Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Comparison of Neurocognitive Outcomes by Clinic Group
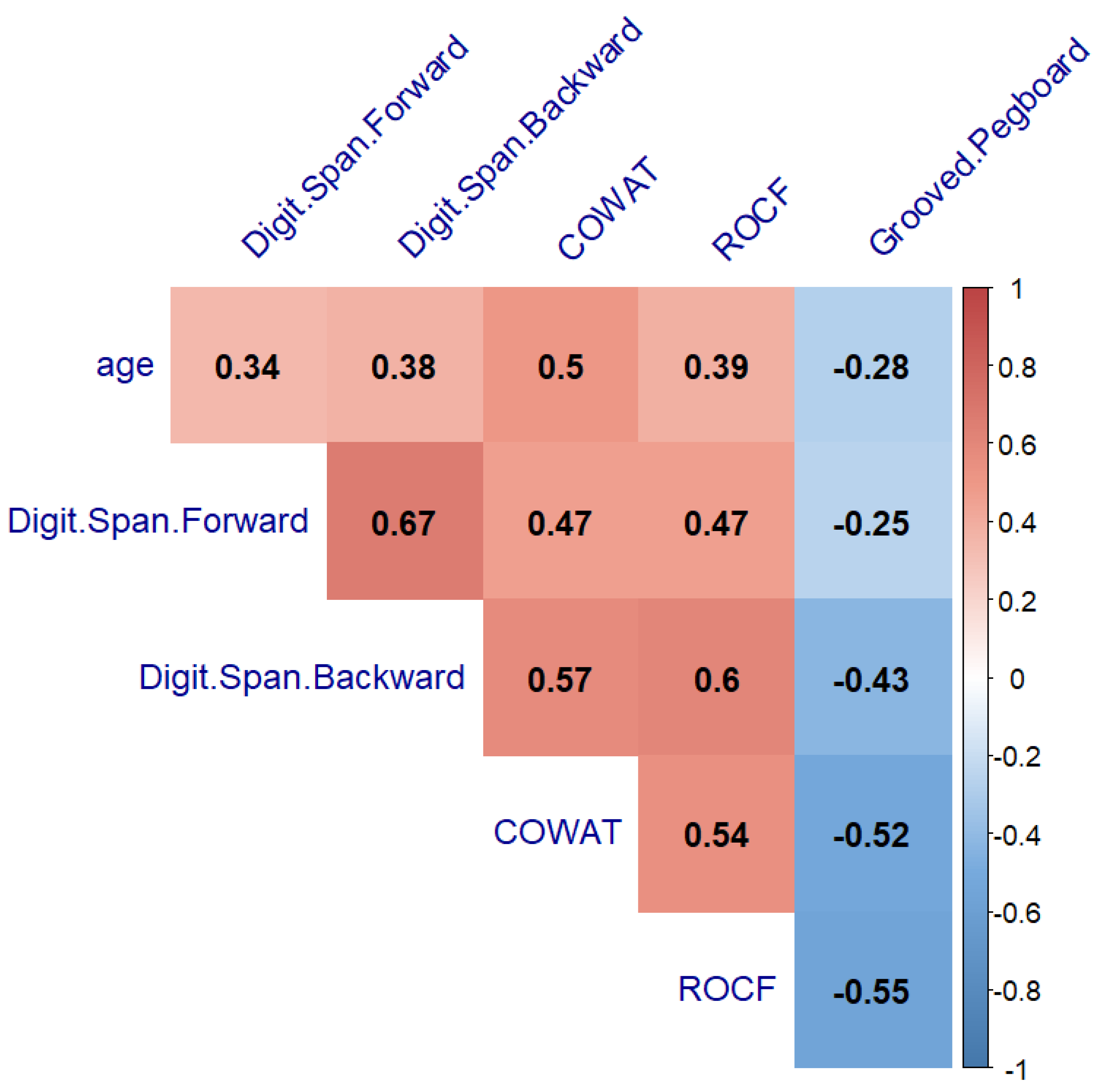
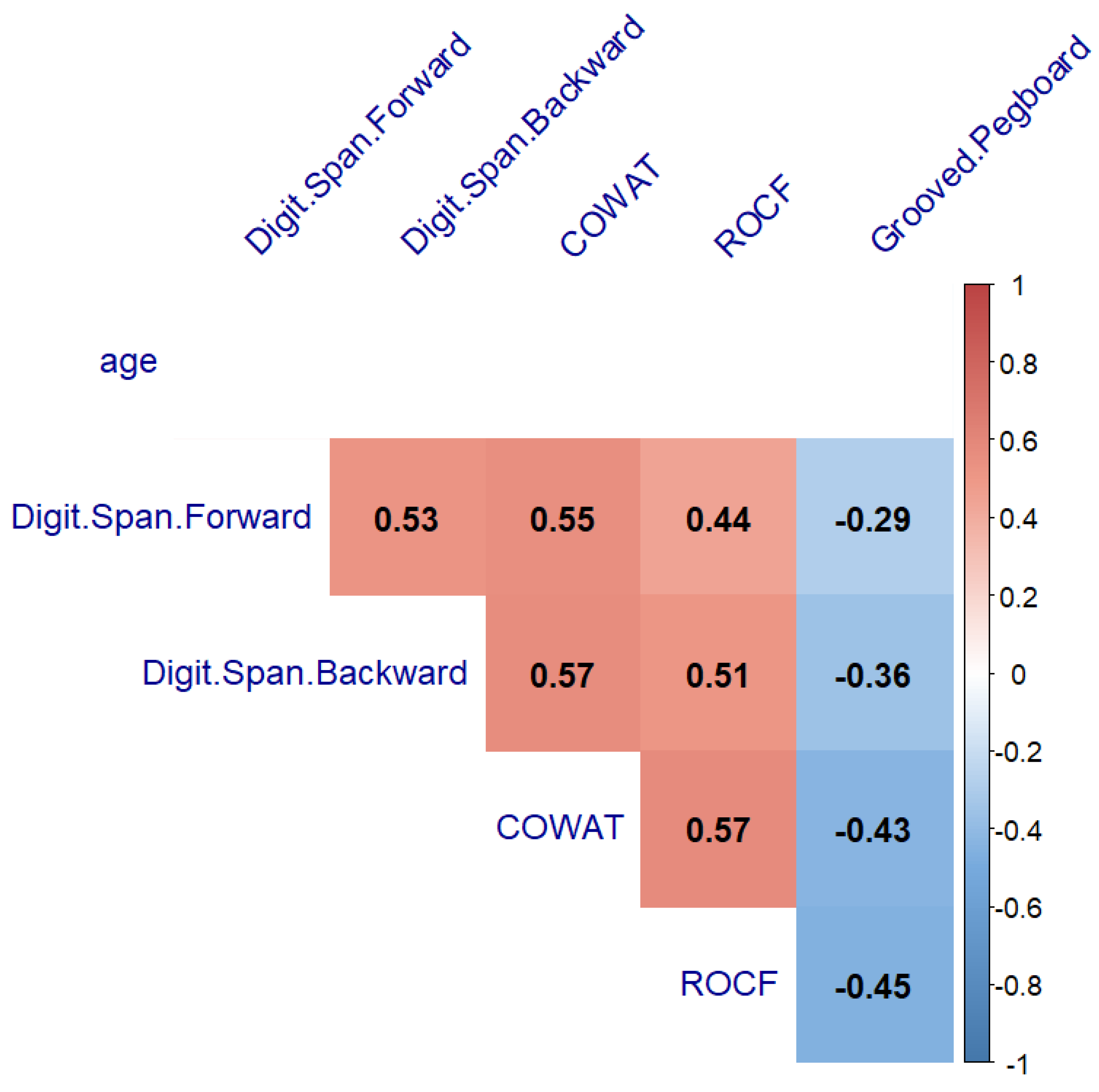
3.2. Comparison of Neurocognitive Measures by Clinic Group between Females and Males, and the Relationship with Age
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.M.; Ward, Z.J.; Chaudhry, A.; Liu, Q.; Yasui, Y.; Armstrong, G.T.; Gibson, T.M.; Howell, R.; Hudson, M.M.; Krull, K.R.; et al. Life Expectancy of Adult Survivors of Childhood Cancer Over 3 Decades. JAMA Oncol. 2020, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Skinner, R.; Wallace, W.H.; Hjorth, L.; Mulder, R.L.; Wong, F.L.; Yasui, Y.; Bhakta, N.; Constine, L.S.; Bhatia, S.; et al. Surveillance for Late Effects in Childhood Cancer Survivors. JCO 2018, 36, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Hudson, M.M. Survivors of Childhood and Adolescent Cancer: Life-Long Risks and Responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.S.; Duke, E.S.; Schofield, H.-L.T.; Ullrich, N.J. Neurotoxic Effects of Childhood Cancer Therapy and Its Potential Neurocognitive Impact. JCO 2021, 39, 1752–1765. [Google Scholar] [CrossRef]
- Kesler, S.R.; Ogg, R.; Reddick, W.E.; Phillips, N.; Scoggins, M.; Glass, J.O.; Cheung, Y.T.; Pui, C.-H.; Robison, L.L.; Hudson, M.M.; et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect. 2018, 8, 333–342. [Google Scholar] [CrossRef]
- Askins, M.A.; Moore, B.D. Preventing Neurocognitive Late Effects in Childhood Cancer Survivors. J. Child. Neurol. 2008, 23, 1160–1171. [Google Scholar] [CrossRef]
- Jacola, L.M.; Partanen, M.; Lemiere, J.; Hudson, M.M.; Thomas, S. Assessment and Monitoring of Neurocognitive Function in Pediatric Cancer. JCO 2021, 39, 1696–1704. [Google Scholar] [CrossRef]
- Ventura, L.M.; Grieco, J.A.; Evans, C.L.; Kuhlthau, K.A.; MacDonald, S.M.; Tarbell, N.J.; Yock, T.I.; Pulsifer, M.B. Executive Functioning, Academic Skills, and Quality of Life in Pediatric Patients with Brain Tumors Post-Proton Radiation Therapy. J. Neurooncol. 2018, 137, 119–126. [Google Scholar] [CrossRef]
- Siegwart, V.; Benzing, V.; Spitzhuettl, J.; Schmidt, M.; Grotzer, M.; Steinlin, M.; Leibundgut, K.; Roebers, C.; Everts, R. Cognition, Psychosocial Functioning, and Health-Related Quality of Life among Childhood Cancer Survivors. Neuropsychol. Rehabil. 2022, 32, 922–945. [Google Scholar] [CrossRef]
