A High Percentage of CD16+ Monocytes Correlates with the Extent of Bone Erosion in Chronic Lymphocytic Leukemia Patients: The Impact of Leukemic B Cells in Monocyte Differentiation and Osteoclast Maturation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
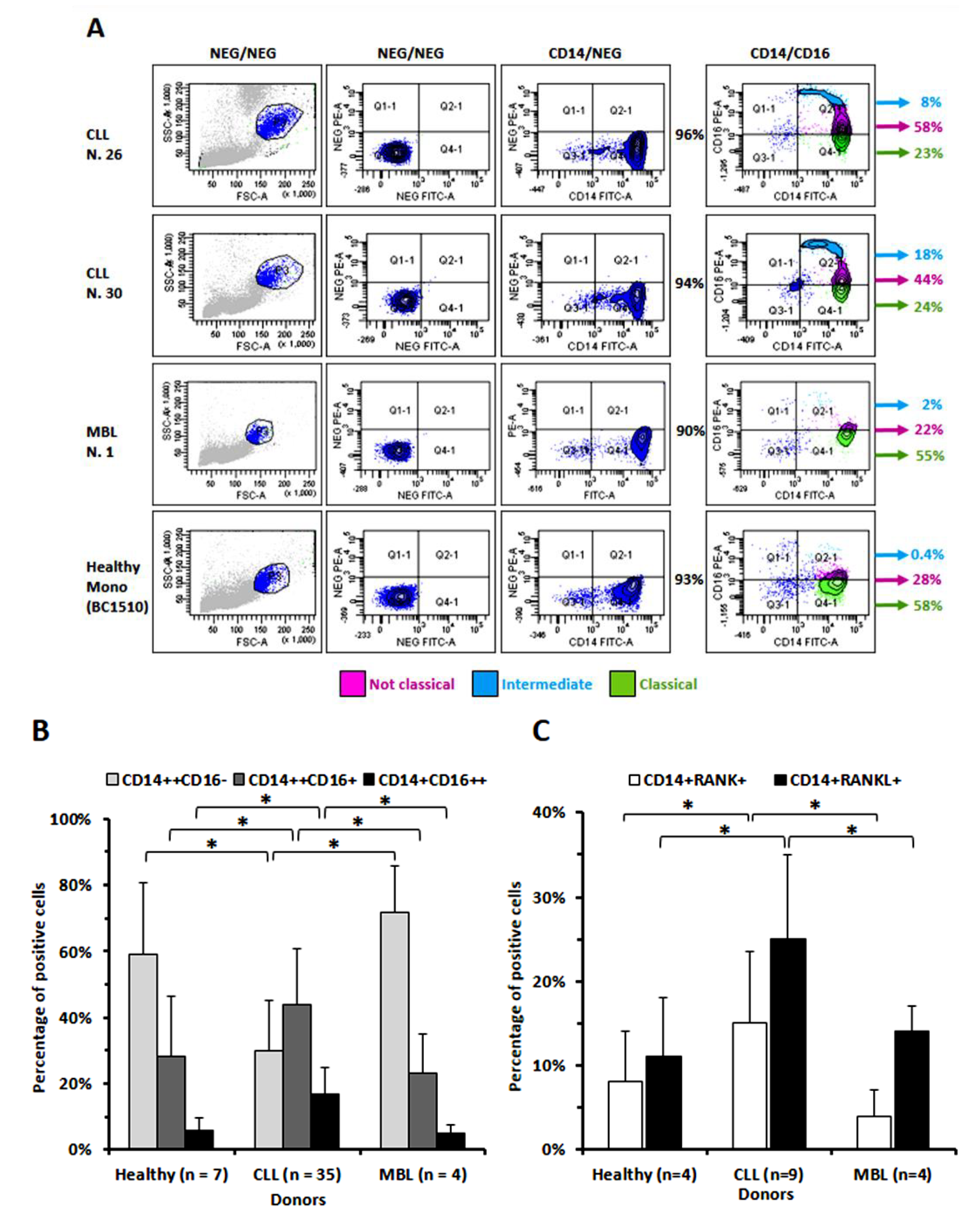
2.2. Determination of the Percentage of CD16-, RANK- and RANKL-Positive Monocytes from Peripheral Blood of CLL or of MBL Patients or of Healthy Donors
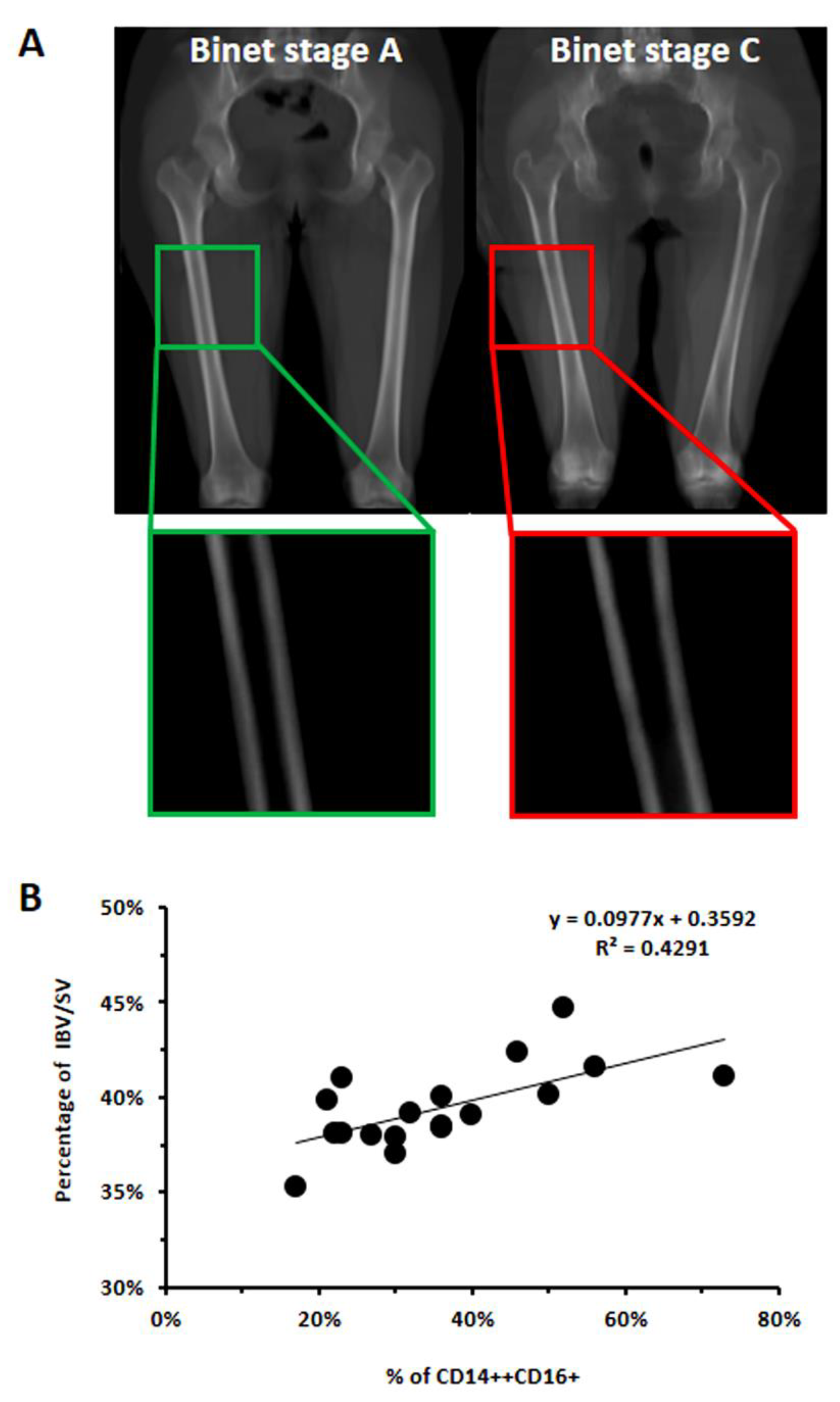
2.3. Evaluation of Trabecular and Compact Bone Volume (IBV and CBV) and Correlation with CD16 Expression
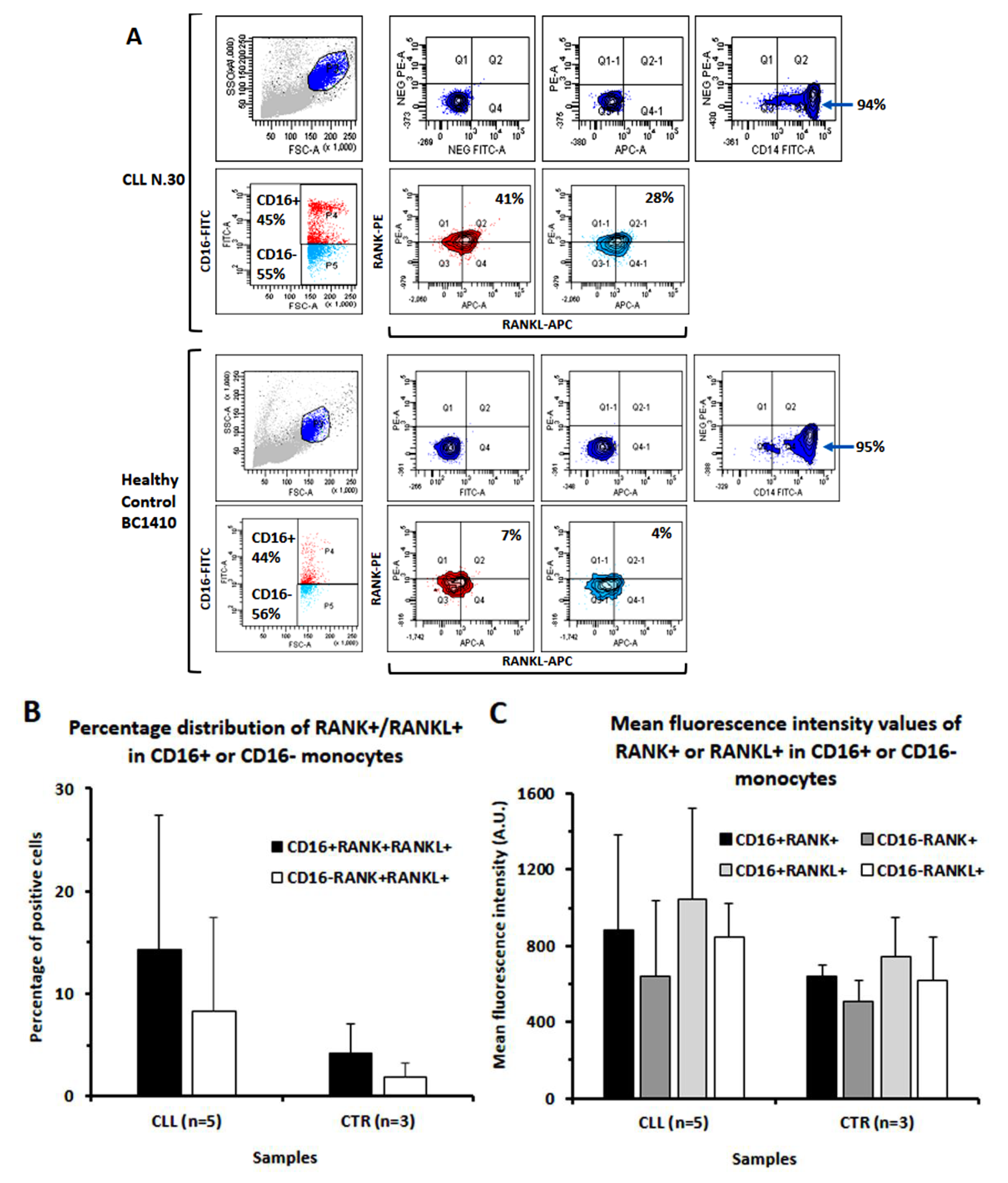
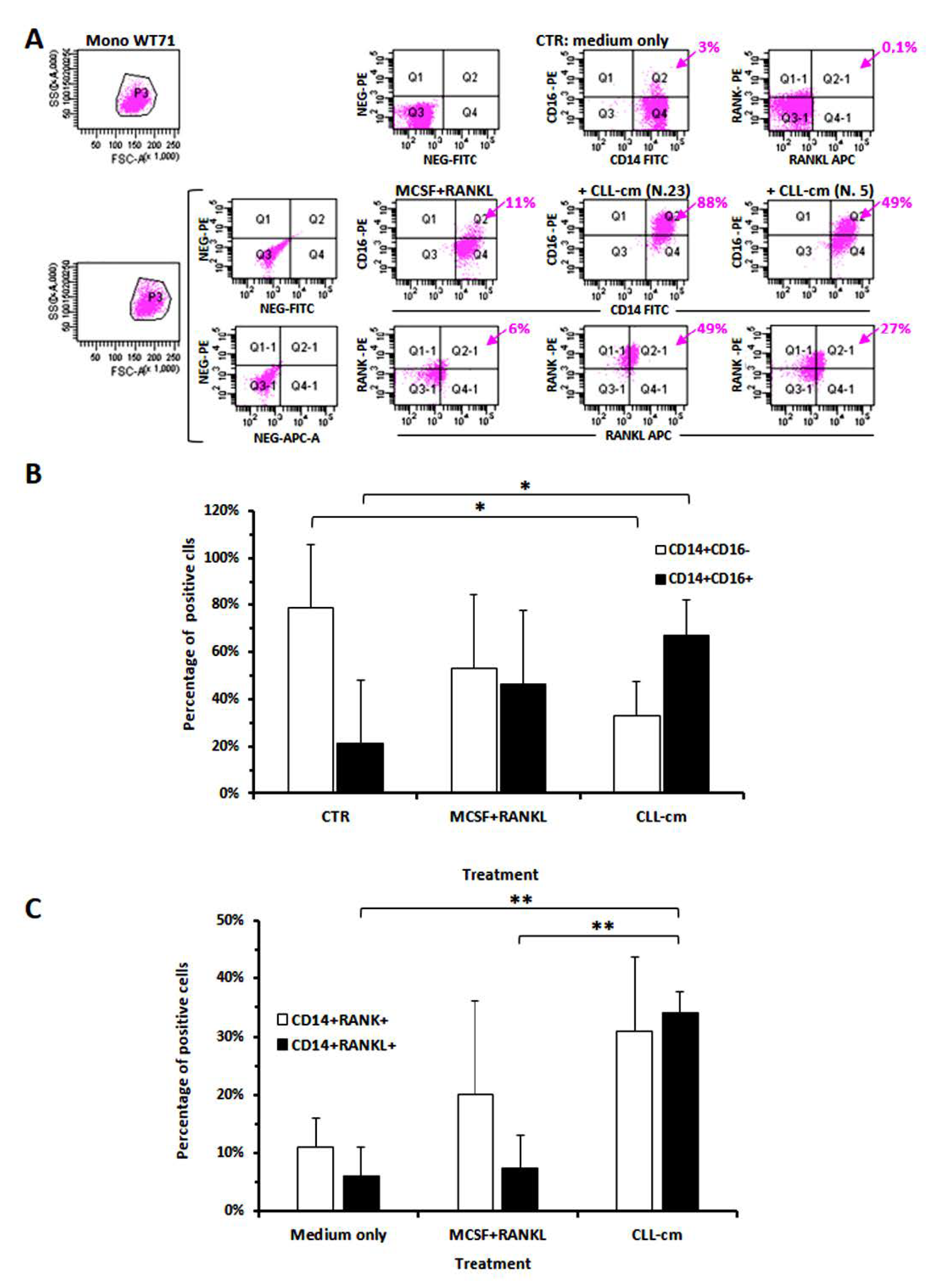
2.4. Determination of the Expression of CD16, RANK and RANKL on Healthy Monocytes following Their Cultures with Conditioned Media or Sera from CLL B Cells by Cyto-Fluorographic Analysis
2.5. Evaluation of Proliferation and Migration of Healthy Monocytes in Response to Conditioned Media from CLL B Cells
2.6. Generation of Osteoclasts and Determination of the Number of Osteoclasts after TRAP Staining
2.7. TRAP Staining and Bone Resorption Assay
2.8. Determination of IL-10 and TGFβ Levels in CLL-Conditioned Media
2.9. Immuno-Histochemical Analyses of Bone Biopsies from CLL Patients
2.10. In Silico Interrogation of Public Data on Human Monocytes from Healthy Donors or from CLL Patients
2.11. Statistics
3. Results
3.1. The Percentage of Circulating Monocytes Expressing CD16 Is Significantly Higher in CLL Than in MBL Patients or in Healthy Donors
3.2. In CLL Patients, the Percentage of CD16+ Monocytes Directly Correlated with the Levels of Bone Erosion
3.3. CLL-Conditioned Media Derived from Cultures of CLL B Cells Up-Regulated CD16 on Healthy Monocytes
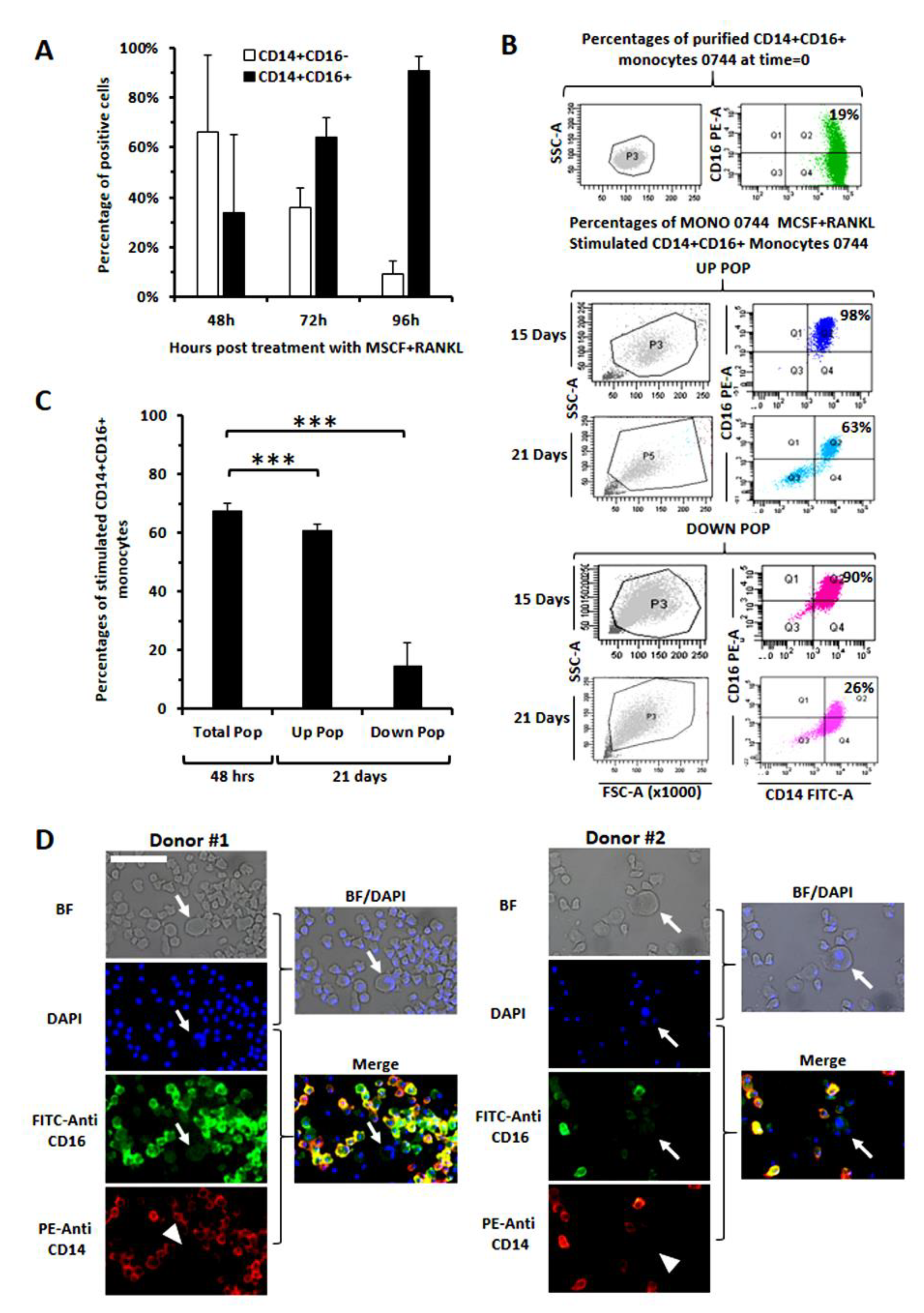
3.4. Evaluation of CD16 Expression along Osteoclast Maturation in Healthy Monocytes
