Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer
Abstract
Simple Summary
Abstract
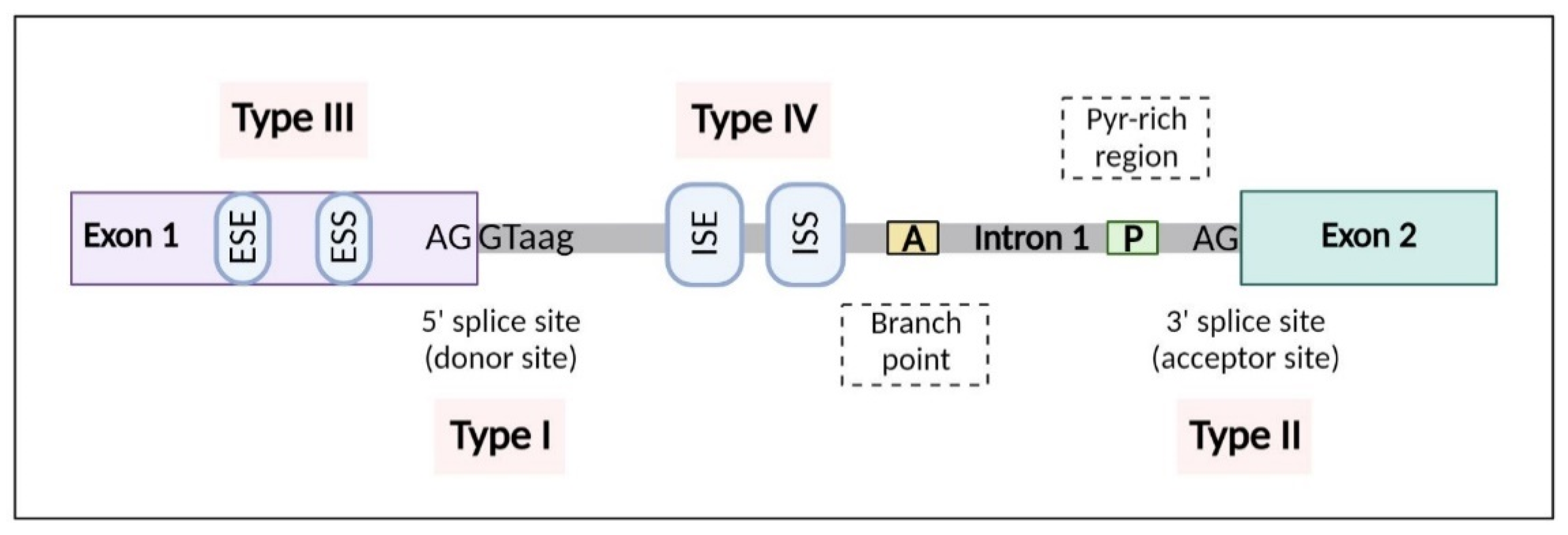
1. Alternative Splicing
2. Cancer Predisposition Genes
3. Pediatric Solid Tumors
3.1. CNS Tumors
3.1.1. Medulloblastoma
3.1.2. Gliomas
Low-Grade Gliomas (LGGs)
- a.
- Astrocytoma
- b.
- Ependymoma
- c.
- Optic glioma
High-Grade Gliomas (HGGs)
3.1.3. Other CNS Tumors
Pinealoblastoma
Atypical Teratoid/Rhabdoid Tumors
3.2. Sarcomas
3.2.1. Osteosarcoma
3.2.2. Ewing Sarcoma
3.2.3. Rhabdomyosarcoma
3.3. Neuroblastoma
3.4. Retinoblastoma
3.5. Wilms Tumors
4. Therapeutic Targeting of Splicing in Cancer
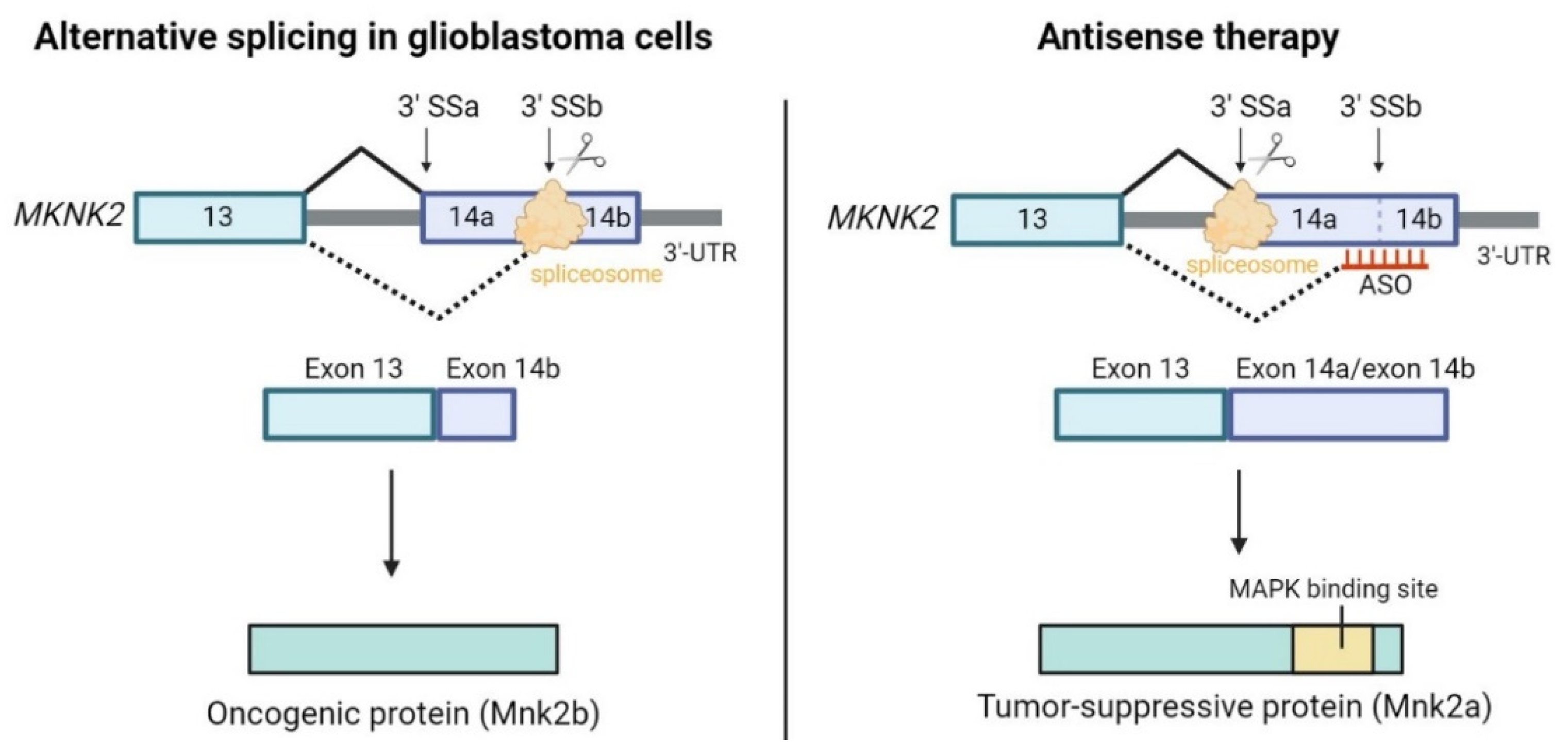
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Modrek, B.; Lee, C. A Genomic View of Alternative Splicing. Nat. Genet. 2002, 30, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Aifantis, I. RNA Splicing and Cancer. Trends Cancer 2020, 6, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Kahles, A.; Lehmann, K.-V.; Toussaint, N.C.; Hüser, M.; Stark, S.G.; Sachsenberg, T.; Stegle, O.; Kohlbacher, O.; Sander, C.; Rätsch, G.; et al. Comprehensive Analysis of Alternative Splicing Across Tumors from 8705 Patients. Cancer Cell 2018, 34, 211–224.e6. [Google Scholar] [CrossRef] [PubMed]
- Frankiw, L.; Baltimore, D.; Li, G. Alternative MRNA Splicing in Cancer Immunotherapy. Nat. Rev. Immunol. 2019, 19, 675–687. [Google Scholar] [CrossRef]
- Gimeno-Valiente, F.; López-Rodas, G.; Castillo, J.; Franco, L. Alternative Splicing, Epigenetic Modifications and Cancer: A Dangerous Triangle, or a Hopeful One? Cancers 2022, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-D.; Lee, N.H. Aberrant RNA Splicing in Cancer and Drug Resistance. Cancers 2018, 10, 458. [Google Scholar] [CrossRef]
- Menghi, F.; Jacques, T.S.; Barenco, M.; Schwalbe, E.C.; Clifford, S.C.; Hubank, M.; Ham, J. Genome-Wide Analysis of Alternative Splicing in Medulloblastoma Identifies Splicing Patterns Characteristic of Normal Cerebellar Development. Cancer Res. 2011, 71, 2045–2055. [Google Scholar] [CrossRef]
- Wei, R.; Yao, Y.; Yang, W.; Zheng, C.-H.; Zhao, M.; Xia, J. DbCPG: A Web Resource for Cancer Predisposition Genes. Oncotarget 2016, 7, 37803–37811. [Google Scholar] [CrossRef]
- Johnson, L.-M.; Hamilton, K.V.; Valdez, J.M.; Knapp, E.; Baker, J.N.; Nichols, K.E. Ethical Considerations Surrounding Germline Next-Generation Sequencing of Children with Cancer. Expert Rev. Mol. Diagn. 2017, 17, 523–534. [Google Scholar] [CrossRef]
- Plon, S.E.; Lupo, P.J. Genetic Predisposition to Childhood Cancer in the Genomic Era. Annu. Rev. Genom. Hum. Genet. 2019, 20, 241–263. [Google Scholar] [CrossRef]