- Zheng, D.J.; Lu, X.; Schore, R.J.; Balsamo, L.; Devidas, M.; Winick, N.J.; Raetz, E.A.; Loh, M.L.; Carroll, W.L.; Sung, L.; et al. Longitudinal Analysis of Quality-of-Life Outcomes in Children during Treatment for Acute Lymphoblastic Leukemia: A Report from the Children’s Oncology Group AALL0932 Trial: QOL in Children During Treatment for ALL. Cancer 2018, 124, 571–579. [Google Scholar] [CrossRef]
- Frederiksen, L.E.; Mader, L.; Feychting, M.; Mogensen, H.; Madanat-Harjuoja, L.; Malila, N.; Tolkkinen, A.; Hasle, H.; Winther, J.F.; Erdmann, F. Surviving Childhood Cancer: A Systematic Review of Studies on Risk and Determinants of Adverse Socioeconomic Outcomes. Int. J. Cancer 2019, 144, 1796–1823. [Google Scholar] [CrossRef] [PubMed]
- Godono, A.; Felicetti, F.; Conti, A.; Clari, M.; Dionisi-Vici, M.; Gatti, F.; Ciocan, C.; Pinto, T.; Arvat, E.; Brignardello, E.; et al. Employment among Childhood Cancer Survivors: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P. Epidemiology of Childhood Cancer. Cancer Treat. Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Decock, M.; De Wilde, R.; Van der Looven, R.; Vander Linden, C. Motor Functioning and Intelligence Quotient in Paediatric Survivors of a Fossa Posterior Tumor Following a Multidisciplinary Rehabilitation Program. Int. J. Environ. Res. Public Health 2022, 19, 7083. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Gallego, E.A.; Gómez, C.M.; Casares, E.V.; Márquez, J.; Pérez-Santamaría, F.J. Declarative and Procedural Learning in Children and Adolescents with Posterior Fossa Tumours. Behav. Brain Funct. 2006, 2, 9. [Google Scholar] [CrossRef]
- Levitch, C.F.; Holland, A.A.; Bledsoe, J.; Kim, S.Y.; Barnett, M.; Ramjan, S.; Sands, S.A. Comparison of Neuropsychological Functioning in Pediatric Posterior Fossa Tumor Survivors: Medulloblastoma, Low-grade Astrocytoma, and Healthy Controls. Pediatr. Blood Cancer 2022, 69, e29491. [Google Scholar] [CrossRef]
- Major, N.; Patel, N.A.; Bennett, J.; Novakovic, E.; Poloni, D.; Abraham, M.; Brown, N.J.; Gendreau, J.L.; Sahyouni, R.; Loya, J. The Current State of Radiotherapy for Pediatric Brain Tumors: An Overview of Post-Radiotherapy Neurocognitive Decline and Outcomes. JPM 2022, 12, 1050. [Google Scholar] [CrossRef]
- Hanzlik, E.; Woodrome, S.E.; Abdel-Baki, M.; Geller, T.J.; Elbabaa, S.K. A Systematic Review of Neuropsychological Outcomes Following Posterior Fossa Tumor Surgery in Children. Childs Nerv. Syst. 2015, 31, 1869–1875. [Google Scholar] [CrossRef]
