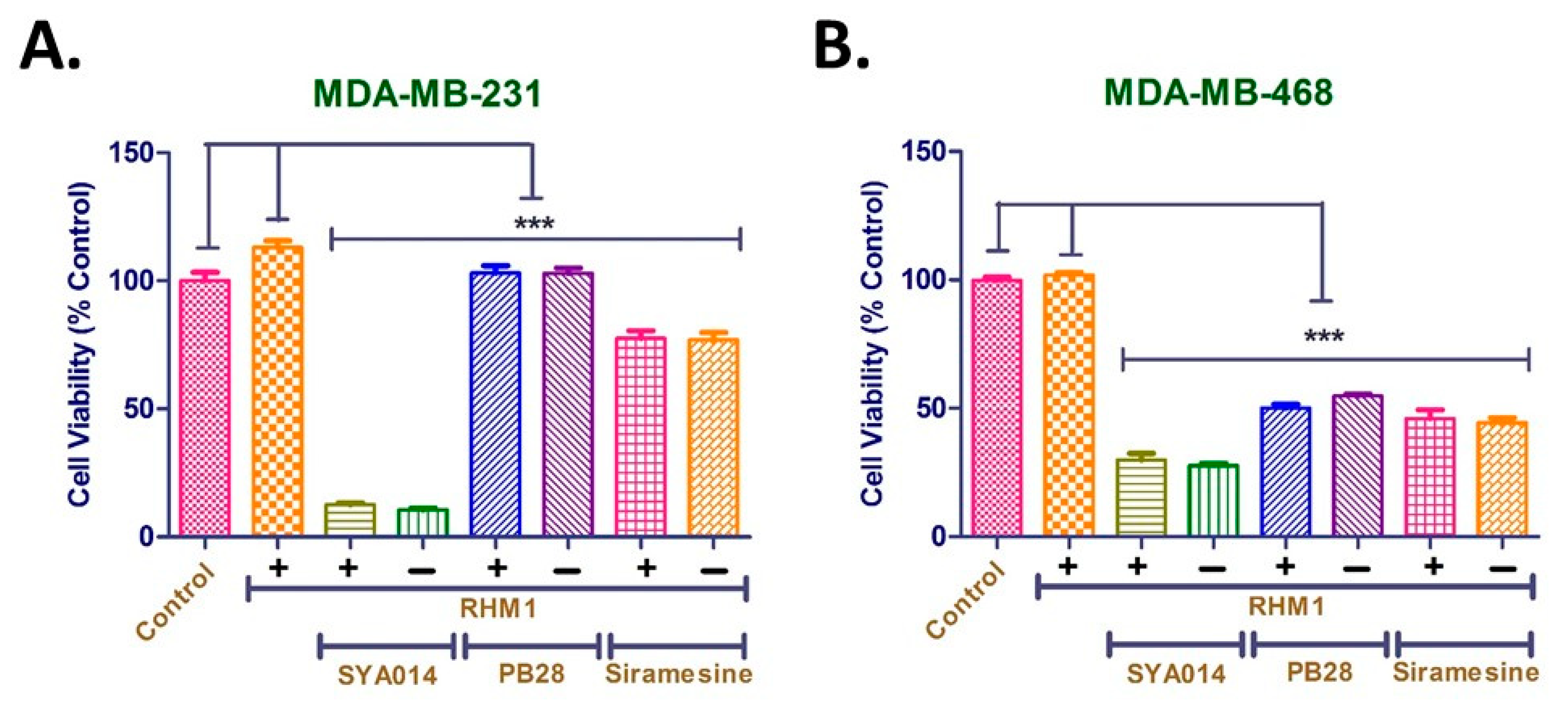
2.11. σ2. R Blockade Experiment with RHM-1
σ2R Blockade was performed according to the method reported previously with slight modifications [
24]. The cells were seeded at a density of 1 × 10
4 cells/well in 96 well plates and allowed to attach over 24 h in complete media. Cells were initially treated with RHM-1 at 10 µM, followed by SYA014 at 25 µM, PB28 at 25 µM and siramesine at 10 µM, either alone or with RHM-1 for 24 h. The control cells were treated with an equivalent volume of the respective solvent. Resazurin reagent (20 µL of 0.05%) was then added to each well and incubated for 1–2 h. The fluorescence was measured at a 560 nm excitation wavelength and a detection wavelength of 590 nm using the FLx 800 microplate fluorescence reader from BioTek (Winooski, VT, USA) to determine the cell viability. Results were plotted using GraphPad Prism version 5.0 software (San Diego, CA. USA). The significance of effect was established using GraphPad Prism version 5.0.