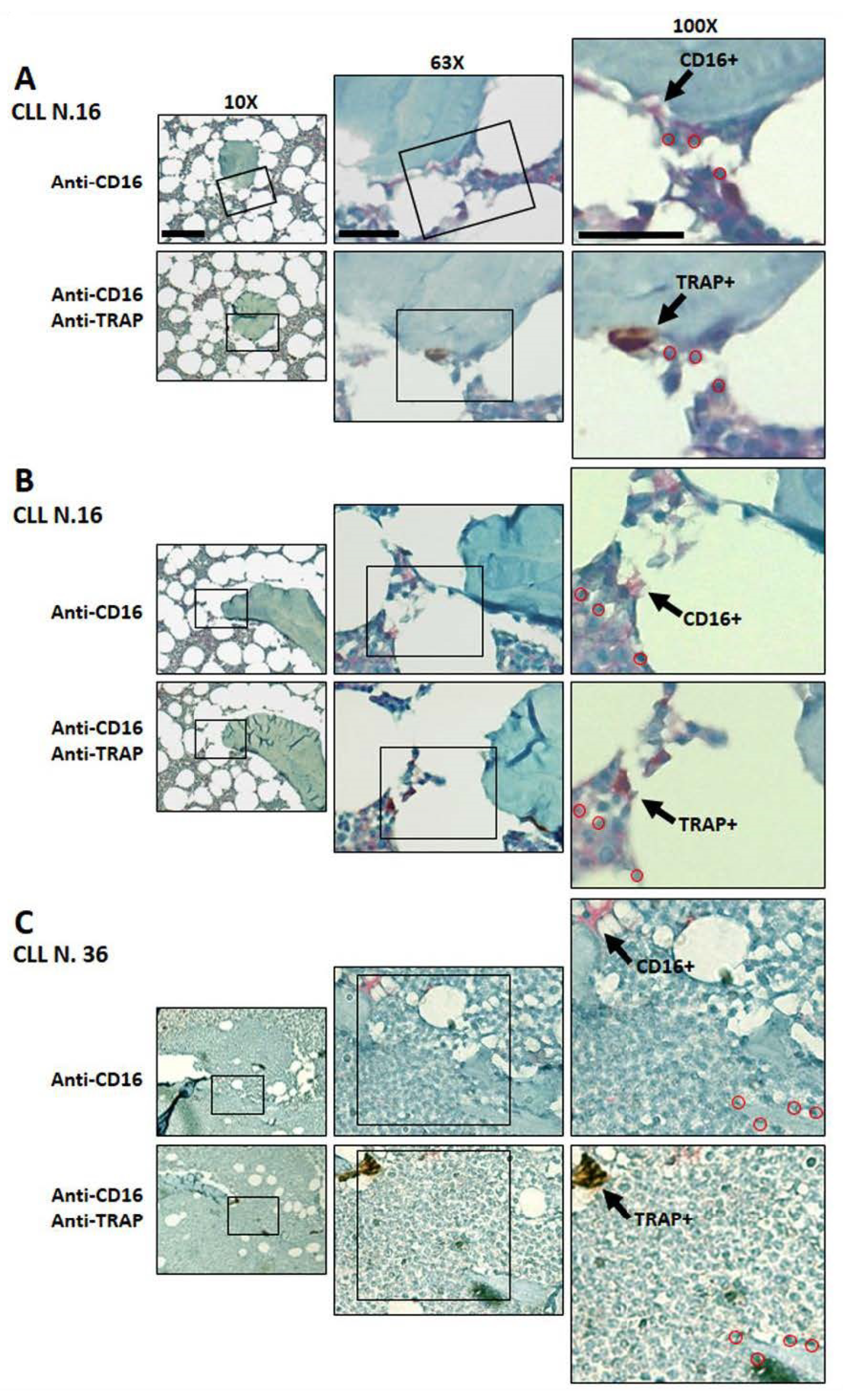
3.5. Immunodetection of Osteoclasts Expressing CD16 and TRAP in Bone Marrow Biopsies
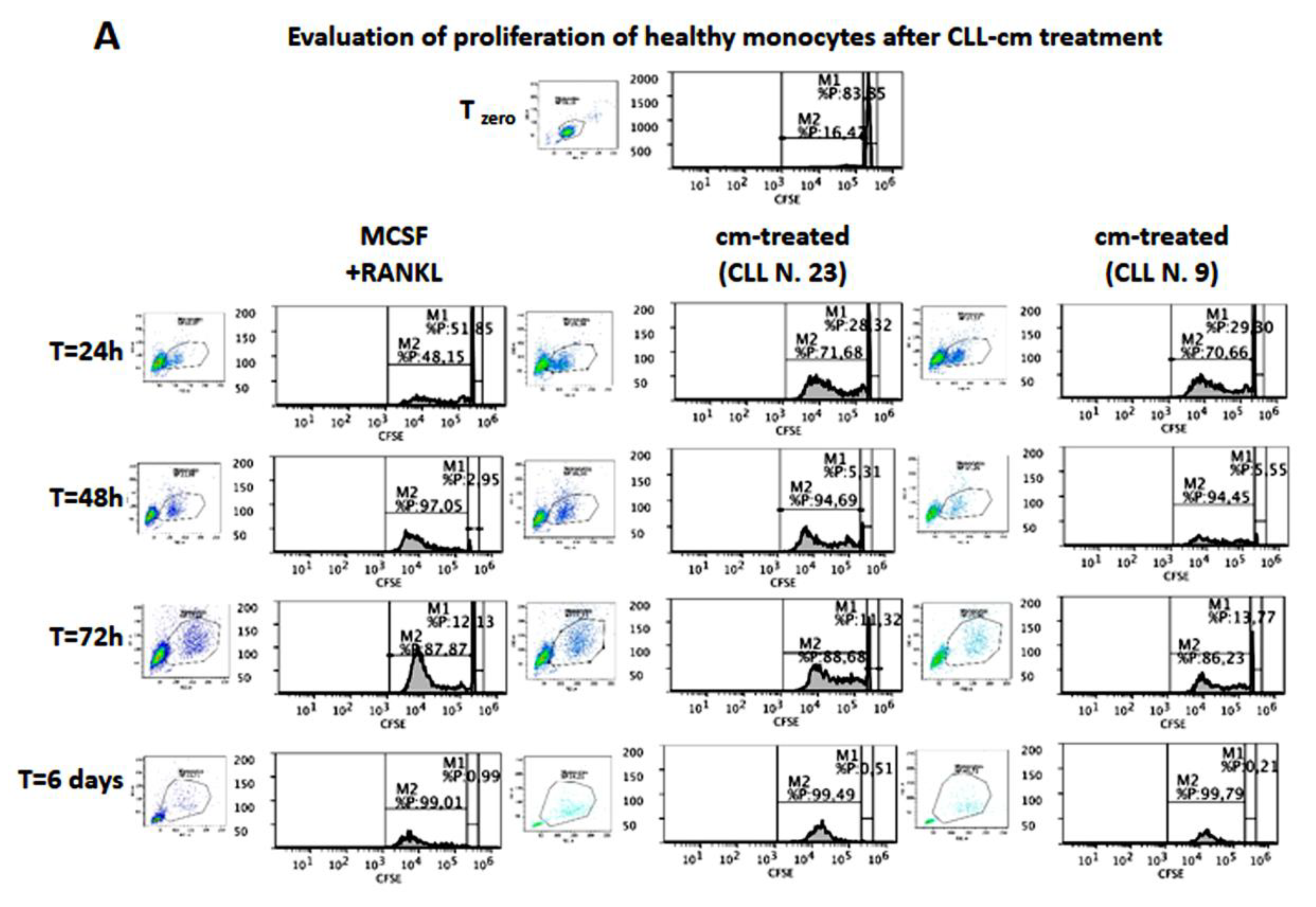
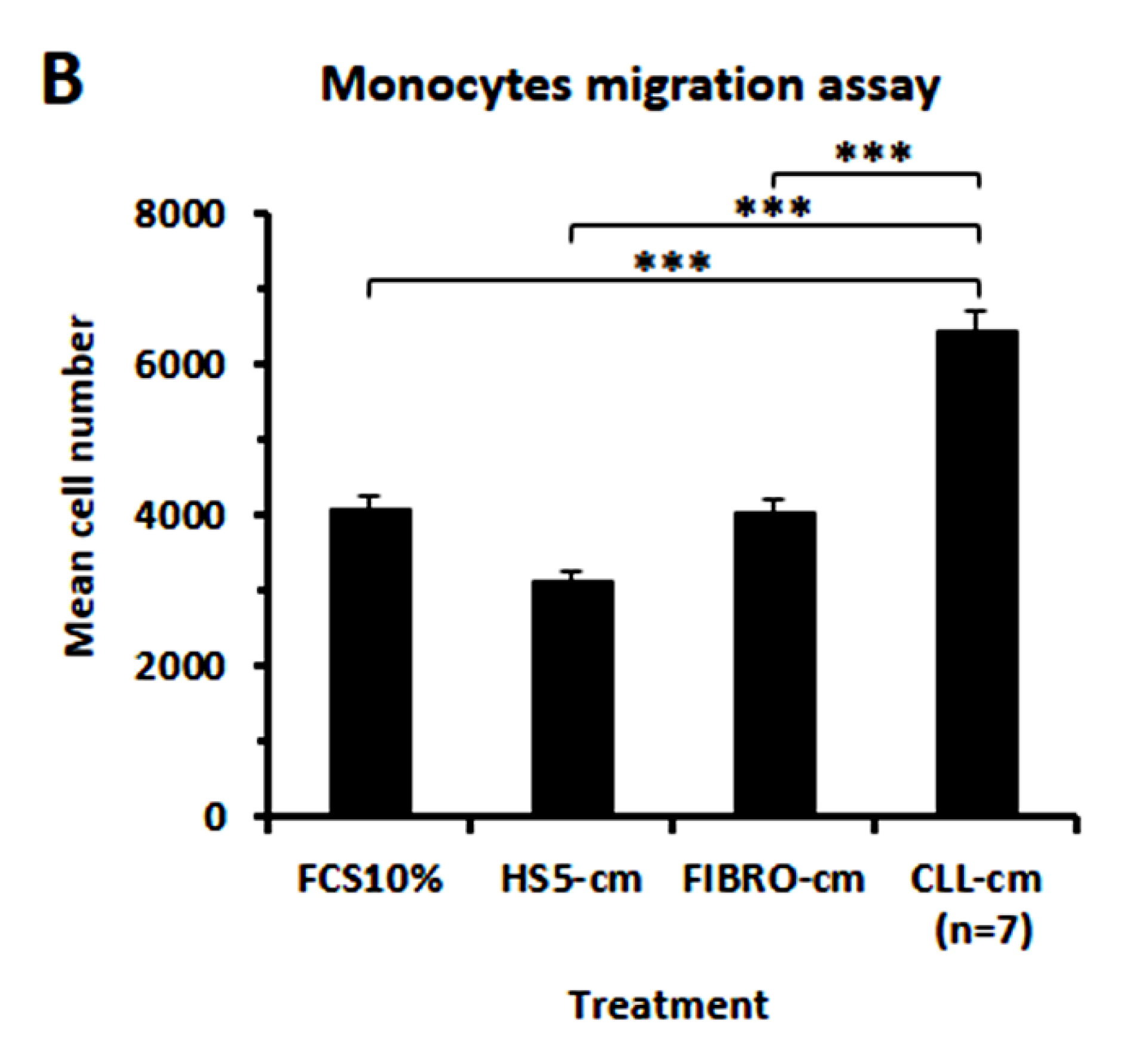
3.6. CLL-Conditioned Media Stimulate Proliferation and Migration of Healthy Monocytes
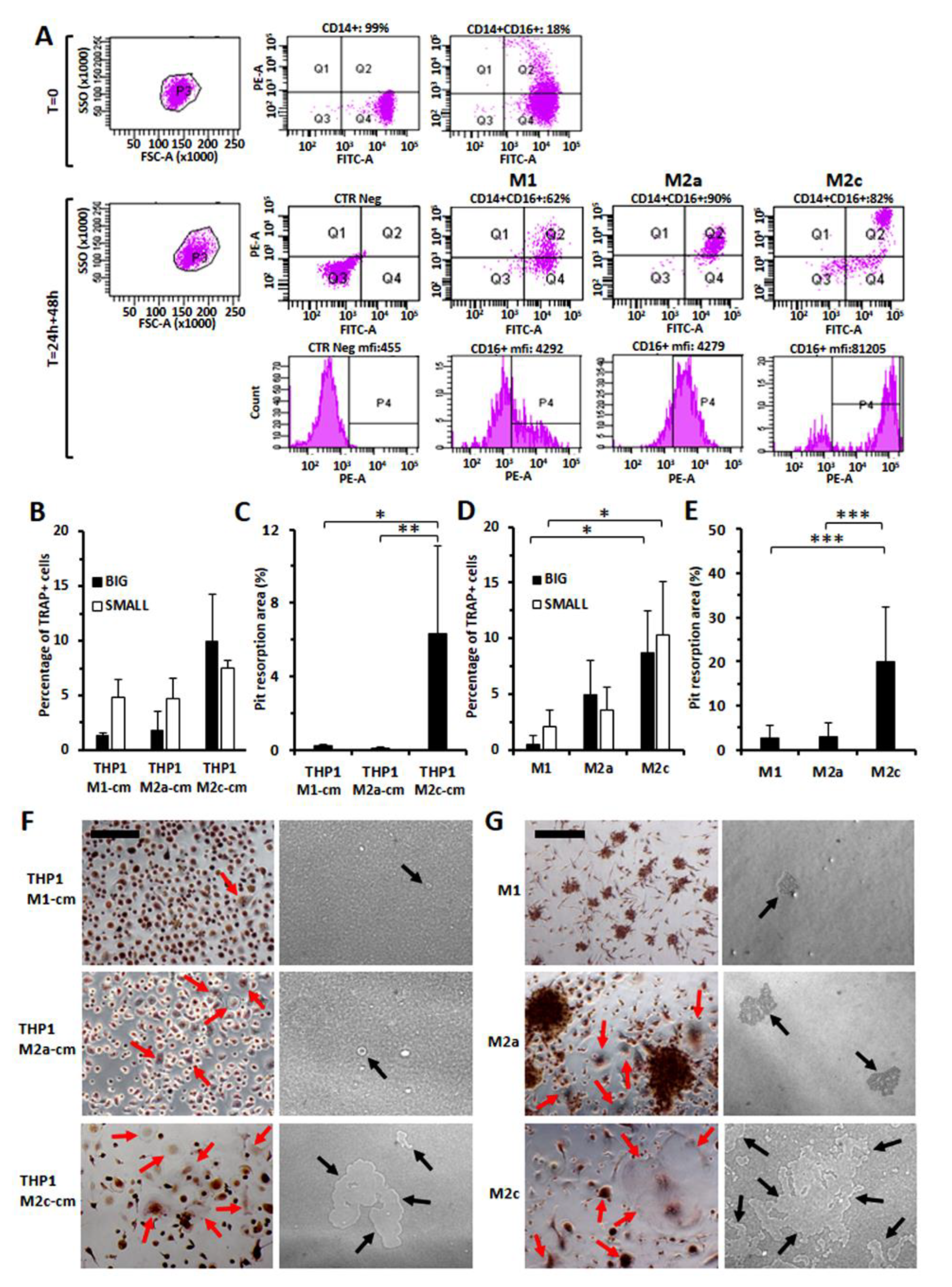
3.7. Increased Osteo-Clastogenesis by M2-Polarized Monocytes
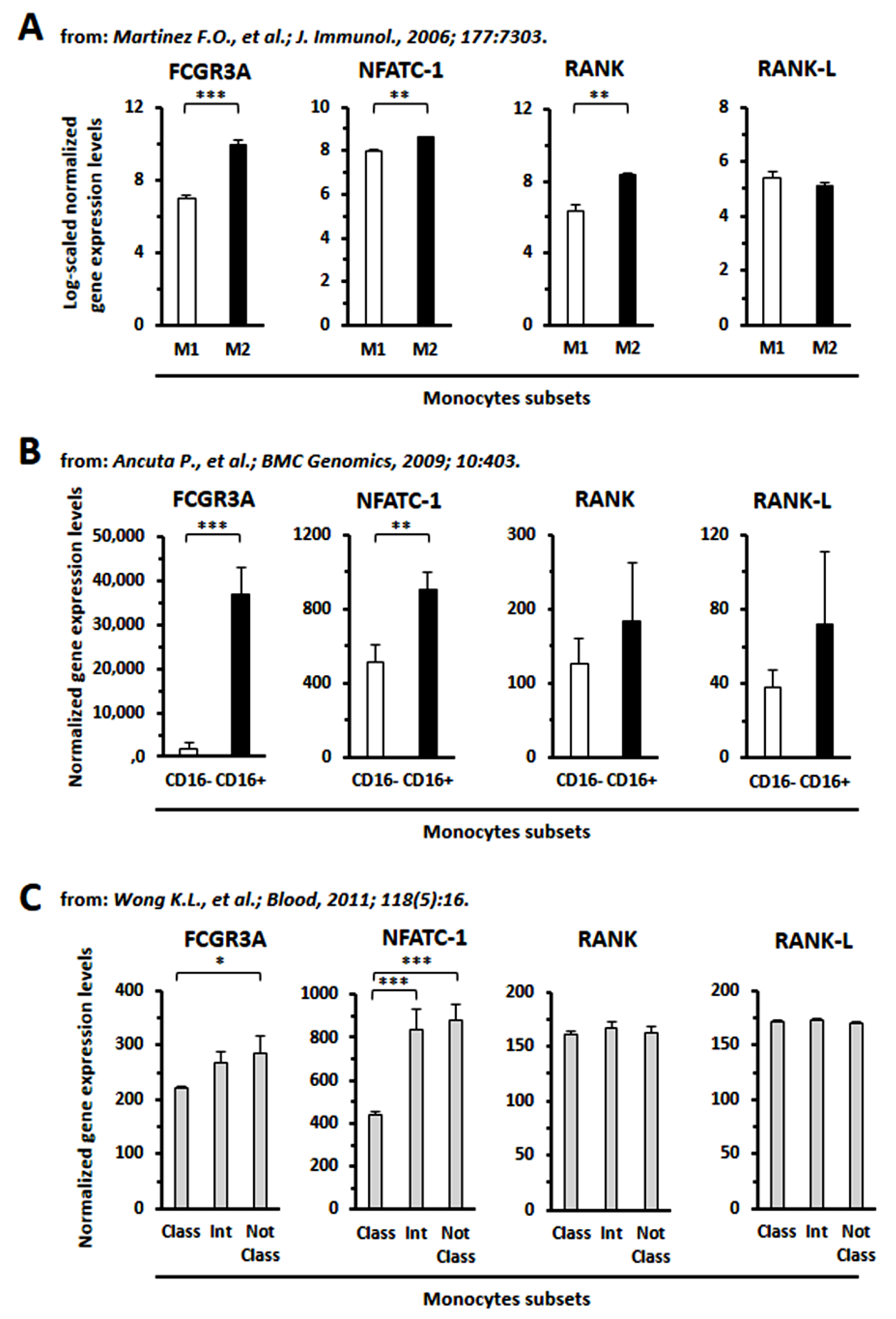
3.8. GEP Analysis
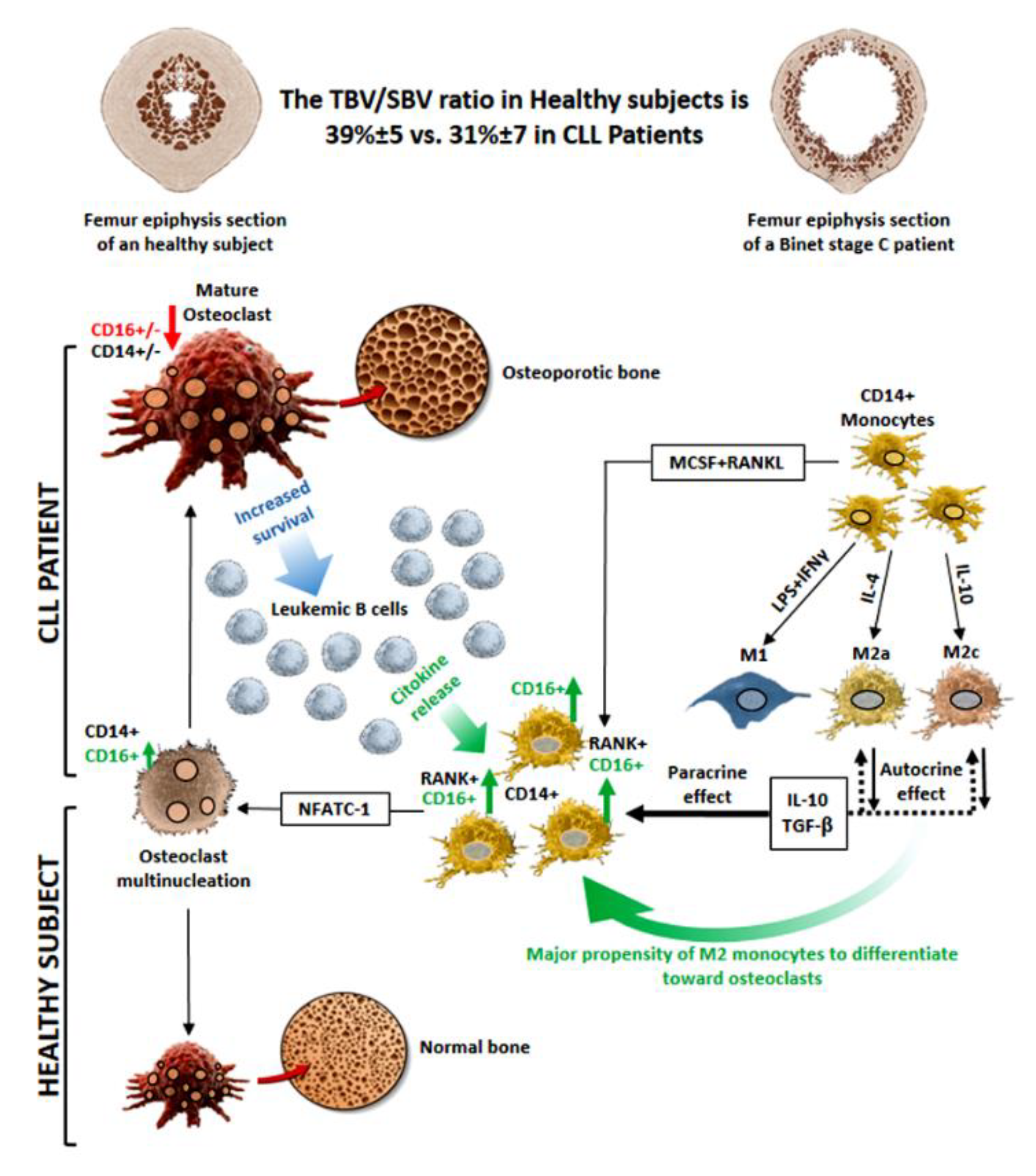
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannoni, P.; Pietra, G.; Travaini, G.; Quarto, R.; Shyti, G.; Benelli, R.; Ottaggio, L.; Mingari, M.C.; Zupo, S.; Cutrona, G.; et al. Chronic Lymphocytic Leukemia Nurse-like cells express the hepatocyte growth factor receptor (c-MET) and indoleamine 2,3-dioxygenase and display features of immunosuppressive type 2 skewed macrophages. Haematologica 2014, 99, 2–11. [Google Scholar] [CrossRef]
- Giannoni, P.; Scaglione, S.; Quarto, R.; Narcisi, R.; Parodi, M.; Balleari, E.; Barbieri, F.; Pattarozzi, A.; Florio, T.; Ferrini, S.; et al. An interaction between hepatocyte growth factor and its receptor (c-MET) prolongs the survival of chronic lymphocytic leukemic cells through STAT3 phosphorylation: A potential role of mesenchymal cells in the disease. Haematologica 2011, 96, 1015–1023. [Google Scholar] [CrossRef]
- Ding, W.; Nowakowski, G.S.; Knox, T.R.; Boysen, J.C.; Maas, M.L.; Schwager, S.M.; Wu, W.; Wellik, L.E.; Dietz, A.B.; Ghosh, A.K.; et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: Implication for CLL disease progression. Br. J. Haematol 2009, 147, 471–483. [Google Scholar] [CrossRef]