- Chieffo, D.P.R.; Lino, F.; Arcangeli, V.; Moriconi, F.; Frassanito, P.; Massimi, L.; Tamburrini, G. Posterior Fossa Tumor Rehabilitation: An Up-to-Date Overview. Children 2022, 9, 904. [Google Scholar] [CrossRef]
- Wolfe, K.R.; Madan-Swain, A.; Kana, R.K. Executive Dysfunction in Pediatric Posterior Fossa Tumor Survivors: A Systematic Literature Review of Neurocognitive Deficits and Interventions. Dev. Neuropsychol. 2012, 37, 153–175. [Google Scholar] [CrossRef]
- Robinson, K.E.; Fraley, C.E.; Pearson, M.M.; Kuttesch, J.F.; Compas, B.E. Neurocognitive Late Effects of Pediatric Brain Tumors of the Posterior Fossa: A Quantitative Review. J. Int. Neuropsychol. Soc. 2013, 19, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.H.; Ha, B.; Lee, E.; Park, B.; Yang, S.E.; Yu, E.; Kim, J. Neurocognitive and Psychological Functioning of Pediatric Brain Tumor Patients Undergoing Proton Beam Therapy for Three Different Tumor Types. Pediatr. Blood Cancer 2022, 69, e29430. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Armstrong, C.; Wileyto, E.P.; Janopaul-Naylor, J.; Sloan, L.; Lustig, R.A.; Kurtz, G.A.; Shabason, J.E.; Dorsey, J.F.; Feinstein, C.; et al. Changes in Neurocognitive Function after Proton versus Photon Radiotherapy for Central Nervous System Tumors. Brain Tumor Res. Treat. 2022, 10, S125. [Google Scholar]
- Malbari, F.; Gill, J.; Daigle, A.; Rodriguez, L.L.; Raghubar, K.P.; Davis, K.C.; Scheurer, M.; Ma, M.M.; Kralik, S.F.; Meoded, A.; et al. Cerebellar Mutism Syndrome in Pediatric Neuro-Oncology: A Multidisciplinary Perspective and Call for Research Priorities. Pediatr. Neurol. 2022, 132, 4–10. [Google Scholar] [CrossRef]
- Hoffmann-Lamplmair, D.; Leiss, U.; Peyrl, A.; Slavc, I.; Czech, T.; Gram, A.; Pletschko, T. Evaluating the Diagnostic Validity of the Cerebellar Cognitive Affective Syndrome (CCAS) in Pediatric Posterior Fossa Tumor Patients. Neuro-Oncol. Adv. 2022, 4, vdac065. [Google Scholar] [CrossRef]
- Schmahmann, J. The Cerebellar Cognitive Affective Syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Ahmadian, N.; van Baarsen, K.; van Zandvoort, M.; Robe, P.A. The Cerebellar Cognitive Affective Syndrome—A Meta-Analysis. Cerebellum 2019, 18, 941–950. [Google Scholar] [CrossRef]
- Slotnick, S.D. The Hippocampus and Long-Term Memory. Cognitive Neuroscience 2022, 13, 113–114. [Google Scholar] [CrossRef]
- Rolls, E.T. The Hippocampus, Ventromedial Prefrontal Cortex, and Episodic and Semantic Memory. Prog. Neurobiol. 2022, 217, 102334. [Google Scholar] [CrossRef]