- Nix, P.; Mundt, E.; Coffee, B.; Goossen, E.; Warf, B.M.; Brown, K.; Bowles, K.; Roa, B. Interpretation of BRCA2 Splicing Variants: A Case Series of Challenging Variant Interpretations and the Importance of Functional RNA Analysis. Fam. Cancer 2021, 21, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, R.G.; Cao, S.; Gao, Q.; Wendl, M.C.; Vo, N.S.; Reynolds, S.M.; Zhao, Y.; Climente-González, H.; Chai, S.; Wang, F.; et al. Systematic Analysis of Splice-Site-Creating Mutations in Cancer. Cell Rep. 2018, 23, 270–281.e3. [Google Scholar] [CrossRef] [PubMed]
- Rhine, C.L.; Cygan, K.J.; Soemedi, R.; Maguire, S.; Murray, M.F.; Monaghan, S.F.; Fairbrother, W.G. Hereditary Cancer Genes Are Highly Susceptible to Splicing Mutations. PLoS Genet. 2018, 14, e1007231. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Gunasekaran, P.; Leung, K.; Bindal, N.; Boutselakis, H.; Ding, M.; Bamford, S.; Cole, C.; Ward, S.; et al. COSMIC: Exploring the World’s Knowledge of Somatic Mutations in Human Cancer. Nucleic Acids Res. 2015, 43, D805–D811. [Google Scholar] [CrossRef] [PubMed]
- Waszak, S.M.; Northcott, P.A.; Buchhalter, I.; Robinson, G.W.; Sutter, C.; Groebner, S.; Grund, K.B.; Brugières, L.; Jones, D.T.W.; Pajtler, K.W.; et al. Spectrum and Prevalence of Genetic Predisposition in Medulloblastoma: A Retrospective Genetic Study and Prospective Validation in a Clinical Trial Cohort. Lancet Oncol. 2018, 19, 785–798. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Liu, B.; Parsons, R.E.; Papadopoulos, N.; Jen, J.; Powell, S.M.; Krush, A.J.; Berk, T.; Cohen, Z.; Tetu, B.; et al. The Molecular Basis of Turcot’s Syndrome. N. Engl. J. Med. 1995, 332, 839–847. [Google Scholar] [CrossRef]
- The St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. The Genomic Landscape of Diffuse Intrinsic Pontine Glioma and Pediatric Non-Brainstem High-Grade Glioma. Nat. Genet. 2014, 46, 444–450. [Google Scholar] [CrossRef]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients with Osteosarcoma. JAMA Oncol. 2020, 6, 724. [Google Scholar] [CrossRef]
- Brohl, A.S.; Patidar, R.; Turner, C.E.; Wen, X.; Song, Y.K.; Wei, J.S.; Calzone, K.A.; Khan, J. Frequent Inactivating Germline Mutations in DNA Repair Genes in Patients with Ewing Sarcoma. Genet. Med. 2017, 19, 955–958. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The Landscape of Genomic Alterations across Childhood Cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Muskens, I.S.; Zhang, C.; de Smith, A.J.; Biegel, J.A.; Walsh, K.M.; Wiemels, J.L. Germline Genetic Landscape of Pediatric Central Nervous System Tumors. Neuro-Oncol. 2019, 21, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Listernick, R.; Charrow, J.; Greenwald, M.; Mets, M. Natural History of Optic Pathway Tumors in Children with Neurofibromatosis Type 1: A Longitudinal Study. J. Pediatr. 1994, 125, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, J.; Nijmeijer, R.; Brouwer, O.F.; Boon, M.; Fock, A.; Hoving, E.W.; Meijer, L.; den Dunnen, W.F.A.; de Bont, E.S.J.M. Neurofibromatosis Type 1 Associated Low Grade Gliomas: A Comparison with Sporadic Low Grade Gliomas. Crit. Rev. Oncol. /Hematol. 