2.12. Cell Viability Study for the Combination Effect of SYA014 and Cisplatin
Cell viability studies were performed to evaluate the cytotoxicity of cisplatin in MDA-MB-231, MDA-MB-468, and MCF10A cells, and also the combination effect of SYA014 with cisplatin in the above-mentioned cell lines. The cells were seeded at a density of 5000 cells/well in 96 well plates and allowed to attach over 24 h in complete media. For the 72 h cytotoxicity studies of cisplatin (0.1–50 µM), 5% FBS containing media was used for MDA-MB-231 and MDA-MB-468 cells, while assay media was used for MCF10A cells. For the combination studies, cells were initially treated with cisplatin at 0.5 µM followed by SYA014 (2–20 µM) for 72 h in the same media as the corresponding cytotoxicity studies. The control cells received equivalent volume of the respective solvent (1:9 ratio DMSO/acetone for SYA014, and normal saline for cisplatin). Cell viability was measured as in
Section 2.3. Results were plotted using GraphPad Prism version 5.0 software (San Diego, CA, USA). The significance of effect was established using GraphPad Prism version 5.0.
2.13. Statistical Analysis
The statistical analysis of data was performed using GraphPad Prism version 5.0 for Windows (San Diego, CA, USA). Data results were expressed as the mean ± standard error of the mean (SEM). Data were also analyzed using one-way analysis of variance (ANOVA). Statistical differences between control and treated groups were determined by Dunnett’s post-test comparisons or Tukey’s multiple comparison test. Significance was defined as * p < 0.05; ** p < 0.01; and *** p < 0.001.
4. Discussion
Triple negative breast cancer (TNBC) is a type of breast cancer which lacks all three standard molecular markers, i.e., estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor type 2 (HER2) expression. TNBC is known to have a higher recurrence rate than other breast cancer subtypes. Due to the lack of these specific receptors, there are limited treatment options for TNBC. Targeted therapies for other subtypes of breast cancer are highly effective with improved survival rate; however, the same is not true for TNBC. Chemotherapy is the only treatment option for TNBC with several limitations such as high relapse rate and toxicity to normal cells. Therefore, there is a dire need for the development of novel therapeutic modalities with no or minimal side effects for the treatment of TNBC. In this regard, we have developed two compounds (SYA013 and SYA014) based on the haloperidol core structure which showed potential anticancer properties. In our previous work, we have reported on the cytotoxicity potentials of SYA013 and SYA014 against two TNBC model cell lines, MDA-MB-231 and MDA-MB-468. We have also demonstrated that these compounds show minimal cytotoxicity against MCF-10A cells, a nontumorigenic breast cell line. Based on those original findings, we further probed the anticancer properties of SYA013 in one of the representative TNBC cells, MDA-MB-231, and made an attempt to decipher the mechanism involved in the antiproliferative property of SYA013 [
15]. In a continuation of these studies, we have now evaluated the anticancer effects of SYA014, an oxime analog of SYA013, against TNBC cells (MDA-MB-231 and MDA-MB-468) and possible mechanisms involved in its apoptotic effects. Initial studies demonstrated that SYA014 inhibits cell proliferation and colony formation in MDA-MB-231 and MDA-MB-468 cells in a concentration-dependent manner, indicating the antiproliferative effects of SYA014 against TNBC cells. SYA014 also prevented MDA-MB-231 spheroid formation and induced disintegration of pre-formed spheroids, as revealed by the cytotoxicity assay and acridine orange/ethidium bromide (AO/EB) staining. Thus, cell proliferation, colony formation assays, and studies with the inhibition of spheroid formation and disintegration of pre-formed spheroids demonstrate the suppressive effects of SYA014. In addition, SYA014 significantly reduced the migration and inhibited the invasion of MDA-MB-231 cells, as shown by the cell migration assay, 2D single cell, and 3D spheroid invasion assays. Results from these studies indicate the anti-invasive effects of SYA014. The metastatic growth of TNBC to distant organs in the body poses a significant clinical challenge. Further, the aggressive and highly invasive nature of TNBC limits treatment options [
25]. Therefore, compounds such as SYA014, with antiproliferative and anti-invasive properties, may have potential for controlling cancer spread.