- Cr, V.; Das, G.; Seth, R.; Sapra, S.; Sri, P.; Meena, J.P.; Gupta, A.K.; Sreenivas, V. Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia: Experience from a Tertiary Care Center in India. Pediatr. Blood Cancer 2022, 69, e29688. [Google Scholar] [CrossRef]
- Iyer, N.S.; Balsamo, L.M.; Bracken, M.B.; Kadan-Lottick, N.S. Chemotherapy-Only Treatment Effects on Long-Term Neurocognitive Functioning in Childhood ALL Survivors: A Review and Meta-Analysis. Blood 2015, 126, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Duffner, P.K.; Armstrong, F.D.; Chen, L.; Helton, K.J.; Brecher, M.L.; Bell, B.; Chauvenet, A.R. Neurocognitive and Neuroradiologic Central Nervous System Late Effects in Children Treated on Pediatric Oncology Group (POG) P9605 (Standard Risk) and P9201 (Lesser Risk) Acute Lymphoblastic Leukemia Protocols (ACCL0131): A Methotrexate Consequence? A Report From the Children’s Oncology Group. J. Pediatr. Hematol./Oncol. 2014, 36, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, M.A.; Ruble, K. Multidisciplinary Care in Pediatric Oncology. JMDH 2011, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vijayanathan, V.; Gulinello, M.; Cole, P.D. Intrathecal Methotrexate Induces Focal Cognitive Deficits and Increases Cerebrospinal Fluid Homocysteine. Pharmacol. Biochem. Behav. 2010, 95, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; White, H.A.; Glass, J.O.; Kun, L.E.; Leigh, L.; Thompson, S.J.; Reddick, W.E. Attentional Functioning and White Matter Integrity among Survivors of Malignant Brain Tumors of Childhood. J. Int. Neuropsychol. Soc. 2004, 10, 180–189. [Google Scholar] [CrossRef]
- Reddick, W.E.; White, H.A.; Glass, J.O.; Wheeler, G.C.; Thompson, S.J.; Gajjar, A.; Leigh, L.; Mulhern, R.K. Developmental Model Relating White Matter Volume to Neurocognitive Deficits in Pediatric Brain Tumor Survivors. Cancer 2003, 97, 2512–2519. [Google Scholar] [CrossRef]
- Robinson, K.E.; Kuttesch, J.F.; Champion, J.E.; Andreotti, C.F.; Hipp, D.W.; Bettis, A.; Barnwell, A.; Compas, B.E. A Quantitative Meta-Analysis of Neurocognitive Sequelae in Survivors of Pediatric Brain Tumors. Pediatr. Blood Cancer 2010, 55, 525–531. [Google Scholar] [CrossRef]
- Campbell, L.K.; Scaduto, M.; Sharp, W.; Dufton, L.; Van Slyke, D.; Whitlock, J.A.; Compas, B. A Meta-Analysis of the Neurocognitive Sequelae of Treatment for Childhood Acute Lymphocytic Leukemia. Pediatr. Blood Cancer 2007, 49, 65–73. [Google Scholar] [CrossRef]