- Giannoni, P.; Marini, C.; Cutrona, G.; Matis, S.; Capra, M.C.; Puglisi, F.; Luzzi, P.; Pigozzi, S.; Gaggero, G.; Neri, A.; et al. Chronic lymphocytic leukemia cells impair osteoblastogenesis and promote osteoclastogenesis: Role of TNFalpha, IL-6 and IL-11 cytokines. Haematologica 2021, 106, 2598–2612. [Google Scholar] [CrossRef]
- Marini, C.; Bruno, S.; Fiz, F.; Campi, C.; Piva, R.; Cutrona, G.; Matis, S.; Nieri, A.; Miglino, M.; Ibatici, A.; et al. Functional Activation of Osteoclast Commitment in Chronic Lymphocytic Leukaemia: A Possible Role for RANK/RANKL Pathway. Sci. Rep. 2017, 7, 14159. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef]
- Chiu, Y.G.; Shao, T.; Feng, C.; Mensah, K.A.; Thullen, M.; Schwarz, E.M.; Ritchlin, C.T. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res. Ther. 2010, 12, R14. [Google Scholar] [CrossRef]
- Mensah, K.A.; Ritchlin, C.T.; Schwarz, E.M. RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens. J. Cell. Physiol. 2010, 223, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Long, C.L.; Humphrey, M.B. Osteoimmunology: The expanding role of immunoreceptors in osteoclasts and bone remodeling. BoneKEy Rep. 2012, 1. [Google Scholar] [CrossRef]
- Mocsai, A.; Humphrey, M.B.; Van Ziffle, J.A.; Hu, Y.; Burghardt, A.; Spusta, S.C.; Majumdar, S.; Lanier, L.L.; Lowell, C.A.; Nakamura, M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 6158–6163. [Google Scholar] [CrossRef]
- Shinohara, M.; Koga, T.; Okamoto, K.; Sakaguchi, S.; Arai, K.; Yasuda, H.; Takai, T.; Kodama, T.; Morio, T.; Geha, R.S.; et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 2008, 132, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Strauss-Ayali, D.; Conrad, S.M.; Mosser, D.M. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007, 82, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, N.; Yabuki, T.; Kumagai, T.; Takagi, S.; Takamoto, S.; Noguchi, H. Selective expansion of the CD14(+)/CD16(bright) subpopulation of circulating monocytes in patients with hemophagocytic syndrome. Ann. Hematol. 