2016, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, M.; Praticò, A.D.; Serra, A.; Maiolino, L.; Cocuzza, S.; Di Mauro, P.; Licciardello, L.; Milone, P.; Privitera, G.; Belfiore, G.; et al. Childhood Neurofibromatosis Type 2 (NF2) and Related Disorders: From Bench to Bedside and Biologically Targeted Therapies. Acta Otorhinolaryngol. Ital. 2016, 36, 345–367. [Google Scholar] [CrossRef]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting from Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef]
- Brichard, B.; Heusterspreute, M.; De Potter, P.; Chantrain, C.; Vermylen, C.; Sibille, C.; Gala, J.-L. Unilateral Retinoblastoma, Lack of Familial History and Older Age Does Not Exclude Germline RB1 Gene Mutation. Eur. J. Cancer 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Broaddus, E.; Topham, A.; Singh, A.D. Incidence of Retinoblastoma in the USA: 1975–2004. Br. J. Ophthalmol. 2009, 93, 21–23. [Google Scholar] [CrossRef]
- Rubenfeld, M.; Abramson, D.H.; Ellsworth, R.M.; Kitchin, F.D. Unilateral vs. Bilateral Retinoblastoma. Ophthalmology 1986, 93, 1016–1019. [Google Scholar] [CrossRef]
- Plowman, P.N.; Pizer, B.; Kingston, J.E. Pineal Parenchymal Tumours: II. Clin. Oncol. 2004, 16, 244–247. [Google Scholar] [CrossRef]
- Dome, J.S.; Rodriguez-Galindo, C.; Spunt, S.L.; Santana, V.M. Pediatric Solid Tumors. In Abeloff’s Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1703–1747.e11. ISBN 978-0-323-47674-4. [Google Scholar]
- Dubuc, A.M.; Northcott, P.A.; Mack, S.; Witt, H.; Pfister, S.; Taylor, M.D. The Genetics of Pediatric Brain Tumors. Curr. Neurol. Neurosci. Rep. 2010, 10, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative Genomic Analysis of Medulloblastoma Identifies a Molecular Subgroup That Drives Poor Clinical Outcome. J. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Koster, J.; Bunt, J.; Hasselt, N.E.; Lakeman, A.; van Sluis, P.; Troost, D.; Meeteren, N.S.; Caron, H.N.; Cloos, J.; et al. Integrated Genomics Identifies Five Medulloblastoma Subtypes with Distinct Genetic Profiles, Pathway Signatures and Clinicopathological Features. PLoS ONE 2008, 3, e3088. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Hielscher, T.; Dubuc, A.; Mack, S.; Shih, D.; Remke, M.; Al-Halabi, H.; Albrecht, S.; Jabado, N.; Eberhart, C.G.; et al. Pediatric and Adult Sonic Hedgehog Medulloblastomas Are Clinically and Molecularly Distinct. Acta Neuropathol. 2011, 122, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.F.; Hatten, M.E. Cerebellum. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 94, pp. 235–282. ISBN 978-0-12-380916-2. [Google Scholar]
- Farini, D.; Marazziti, D.; Geloso, M.C.; Sette, C. Transcriptome Programs Involved in the Development and Structure of the Cerebellum. Cell. Mol. Life Sci. 2021, 78, 6431–6451. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, A.M.; Morrissy, A.S.; Kloosterhof, N.K.; Northcott, P.A.; Yu, E.P.Y.; Shih, D.; Peacock, J.; Grajkowska, W.; van Meter, T.; Eberhart, C.G.; et al. Subgroup-Specific Alternative Splicing in Medulloblastoma. Acta Neuropathol. 2012, 123, 485–499. [Google Scholar] [CrossRef][Green Version]