- Bledsoe, J.C.; Breiger, D.; Breiger, M.; Shonka, S.; Ermoian, R.P.; Ojemann, J.G.; Werny, D.M.; Leary, S.E.S.; Geyer, J.R. Differential Trajectories of Neurocognitive Functioning in Females versus Males Following Treatment for Pediatric Brain Tumors. Neuro-Oncol. 2019, 21, 1310–1318. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale, 4th ed. 2012. Available online: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Wechsler-Adult-Intelligence-Scale-%7C-Fourth-Edition/p/100000392.html (accessed on 1 October 2022).
- Stern, R.A.; Singer, E.A.; Duke, L.M.; Singer, N.G.; Morey, C.E.; Daughtrey, E.W.; Kaplan, E. The Boston Qualitative Scoring System for the Rey-Osterrieth Complex Figure: Description and Interrater Reliability. Clin. Neuropsychol. 1994, 8, 309–322. [Google Scholar] [CrossRef]
- Meyers, J.E.; Meyers, K.R. Rey Complex Figure Test under Four Different Administration Procedures. Clin. Neuropsychol. 1995, 9, 63–67. [Google Scholar] [CrossRef]
- Benton, A.L. Differential Behavioral Effects in Frontal Lobe Disease. Neuropsychologia 1968, 6, 53–60. [Google Scholar] [CrossRef]
- Ruff, R.M.; Parker, S.B. Gender- and Age-Specific Changes in Motor Speed and Eye-Hand Coordination in Adults: Normative Values for the Finger Tapping and Grooved Pegboard Tests. Percept. Mot. Skills 1993, 76, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Green, J.L.; Knight, S.J.; McCarthy, M.; De Luca, C.R. Motor Functioning during and Following Treatment with Chemotherapy for Pediatric Acute Lymphoblastic Leukemia: Motor Skills in Children Treated for ALL. Pediatr. Blood Cancer 2013, 60, 1261–1266. [Google Scholar] [CrossRef]
- Peterson, C.C.; Johnson, C.E.; Ramirez, L.Y.; Huestis, S.; Pai, A.L.H.; Demaree, H.A.; Drotar, D. A Meta-Analysis of the Neuropsychological Sequelae of Chemotherapy-Only Treatment for Pediatric Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2008, 51, 99–104. [Google Scholar] [CrossRef]
- Reinders-Messelink, H.; Schoemaker, M.; Snijders, T.; Göeken, L.; van den Briel, M.; Bökkerink, J.; Kamps, W. Motor Performance of Children during Treatment for Acute Lymphoblastic Leukemia. Med. Pediatr. Oncol. 1999, 33, 545–550. [Google Scholar] [CrossRef]