2007, 86, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Todd, I.; Radford, P.M.; Ziegler-Heitbrock, L.; Ghaemmaghami, A.M.; Powell, R.J.; Tighe, P.J. Elevated CD16 expression by monocytes from patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2007, 56, 4182–4188. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007, 81, 584–592. [Google Scholar] [CrossRef]
- Mucci, J.M.; Cuello, M.F.; Kisinovsky, I.; Larroude, M.; Delpino, M.V.; Rozenfeld, P.A. Proinflammatory and proosteoclastogenic potential of peripheral blood mononuclear cells from Gaucher patients: Implication for bone pathology. Blood Cells Mol. Dis. 2015, 55, 134–143. [Google Scholar] [CrossRef]
- Petitprez, V.; Royer, B.; Desoutter, J.; Guiheneuf, E.; Rigolle, A.; Marolleau, J.P.; Kamel, S.; Guillaume, N. CD14+ CD16+ monocytes rather than CD14+ CD51/61+ monocytes are a potential cytological marker of circulating osteoclast precursors in multiple myeloma. A preliminary study. Int. J. Lab. Hematol. 2015, 37, 29–35. [Google Scholar] [CrossRef]
- Bolzoni, M.; Ronchetti, D.; Storti, P.; Donofrio, G.; Marchica, V.; Costa, F.; Agnelli, L.; Toscani, D.; Vescovini, R.; Todoerti, K.; et al. IL21R expressing CD14(+)CD16(+) monocytes expand in multiple myeloma patients leading to increased osteoclasts. Haematologica 2017, 102, 773–784. [Google Scholar] [CrossRef]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Robinson, J.D.; Lupkiewicz, S.M.; Palenik, L.; Lopez, L.M.; Ariet, M. Determination of ideal body weight for drug dosage calculations. Am. J. Hosp. Pharm. 1983, 40, 1016–1019. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Dougherty, E.R. Binarization of microarray data on the basis of a mixture model. Mol. Cancer 2003, 2, 679–684. [Google Scholar]