- Suzuki, H.; Kumar, S.A.; Shuai, S.; Diaz-Navarro, A.; Gutierrez-Fernandez, A.; De Antonellis, P.; Cavalli, F.M.G.; Juraschka, K.; Farooq, H.; Shibahara, I.; et al. Recurrent Noncoding U1 SnRNA Mutations Drive Cryptic Splicing in SHH Medulloblastoma. Nature 2019, 574, 707–711. [Google Scholar] [CrossRef]
- Waszak, S.M.; Robinson, G.W.; Gudenas, B.L.; Smith, K.S.; Forget, A.; Kojic, M.; Garcia-Lopez, J.; Hadley, J.; Hamilton, K.V.; Indersie, E.; et al. Germline Elongator Mutations in Sonic Hedgehog Medulloblastoma. Nature 2020, 580, 396–401. [Google Scholar] [CrossRef]
- Ilencikova, D.; Sejnova, D.; Jindrova, J.; Babal, P. High-Grade Brain Tumors in Siblings with Biallelic MSH6 Mutations: Biallelic MSH6 Mutations and Malignancies. Pediatr. Blood Cancer 2011, 57, 1067–1070. [Google Scholar] [CrossRef]
- Fujii, M.; Noguchi, K.; Urade, M.; Muraki, Y.; Moridera, K.; Kishimoto, H.; Hashimoto-Tamaoki, T.; Nakano, Y. Novel PTCH1 Mutations in Japanese Nevoid Basal Cell Carcinoma Syndrome Patients: Two Familial and Three Sporadic Cases Including the First Japanese Patient with Medulloblastoma. J. Hum. Genet. 2011, 56, 277–283. [Google Scholar] [CrossRef]
- Pastorino, L.; Ghiorzo, P.; Nasti, S.; Battistuzzi, L.; Cusano, R.; Marzocchi, C.; Garrè, M.L.; Clementi, M.; Scarrà, G.B. Identification of a SUFU Germline Mutation in a Family with Gorlin Syndrome. Am. J. Med. Genet. 2009, 149A, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.J.; Walsh, M.; Rajagopalan, B.; Finlay, M.; Trainer, A.H.; Bonnet, F.; Sevenet, N.; Winship, I.M. Mutations in SUFU and PTCH1 Genes May Cause Different Cutaneous Cancer Predisposition Syndromes: Similar, but Not the Same. Fam. Cancer 2018, 17, 601–606. [Google Scholar] [CrossRef]
- Brugières, L.; Remenieras, A.; Pierron, G.; Varlet, P.; Forget, S.; Byrde, V.; Bombled, J.; Puget, S.; Caron, O.; Dufour, C.; et al. High Frequency of Germline SUFU Mutations in Children With Desmoplastic/Nodular Medulloblastoma Younger Than 3 Years of Age. J. Clin. Oncol. 2012, 30, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Liu, L.; Raffel, C.; Hui, C.; Mainprize, T.G.; Zhang, X.; Agatep, R.; Chiappa, S.; Gao, L.; Lowrance, A.; et al. Mutations in SUFU Predispose to Medulloblastoma. Nat. Genet. 2002, 31, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Chompret, A.; Brugières, L.; Ronsin, M.; Gardes, M.; Dessarps-Freichey, F.; Abel, A.; Hua, D.; Ligot, L.; Dondon, M.G.; Bressac-de Paillerets, B.; et al. P53 Germline Mutations in Childhood Cancers and Cancer Risk for Carrier Individuals. Br. J. Cancer 2000, 82, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Venkataramany, A.S.; Schieffer, K.M.; Lee, K.; Cottrell, C.E.; Wang, P.Y.; Mardis, E.R.; Cripe, T.P.; Chandler, D.S. Alternative RNA Splicing Defects in Pediatric Cancers: New Insights in Tumorigenesis and Potential Therapeutic Vulnerabilities. Ann. Oncol. 2022, 33, 578–592. [Google Scholar] [CrossRef]