- Lehtinen, S.S.; Huuskonen, U.E.; Harila-Saari, A.H.; Tolonen, U.; Vainionpää, L.K.; Lanning, B.M. Motor Nervous System Impairment Persists in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia: Motor Pathway Lesions in Patients with ALL. Cancer 2002, 94, 2466–2473. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, J.B.; Byun, D.H.; Son, S.M. Disruption of the Corticospinal Tract in Patients with Acute Lymphoblastic Leukemia: A Case Series. Children 2022, 9, 1223. [Google Scholar] [CrossRef]
- Goodenough, C.G.; Diouf, B.; Yang, W.; Sapkota, Y.; Finch, E.R.; Lu, L.; Partin, R.E.; Wogksch, M.D.; Hudson, M.M.; Robison, L.L.; et al. Association between CEP72 Genotype and Persistent Neuropathy in Survivors of Childhood Acute Lymphoblastic Leukemia. Leukemia 2022, 36, 1160–1163. [Google Scholar] [CrossRef]
- Cámara, S.; Fournier, M.C.; Cordero, P.; Melero, J.; Robles, F.; Esteso, B.; Vara, M.T.; Rodríguez, S.; Lassaletta, Á.; Budke, M. Neuropsychological Profile in Children with Posterior Fossa Tumors with or Without Postoperative Cerebellar Mutism Syndrome (CMS). Cerebellum 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Kristiansen, I.; Frykberg, G.E.; Höglund, A.; Sondell, A.; Strömberg, B.; Frisk, P. Motor Performance after Treatment of Pilocytic Astrocytoma in the Posterior Fossa in Childhood. Cancer Rep. 2022, 5, e1548. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.C.; Becker, N.; Apps, R.; Jones, M.W. Back to Front: Cerebellar Connections and Interactions with the Prefrontal Cortex. Front. Syst. Neurosci. 2014, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.C. Electrophysiological Mapping of Novel Prefrontal—Cerebellar Pathways. Front. Integr. Neurosci. 2009, 3, 18. [Google Scholar] [CrossRef]
- Krull, K.R.; Brinkman, T.M.; Li, C.; Armstrong, G.T.; Ness, K.K.; Srivastava, D.K.; Gurney, J.G.; Kimberg, C.; Krasin, M.J.; Pui, C.-H.; et al. Neurocognitive Outcomes Decades After Treatment for Childhood Acute Lymphoblastic Leukemia: A Report From the St Jude Lifetime Cohort Study. JCO 2013, 31, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Krull, K.R. Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated on Contemporary Treatment Protocols: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef]
- Steinlin, M. Neuropsychological Long-Term Sequelae after Posterior Fossa Tumour Resection during Childhood. Brain 2003, 126, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Manfredi, V.; Massimino, M.; Bardoni, A.; Borgatti, R.; Poggi, G. Cognitive Functioning of Pediatric Patients with Brain Tumor: An Investigation of the Role of Gender. Childs Nerv. Syst. 2018, 34, 2415–2423. [Google Scholar] [CrossRef]