The fragmentation of nuclear DNA into oligonucleosomal size fragments is one of the key biochemical hallmarks of the late-stage apoptosis [
26]. We have also shown that SYA014 induces DNA fragmentation in MDA-MB-231 and MDA-MB-468 cells, as demonstrated by AO/EB staining results. The effect of SYA014 in triggering apoptosis in TNBC cells was confirmed by the propidium iodide/annexin V-FITC apoptosis assay followed by flow cytometric analyses. Cell cycle analysis showed that SYA014 arrests the cell cycle at the G0/G1 phase in both TNBC cell lines. To illustrate the apoptosis-inducing effect of SYA014, we evaluated the mode of cell death by measuring the activation of caspases, namely caspase 8, 9 and, 3/7. Activation of caspases is triggered by the intrinsic and extrinsic signaling cascade, inducing the fragmentation of genomic DNA, thus leading to apoptotic cell death [
27]. We observed a significant upregulation in caspase 3/7 (an apoptosis effector caspase) and a moderate increase in caspase 9 (an apoptosis initiator caspase). Caspase-9 is an essential initiator caspase for the intrinsic apoptotic pathway [
28]. The decreased levels of procaspase-9 and down-regulation of procaspase-3, an executor caspase, indicates the triggering of the intrinsic apoptotic mechanism in TNBC cells. PARP also functions as an important marker for apoptosis [
29]. The cleavage of PARP, a substrate of active caspase-3 from a 116 kDa band to a 89 kDa fragment, further supported the activation of caspase-3 [
30]. The intrinsic apoptotic mechanism was further confirmed by western blot analysis with upregulation of other pro-apoptotic proteins, Bax and, Bak expression. In addition, anti-apoptotic family proteins such as B-cell lymphoma 2 (Bcl-2), myeloid cell leukemia 1 (Mcl-1), and Bcl-2 like protein X (Bcl-XL) expression was decreased. The evaluation of death receptor proteins, DR5, TNF-R1 and RIP and cell cycle regulator proteins, p21, p27, Cyclin D1, Cdk4 and Cdk4 did not show any significant effects, suggesting that the apoptosis induced by SYA014 was not by extrinsic pathway. Thus, the present data indicate the possibility that SYA014 induced the intrinsic mechanism involved in the apoptotic pathway of TNBC cell death. Siramesine, a well-known σ2R, follows the intrinsic apoptotic pathway and induces cell death by oxidative stress and mitochondria destabilization [
31]. Another σ2R agonist and σ1R antagonist, PB28, induces caspase-independent apoptosis [
32]. Other σ2R ligands such as WC-26, SV119, and RHM-138, with cytotoxic effects in different cancer cells, induce cell death by multiple signaling pathways [
33]. Based on the results obtained in our previous study with SYA013 in MDA-MB-231 cells, we have outlined a possible apoptotic pathway as intrinsic in nature [
15] and SYA014 seems to follow the same mechanism of action in inducing apoptosis.
There are several σ2R binding ligands (agonists/antagonists), such as siramesine, PB28, SV119 and WC-26, which showed significant in vitro antiproliferative effects [
34,
35]. However, it is still not clear whether σ2R binding of these ligands has any role in imparting/enhancing their anticancer properties. Recently, an interesting study involved widely known σ1 and σ2 ligands being screened in a series of cell lines, and the results revealed that there is no correlation between σ2R expression and the binding of ligands to their receptors, and observed antiproliferative properties with a few exceptions [
36]. Our results with SYA014 in σ2R blockade are consistent with these earlier findings, where blocking of σ2R in MDA-MB-231 and MDA-MB-468 cells with the RHM-1 ligand had no significant impact on the cytotoxic effect of SYA014. Furthermore, siramesine and PB28 exhibited similar cytotoxicities, either alone or in the presence of the σ2R blocking ligand, RHM-1. Thus, it is evident from the results that the σ2R is unlikely to play a significant role in the anticancer effects of SYA014 in the representative TNBC cells.
Combination therapy is a therapeutic modality for the effective treatment of cancers. The use of two or more drugs certainly enhances the therapeutic efficacy compared to monotherapy, due to several factors such as targeting multiple signaling pathways in tumor cells, additive/synergistic effect and reducing a single drug overdose while addressing the multidrug resistance. Thus, the use of combination therapy offers multiple advantages over monotherapy. In particular, novel agents with different therapeutic targets provide an opportunity for developing new combination therapies. SYA014 exhibited significant antiproliferative effect in representative TNBC cell types and has the potential for exploration as a monotherapeutic agent. In addition, different doses of SYA014 (20, 10, 5 and 2 µM) in combination with cisplatin (0.5 µM) enhanced the cytotoxic effects in MDA-MB231 cells. Similar effects were observed in MDA-MB-468 cells with combination treatment of cisplatin with SYA014 at 10, 5 and 2 µM concentrations. Interestingly, co-treatment of cisplatin with SYA014 at 10, 5 and 2 µM concentrations in normal breast epithelial tissue cells (MCF10A) did not exhibit significantly increased cytotoxicity as compared to cisplatin treatment alone. Therefore, it is evident that the combination of cisplatin with SYA014 at 10, 5 and 2 µM concentrations did not increase toxicity to normal cells compared to cisplatin alone. Thus, SYA014 is effective in killing tumor cell types either alone or in combination with standard chemotherapeutic drugs.