- D’Angelo, F.; Ceccarelli, M.; Tala; Garofano, L.; Zhang, J.; Frattini, V.; Caruso, F.P.; Lewis, G.; Alfaro, K.D.; Bauchet, L.; et al. The Molecular Landscape of Glioma in Patients with Neurofibromatosis 1. Nat. Med. 2019, 25, 176–187. [Google Scholar] [CrossRef]
- Nemethova, M.; Bolcekova, A.; Ilencikova, D.; Durovcikova, D.; Hlinkova, K.; Hlavata, A.; Kovacs, L.; Kadasi, L.; Zatkova, A. Thirty-Nine Novel Neurofibromatosis 1 ( NF1) Gene Mutations Identified in Slovak Patients: NF1 in Slovakia. Ann. Hum. Genet. 2013, 77, 364–379. [Google Scholar] [CrossRef]
- Kline, C.N.; Joseph, N.M.; Grenert, J.P.; van Ziffle, J.; Talevich, E.; Onodera, C.; Aboian, M.; Cha, S.; Raleigh, D.R.; Braunstein, S.; et al. Targeted Next-Generation Sequencing of Pediatric Neuro-Oncology Patients Improves Diagnosis, Identifies Pathogenic Germline Mutations, and Directs Targeted Therapy. Neuro-Oncol. 2016, 19, 699–709. [Google Scholar] [CrossRef]
- Kline, C.N.; Joseph, N.M.; Grenert, J.P.; van Ziffle, J.; Yeh, I.; Bastian, B.C.; Mueller, S.; Solomon, D.A. Inactivating MUTYH Germline Mutations in Pediatric Patients with High-Grade Midline Gliomas. Neuro Oncol. 2016, 18, 752–753. [Google Scholar] [CrossRef]
- Kordes, U.; Gesk, S.; Frühwald, M.C.; Graf, N.; Leuschner, I.; Hasselblatt, M.; Jeibmann, A.; Oyen, F.; Peters, O.; Pietsch, T.; et al. Clinical and Molecular Features in Patients with Atypical Teratoid Rhabdoid Tumor or Malignant Rhabdoid Tumor: Molecular Features in Patients with ATRT. Genes Chromosom. Cancer 2010, 49, 176–181. [Google Scholar] [CrossRef] [PubMed]
- de Kock, L.; Sabbaghian, N.; Druker, H.; Weber, E.; Hamel, N.; Miller, S.; Choong, C.S.; Gottardo, N.G.; Kees, U.R.; Rednam, S.P.; et al. Germ-Line and Somatic DICER1 Mutations in Pineoblastoma. Acta Neuropathol. 2014, 128, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Jolly, K.W.; Malkin, D.; Douglass, E.C.; Brown, T.F.; Sinclair, A.E.; Look, A.T. Splice-Site Mutation of the P53 Gene in a Family with Hereditary Breast-Ovarian Cancer. Oncogene 1994, 9, 97–102. [Google Scholar] [PubMed]
- Hottinger, A.F.; Khakoo, Y. Neurooncology of Familial Cancer Syndromes. J. Child Neurol. 2009, 24, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Beetz, C.; Williams, S.G.; Bhaskar, S.S.; O’Sullivan, J.; Anderson, B.; Daly, S.B.; Urquhart, J.E.; Bholah, Z.; Oudit, D.; et al. Germline Mutations in SUFU Cause Gorlin Syndrome–Associated Childhood Medulloblastoma and Redefine the Risk Associated With PTCH1 Mutations. J. Clin. Oncol. 2014, 32, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.H.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-Specific Prognostic Implications of TP53 Mutation in Medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Jones, D.T.W.; Jäger, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Mastronuzzi, A.; Po, A.; Carai, A.; Alfano, V.; Serra, A.; Colafati, G.S.; Strocchio, L.; Antonelli, M.; Buttarelli, F.R.; et al. Characterization of Medulloblastoma in Fanconi Anemia: A Novel Mutation in the BRCA2 Gene and SHH Molecular Subgroup. Biomark. Res. 2015, 3, 13. [Google Scholar] [CrossRef]