- Tonning Olsson, I.; Perrin, S.; Lundgren, J.; Hjorth, L.; Johanson, A. Long-Term Cognitive Sequelae After Pediatric Brain Tumor Related to Medical Risk Factors, Age, and Sex. Pediatr. Neurol. 2014, 51, 515–521. [Google Scholar] [CrossRef]
- Dean, D.C.; O’Muircheartaigh, J.; Dirks, H.; Waskiewicz, N.; Walker, L.; Doernberg, E.; Piryatinsky, I.; Deoni, S.C.L. Characterizing Longitudinal White Matter Development during Early Childhood. Brain Struct. Funct. 2015, 220, 1921–1933. [Google Scholar] [CrossRef]


| Demographic Characteristics and Methods | Descriptive Statistics | Control | PBT | THL |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 11.36 (2.79) | 11.39 (2.97) | 10.56 (3.08) | |
| Range | 7–16 | 6–17 | 6–17 | |
| Sex | ||||
| Females | 310 | 72 | 168 | |
| Males | 336 | 65 | 199 | |
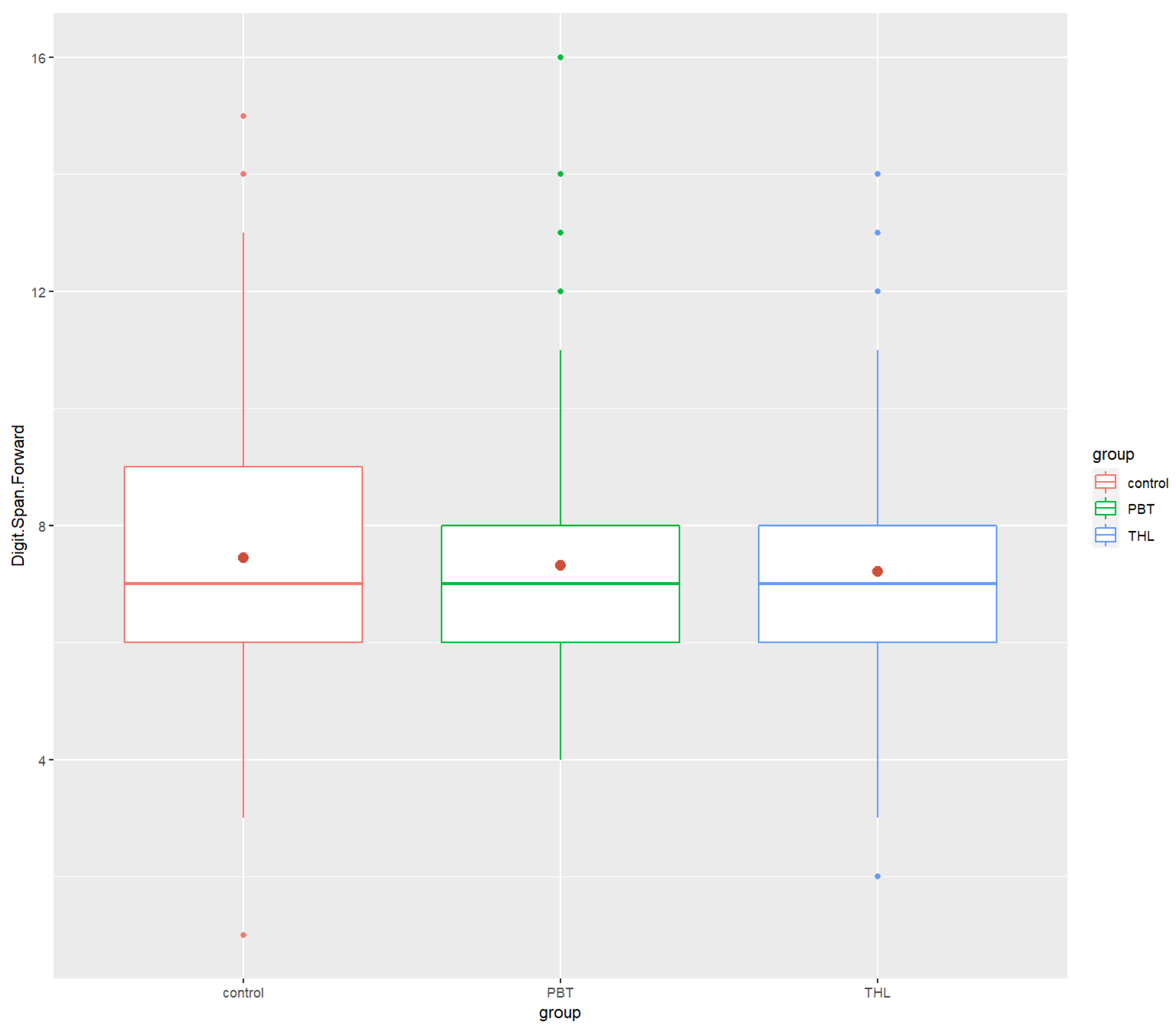
| Digit Span Forward | ||||
| Mean (SD) | 8.14 (1.80) | 7.94 (1.87) | 7.70 (1.87) | |
| Median | 8 | 8 | 8 | |
| Min/Max | 1.0/15 | 4.0/14 | 3.0/14 | |
| Digit Span Backward | ||||
| Mean (SD) | 6.75 (1.82) | 6.70 (2.12) | 6.73 (1.88) | |
| Median | 7.0 | 6 | 6 | |
| Min/Max | 3.0/14 | 4.0/16 | 2.0/13 | |
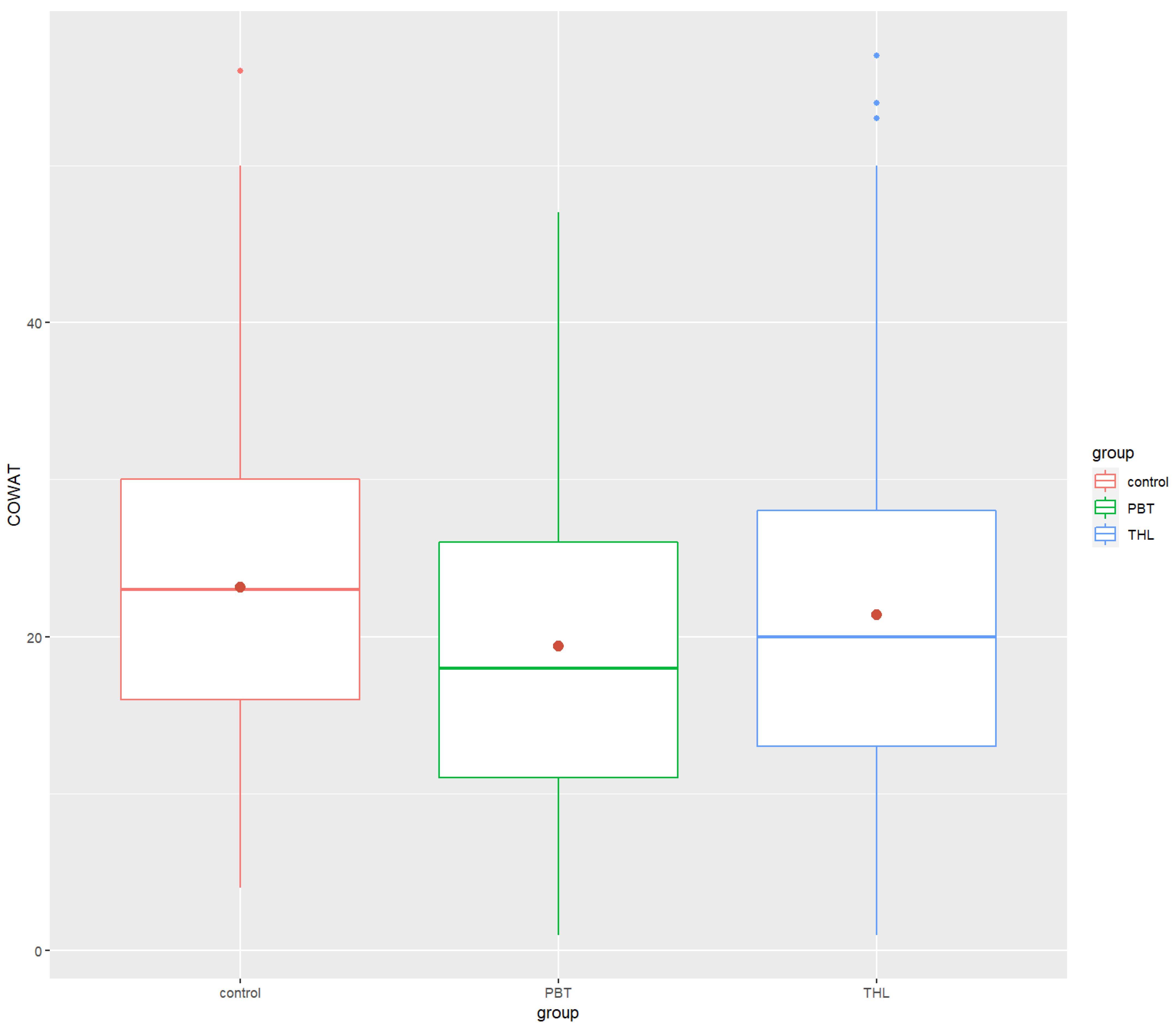
| COWAT | ||||
| Mean (SD) | 23.17 (9.39) | 19.39 (10.39) | 21.37 (11.13) | |
| Median | 23.0 | 18 | 20 | |
| Min/Max | 4.0/56 | 1.0/47 | 1.0/57 | |
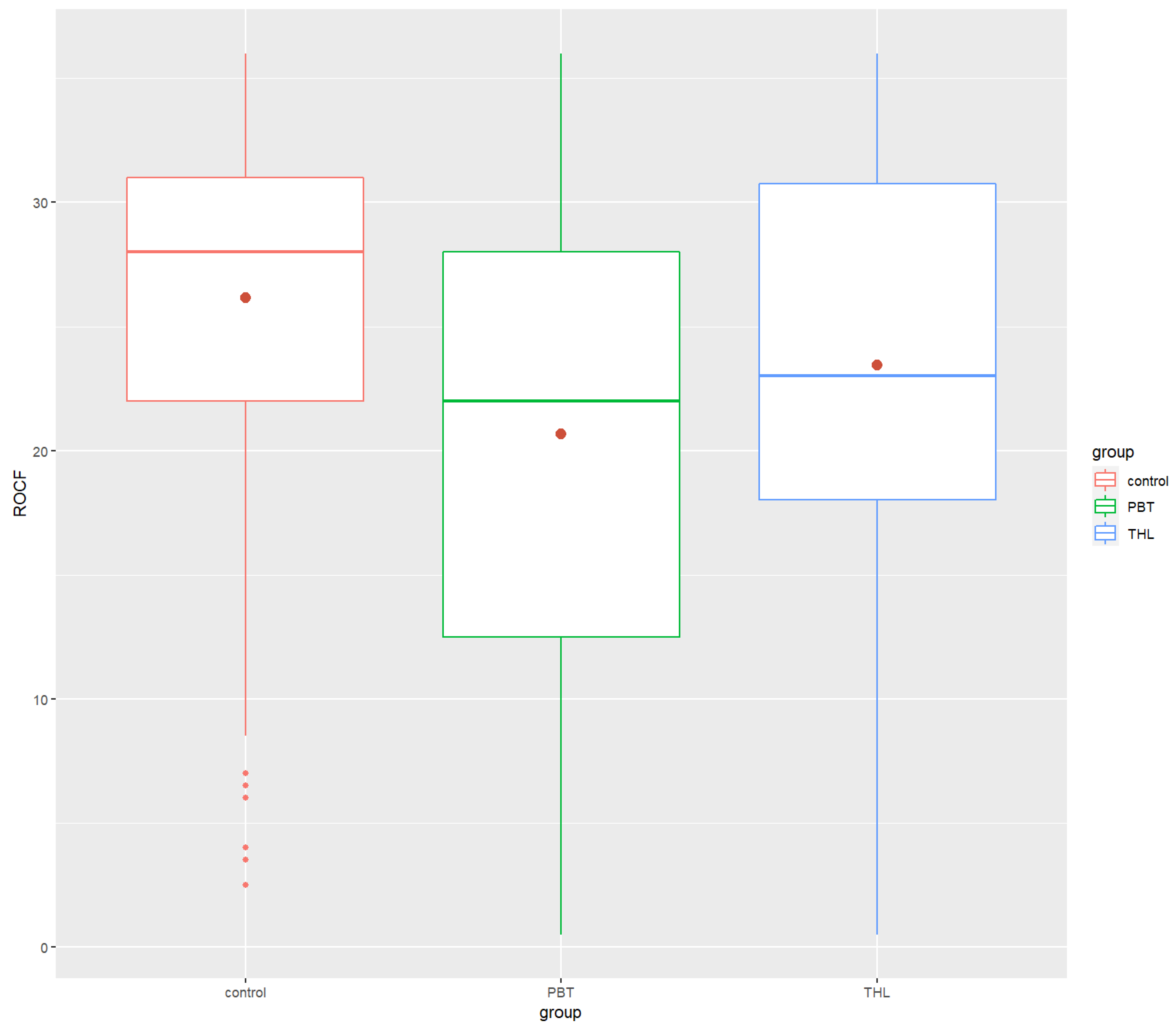
| ROCF | ||||
| Mean (SD) | 26.15 (6.60) | 20.66 (9.40) | 23.44 (7.97) | |
| Median | 28.0 | 22 | 23 | |
| Min/Max | 2.5/36 | 0.5/36 | 0.5/36 | |
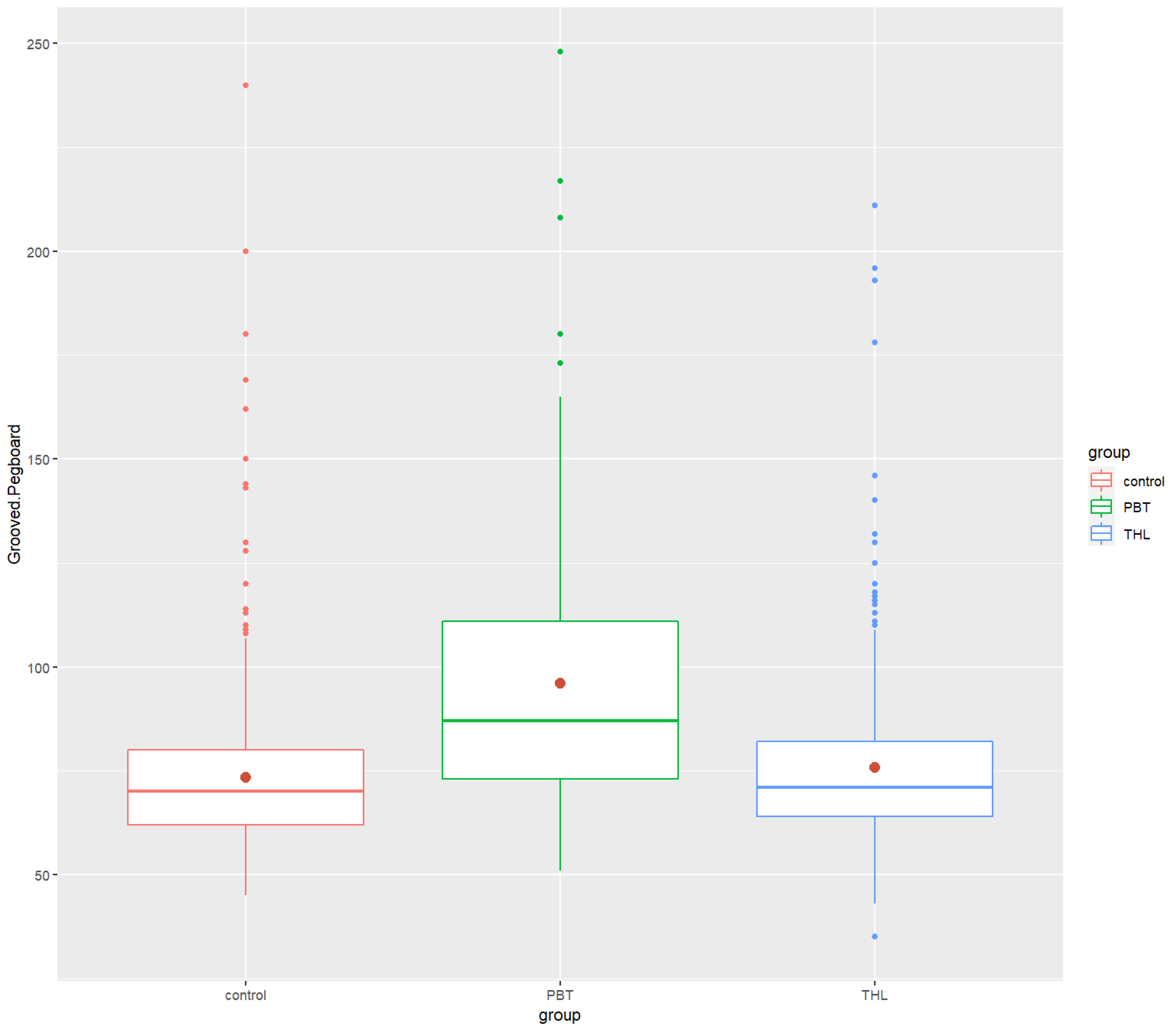
| Grooved Pegboard | ||||
| Mean (SD) | 73.39 (18.65) | 96.01 (32.87) | 75.79 (20.62) | |
| Median | 70.0 | 87 | 71 | |
| Min/Max | 45.0/240 | 51.0/248 | 35.0/211 |
| Neurocognitive Measures | Control | PBT | THL | Kruskal–Wallis One-Way ANOVA | Dunn Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | p-Value | η2 | Control—PBT | Control—THL | PBT—THL | |
| Digit Span Forward | 8.14 (1.80) | 8 | 7.94 (1.87) | 8 | 7.70 (1.87) | 8 | 0.0006 *** | 0.0111 | 0.349 (ns) | 0.000474 *** | 0.965 (ns) |
| Digit Span Backward | 6.75 (1.82) | 7 | 6.70 (2.12) | 6 | 6.73 (1.88) | 6 | 0.653 (ns) | −0.00100 | 0.374 (ns) | 0.642 (ns) | 0.596 (ns) |
| COWAT | 23.17 (9.39) | 23 | 19.39 (10.39) | 18 | 21.37 (11.13) | 20 | 0.000004 *** | 0.0199 | 0.0000534 **** | 0.00128 ** | 0.250 (ns) |
| ROCF | 26.15 (6.60) | 28 | 20.66 (9.40) | 22 | 23.44 (7.97) | 23 | 1.59 × 10−12 *** | 0.0456 | 5.65 × 10 −10 **** | 6.24 × 10−7 **** | 2.84 × 10−2 * |