- Ancuta, P.; Liu, K.Y.; Misra, V.; Wacleche, V.S.; Gosselin, A.; Zhou, X.; Gabuzda, D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genom. 2009, 10, 403. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Marini, C.; Piva, R.; Miglino, M.; Massollo, M.; Bongioanni, F.; Morbelli, S.; Bottoni, G.; Campi, C.; Bacigalupo, A.; et al. Adult advanced chronic lymphocytic leukemia: Computational analysis of whole-body CT documents a bone structure alteration. Radiology 2014, 271, 805–813. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Mensah, K.A.; Schwarz, E.M.; Ju, Y.; Takahata, M.; Feng, C.; McMahon, L.A.; Hicks, D.G.; Panepento, B.; Keng, P.C.; et al. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP). J. Bone Min. Res. 2012, 27, 79–92. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Seta, N.; Yoshimoto, K.; Suzuki, K.; Yamaoka, K.; Takeuchi, T. CD14(bright)CD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Maffei, R.; Bulgarelli, J.; Fiorcari, S.; Bertoncelli, L.; Martinelli, S.; Guarnotta, C.; Castelli, I.; Deaglio, S.; Debbia, G.; De Biasi, S.; et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica 2013, 98, 1115–1123. [Google Scholar] [CrossRef]
- Lari, R.; Kitchener, P.D.; Hamilton, J.A. The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res. Ther. 2009, 11, R23. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tsukada, N.; Burger, M.; Zvaifler, N.J.; Dell’Aquila, M.; Kipps, T.J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000, 96, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Maffei, R.; Audrito, V.; Martinelli, S.; Ten Hacken, E.; Zucchini, P.; Grisendi, G.; Potenza, L.; Luppi, M.; Burger, J.A.; et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016, 7, 65968–65981. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, O.; Jimbu, L.; Neaga, A.; Popescu, C.; Berceanu, I.; Tomuleasa, C.; Fetica, B.; Zdrenghea, M. Macrophage Polarization in Chronic Lymphocytic Leukemia: Nurse-Like Cells Are the Caretakers of Leukemic Cells. Biomedicines 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Cutucache, C.E. Tumor-induced host immunosuppression: Special focus on CLL. Int. Immunopharmacol. 2013, 17, 35–41. [Google Scholar] [CrossRef]
- Fiorcari, S.; Maffei, R.; Atene, C.G.; Potenza, L.; Luppi, M.; Marasca, R. Nurse-Like Cells and Chronic Lymphocytic Leukemia B Cells: A Mutualistic Crosstalk inside Tissue Microenvironments. Cells 2021, 10, 217. [Google Scholar] [CrossRef]
- Jitschin, R.; Braun, M.; Buttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; Le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef]
- Komano, Y.; Nanki, T.; Hayashida, K.; Taniguchi, K.; Miyasaka, N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 2006, 8, R152. [Google Scholar] [CrossRef]
- Kowalska, W.; Bojarska-Junak, A. Monocytic MDSC as a source of immunosuppressive cytokines in chronic lymphocytic leukemia (CLL) microenvironment. Folia Histochem. Et Cytobiol. 2020, 58, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, O.J.; Kim, J.; Kwon, Y.; Yun, C.H.; Han, S.H. Modulation of macrophage subtypes by IRF5 determines osteoclastogenic potential. J. Cell. Physiol. 2019, 234, 23033–23042. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, W.; Zarobkiewicz, M.; Tomczak, W.; Wos, J.; Morawska, I.; Bojarska-Junak, A. Reduced Percentage of CD14(dim)CD16(+)SLAN(+) Monocytes Producing TNF and IL-12 as an Immunological Sign of CLL Progression. Int. J. Mol. Sci. 2022, 23, 3029. [Google Scholar] [CrossRef]
- Kirkwood, K.L.; Zhang, L.; Thiyagarajan, R.; Seldeen, K.L.; Troen, B.R. Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility. Immunol. Investig. 2018, 47, 844–854. [Google Scholar] [CrossRef]