- Xu, J.; Margol, A.S.; Shukla, A.; Ren, X.; Finlay, J.L.; Krieger, M.D.; Gilles, F.H.; Couch, F.J.; Aziz, M.; Fung, E.T.; et al. Disseminated Medulloblastoma in a Child with Germline BRCA2 6174delT Mutation and without Fanconi Anemia. Front. Oncol. 2015, 5, 191. [Google Scholar] [CrossRef]
- Amlashi, S.F.A.; Riffaud, L.; Brassier, G.; Morandi, X. Nevoid Basal Cell Carcinoma Syndrome: Relation with Desmoplastic Medulloblastoma in Infancy: A Population-Based Study and Review of the Literature. Cancer 2003, 98, 618–624. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F.; Mulvihill, J.J.; Blattner, W.A.; Dreyfus, M.G.; Tucker, M.A.; Miller, R.W. A Cancer Family Syndrome in Twenty-Four Kindreds. Cancer Res. 1988, 48, 5358–5362. [Google Scholar] [PubMed]
- Fromentel, C.C.D.; Soussi, T. TP53 Tumor Suppressor Gene: A Model for Investigating Human Mutagenesis. Genes Chromosom. Cancer 1992, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kouidou, S.; Malousi, A.; Maglaveras, N. Li-Fraumeni and Li-Fraumeni-like Syndrome Mutations in P53 Are Associated with Exonic Methylation and Splicing Regulatory Elements: LF/LFL SYNDROME MUTATIONS IN P53. Mol. Carcinog. 2009, 48, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Warneford, S.G.; Witton, L.J.; Townsend, M.L.; Rowe, P.B.; Reddel, R.R.; Dalla-Pozza, L.; Symonds, G. Germ-Line Splicing Mutation of the P53 Gene in a Cancer-Prone Family. Cell Growth Differ. 1992, 3, 839–846. [Google Scholar]
- Felix, C.A.; Strauss, E.A.; D’Amico, D.; Tsokos, M.; Winter, S.; Mitsudomi, T.; Nau, M.M.; Brown, D.L.; Leahey, A.M.; Horowitz, M.E. A Novel Germline P53 Splicing Mutation in a Pediatric Patient with a Second Malignant Neoplasm. Oncogene 1993, 8, 1203–1210. [Google Scholar]
- Haque, M.M.; Kowtal, P.; Sarin, R. Identification and Characterization of TP53 Gene Allele Dropout in Li-Fraumeni Syndrome and Oral Cancer Cohorts. Sci. Rep. 2018, 8, 11705. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The Whole-Genome Landscape of Medulloblastoma Subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef]
- Hart, R.M.; Kimler, B.F.; Evans, R.G.; Park, C.H. Radiotherapeutic Management of Medulloblastoma in a Pediatric Patient with Ataxia Telangiectasia. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1237–1240. [Google Scholar] [CrossRef]