| Grooved Pegboard | 73.39 (18.65) | 70 | 96.01 (32.87) | 87 | 75.79 (20.62) | 71 | 8.36 × 10−20 *** | 0.0749 | 3.08 × 10 −20 **** | 3.90 × 10−1 (ns) | 2.18 × 10−14 **** |
| Methods and Descriptive Statistics | Control | PBT | THL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | p-Value | r | Females | Males | p-Value | r | Females | Males | p-Value | r | ||
| Digit Span Forward | Mean (SD) | 8.20 (1.84) | 8.07 (1.75) | 0.324 (ns) | 0.0388 | 7.76 (1.67) | 8.14 (2.08) | 0.406 (ns) | 0.713 | 7.76 (1.78) | 7.66 (1.96) | 0.327 (ns) | 0.0512 |
| Median | 8 | 8 | 7 | 8 | 8 | 7 | |||||||
| Digit Span Backward | Mean (SD) | 6.80 (1.85) | 6.7 (1.79) | 0.563 (ns) | 0.0227 | 6.49 (1.78) | 6.94 (2.44) | 0.555 (ns) | 0.0506 | 6.67 (1.89) | 6.78 (1.87) | 0.674 (ns) | 0.0220 |
| Median | 7 | 7 | 6 | 6 | 6 | 6 | |||||||
| COWAT | Mean (SD) | 24.3 (10.1) | 21.9 (8.44) | 0.003 ** | 0.115 | 20.4 (10.9) | 18.3 (9.82) | 0.156 (ns) | 0.121 | 20.8 (11.0) | 21.9 (11.2) | 0.387 (ns) | 0.0452 |
| Median | 24 | 22 | 19.5 | 16 | 19 | 20 | |||||||
| ROCF | Mean (SD) | 27.0 (6.07) | 25.3 (7.03) | 0.005 ** | 0.112 | 20.7 (9.72) | 20.6 (9.1) | 0.855 (ns) | 0.0158 | 22.5 (8.29) | 24.2 (7.64) | 0.059 (ns) | 0.0988 |
| Median | 28 | 27 | 22.5 | 22 | 23 | 24 | |||||||
| Grooved Pegboard | Mean (SD) | 72.5 (19.1) | 74.3 (18.2) | 0.117 (ns) | 0.0617 | 91.5 (31.8) | 101 (33.5) | 0.043 * | 0.173 | 77.3 (20.8) | 74.5 (20.4) | 0.062 (ns) | 0.0974 |
| Median | 70 | 71 | 84 | 92 | 72 | 69 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chipeeva, N.; Deviaterikova, A.; Glebova, E.; Romanova, E.; Karelin, A.; Kasatkin, V. Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children. Cancers 2022, 14, 5982. https://doi.org/10.3390/cancers14235982
Chipeeva N, Deviaterikova A, Glebova E, Romanova E, Karelin A, Kasatkin V. Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children. Cancers. 2022; 14(23):5982. https://doi.org/10.3390/cancers14235982
Chicago/Turabian StyleChipeeva, Nadezda, Alena Deviaterikova, Elena Glebova, Elizaveta Romanova, Alexander Karelin, and Vladimir Kasatkin. 2022. "Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children" Cancers 14, no. 23: 5982. https://doi.org/10.3390/cancers14235982
APA StyleChipeeva, N., Deviaterikova, A., Glebova, E., Romanova, E., Karelin, A., & Kasatkin, V. (2022). Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children. Cancers, 14(23), 5982. https://doi.org/10.3390/cancers14235982