- Yan, L.; Liang, M.; Yang, T.; Ji, J.; Jose Kumar Sreena, G.S.; Hou, X.; Cao, M.; Feng, Z. The Immunoregulatory Role of Myeloid-Derived Suppressor Cells in the Pathogenesis of Rheumatoid Arthritis. Front. Immunol. 2020, 11, 568362. [Google Scholar] [CrossRef]
- Sawant, A.; Deshane, J.; Jules, J.; Lee, C.M.; Harris, B.A.; Feng, X.; Ponnazhagan, S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013, 73, 672–682. [Google Scholar] [CrossRef]
- Sawant, A.; Ponnazhagan, S. Myeloid-derived suppressor cells as osteoclast progenitors: A novel target for controlling osteolytic bone metastasis. Cancer Res. 2013, 73, 4606–4610. [Google Scholar] [CrossRef]
- Danilin, S.; Merkel, A.R.; Johnson, J.R.; Johnson, R.W.; Edwards, J.R.; Sterling, J.A. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology 2012, 1, 1484–1494. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Wang, S.; Fu, R.; Guo, C.; Wang, H.; Zhao, J.; Gaskin, F.; Chen, J.; Yang, N.; et al. Myeloid-derived suppressor cells contribute to bone erosion in collagen-induced arthritis by differentiating to osteoclasts. J. Autoimmun. 2015, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhang, J.; Lwin, S.T.; Edwards, J.R.; Edwards, C.M.; Mundy, G.R.; Yang, X. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS ONE 2012, 7, e48871. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Moeen, S.M.; Thabet, A.F.; Rayan, A.; Abdel-Rahim, M.H.; Mohamed, W.M.Y.; Hetta, H.F. Monocytic myeloid-derived suppressor cells in chronic lymphocytic leukemia patients: A single center experience. Leuk Lymphoma 2020, 61, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.; Kowalska, W.; Chocholska, S.; Tomczak, W.; Szymanska, A.; Morawska, I.; Wojciechowska, A.; Bojarska-Junak, A. High M-MDSC Percentage as a Negative Prognostic Factor in Chronic Lymphocytic Leukaemia. Cancers 2020, 12, 2614. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoni, P.; Marini, C.; Cutrona, G.; Todoerti, K.; Neri, A.; Ibatici, A.; Sambuceti, G.; Pigozzi, S.; Mora, M.; Ferrarini, M.; et al. A High Percentage of CD16+ Monocytes Correlates with the Extent of Bone Erosion in Chronic Lymphocytic Leukemia Patients: The Impact of Leukemic B Cells in Monocyte Differentiation and Osteoclast Maturation. Cancers 2022, 14, 5979. https://doi.org/10.3390/cancers14235979
Giannoni P, Marini C, Cutrona G, Todoerti K, Neri A, Ibatici A, Sambuceti G, Pigozzi S, Mora M, Ferrarini M, et al. A High Percentage of CD16+ Monocytes Correlates with the Extent of Bone Erosion in Chronic Lymphocytic Leukemia Patients: The Impact of Leukemic B Cells in Monocyte Differentiation and Osteoclast Maturation. Cancers. 2022; 14(23):5979. https://doi.org/10.3390/cancers14235979
Chicago/Turabian StyleGiannoni, Paolo, Cecilia Marini, Giovanna Cutrona, Katia Todoerti, Antonino Neri, Adalberto Ibatici, Gianmario Sambuceti, Simona Pigozzi, Marco Mora, Manlio Ferrarini, and et al. 2022. "A High Percentage of CD16+ Monocytes Correlates with the Extent of Bone Erosion in Chronic Lymphocytic Leukemia Patients: The Impact of Leukemic B Cells in Monocyte Differentiation and Osteoclast Maturation" Cancers 14, no. 23: 5979. https://doi.org/10.3390/cancers14235979
APA StyleGiannoni, P., Marini, C., Cutrona, G., Todoerti, K., Neri, A., Ibatici, A., Sambuceti, G., Pigozzi, S., Mora, M., Ferrarini, M., Fais, F., & de Totero, D. (2022). A High Percentage of CD16+ Monocytes Correlates with the Extent of Bone Erosion in Chronic Lymphocytic Leukemia Patients: The Impact of Leukemic B Cells in Monocyte Differentiation and Osteoclast Maturation. Cancers, 14(23), 5979. https://doi.org/10.3390/cancers14235979