- Sturm, D.; Pfister, S.M.; Jones, D.T.W. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J. Clin. Oncol. 2017, 35, 2370–2377. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015, 17, iv1–iv62. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncol. 2015, 16, x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, L.; Taniguchi, K.; Wang, X.; Cunningham, J.M.; McDonnell, S.K.; Qian, C.; Marks, A.F.; Slager, S.L.; Peterson, B.J.; et al. Mutations in CHEK2 Associated with Prostate Cancer Risk. Am. J. Hum. Genet. 2003, 72, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Partap, S. Pediatric Ependymoma. J. Child Neurol. 2016, 31, 1354–1366. [Google Scholar] [CrossRef]
- Foss-Skiftesvik, J.; Stoltze, U.K.; van Overeem Hansen, T.; Ahlborn, L.B.; Sørensen, E.; Ostrowski, S.R.; Kullegaard, S.M.A.; Laspiur, A.O.; Melchior, L.C.; Scheie, D.; et al. Redefining Germline Predisposition in Children with Molecularly Characterized Ependymoma: A Population-Based 20-Year Cohort. Acta Neuropathol. Commun. 2022, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Baser, M.E.; Kuramoto, L.; Joe, H.; Friedman, J.M.; Wallace, A.J.; Gillespie, J.E.; Ramsden, R.T.; Evans, D.G.R. Genotype-Phenotype Correlations for Nervous System Tumors in Neurofibromatosis 2: A Population-Based Study. Am. J. Hum. Genet. 2004, 75, 231–239. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Molecular Insights into NF2 /Merlin Tumor Suppressor Function. FEBS Lett. 2014, 588, 2743–2752. [Google Scholar] [CrossRef]
- Painter, S.L.; Sipkova, Z.; Emmanouil, B.; Halliday, D.; Parry, A.; Elston, J.S. Neurofibromatosis Type 2–Related Eye Disease Correlated With Genetic Severity Type. J. Neuro-Ophthalmol. 2019, 39, 44–49. [Google Scholar] [CrossRef]
- Stuckert, A.; Bertrand, K.C.; Wang, P.; Smith, A.; Mack, S.C. Weighing Ependymoma as an Epigenetic Disease. J. Neurooncol. 2020, 150, 57–61. [Google Scholar] [CrossRef]
- Jain, S.U.; Do, T.J.; Lund, P.J.; Rashoff, A.Q.; Diehl, K.L.; Cieslik, M.; Bajic, A.; Juretic, N.; Deshmukh, S.; Venneti, S.; et al. PFA Ependymoma-Associated Protein EZHIP Inhibits PRC2 Activity through a H3 K27M-like Mechanism. Nat. Commun. 2019, 10, 2146. [Google Scholar] [CrossRef]
- Uusitalo, E.; Rantanen, M.; Kallionpää, R.A.; Pöyhönen, M.; Leppävirta, J.; Ylä-Outinen, H.; Riccardi, V.M.; Pukkala, E.; Pitkäniemi, J.; Peltonen, S.; et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J. Clin. Oncol. 2016, 34, 1978–1986. [Google Scholar] [CrossRef]
- Seminog, O.O.; Goldacre, M.J. Risk of Benign Tumours of Nervous System, and of Malignant Neoplasms, in People with Neurofibromatosis: Population-Based Record-Linkage Study. Br. J. Cancer 2013, 108, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, G.; Lafforgue, M.-P.; Lion-François, L.; Kemlin, I.; Rodriguez, D.; Castelnau, P.; Carneiro, M.; Meyer, P.; Rivier, F.; Barbarot, S.; et al. Systematic MRI in NF1 Children under Six Years of Age for the Diagnosis of Optic Pathway Gliomas. Study and Outcome of a French Cohort. Eur. J. Paediatr. Neurol. 2016, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Toonen, J.A.; Cimino, P.J.; Gianino, S.M.; Gutmann, D.H. Akt- or MEK-Mediated MTOR Inhibition Suppresses Nf1 Optic Glioma Growth. Neuro-Oncol. 2015, 17, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.; Raleigh, D.; Bindra, R.; Mueller, S.; Haas-Kogan, D. Pediatric High-Grade Glioma: Current Molecular Landscape and Therapeutic Approaches. J. Neurooncol. 2017, 134, 541–549. [Google Scholar] [CrossRef]
- Wimmer, K.; Kratz, C.P.; Vasen, H.F.A.; Caron, O.; Colas, C.; Entz-Werle, N.; Gerdes, A.-M.; Goldberg, Y.; Ilencikova, D.; Muleris, M.; et al. Diagnostic Criteria for Constitutional Mismatch Repair Deficiency Syndrome: Suggestions of the European Consortium ‘Care for CMMRD’ (C4CMMRD). J. Med. Genet. 2014, 51, 355–365. [Google Scholar] [CrossRef]
- Wimmer, K.; Etzler, J. Constitutional Mismatch Repair-Deficiency Syndrome: Have We so Far Seen Only the Tip of an Iceberg? Hum. Genet. 2008, 124, 105–122. [Google Scholar] [CrossRef]
- Hill, D.A.; Ivanovich, J.; Priest, J.R.; Gurnett, C.A.; Dehner, L.P.; Desruisseau, D.; Jarzembowski, J.A.; Wikenheiser-Brokamp, K.A.; Suarez, B.K.; Whelan, A.J.; et al. DICER1 Mutations in Familial Pleuropulmonary Blastoma. Science 2009, 325, 965. [Google Scholar] [CrossRef]
- Smith, M.J.; Wallace, A.J.; Bowers, N.L.; Eaton, H.; Evans, D.G.R. SMARCB1 Mutations in Schwannomatosis and Genotype Correlations with Rhabdoid Tumors. Cancer Genet. 2014, 207, 373–378. [Google Scholar] [CrossRef]
- Taylor, M.D.; Gokgoz, N.; Andrulis, I.L.; Mainprize, T.G.; Drake, J.M.; Rutka, J.T. Familial Posterior Fossa Brain Tumors of Infancy Secondary to Germline Mutation of the HSNF5 Gene. Am. J. Hum. Genet. 2000, 66, 1403–1406. [Google Scholar] [CrossRef]
- Sabatella, M.; Mantere, T.; Waanders, E.; Neveling, K.; Mensenkamp, A.R.; Dijk, F.; Hehir-Kwa, J.Y.; Derks, R.; Kwint, M.; O’Gorman, L.; et al. Optical Genome Mapping Identifies a Germline Retrotransposon Insertion in SMARCB1 in Two Siblings with Atypical Teratoid Rhabdoid Tumors. J. Pathol. 2021, 255, 202–211. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours; International Agency for Research on Cancer: Lyon, France, 2020; ISBN 978-92-832-4502-5. [Google Scholar]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Calvert, G.T.; Randall, R.L.; Jones, K.B.; Cannon-Albright, L.; Lessnick, S.; Schiffman, J.D. At-Risk Populations for Osteosarcoma: The Syndromes and Beyond. Sarcoma 2012, 2012, 152382. [Google Scholar] [CrossRef] [PubMed]
- Tinat, J.; Bougeard, G.; Baert-Desurmont, S.; Vasseur, S.; Martin, C.; Bouvignies, E.; Caron, O.; Bressac-de Paillerets, B.; Berthet, P.; Dugast, C.; et al. 2009 Version of the Chompret Criteria for Li Fraumeni Syndrome. J. Clin. Oncol. 2009, 27, e108–e109. [Google Scholar] [CrossRef] [PubMed]
- Chauveinc, L.; Mosseri, V.; Quintana, E.; Desjardins, L.; Schlienger, P.; Doz, F.; Dutrillaux, B. Osteosarcoma Following Retinoblastoma: Age at Onset and Latency Period. Ophthalmic Genet. 2001, 22, 77–88. [Google Scholar] [CrossRef]
- Siitonen, H.A.; Sotkasiira, J.; Biervliet, M.; Benmansour, A.; Capri, Y.; Cormier-Daire, V.; Crandall, B.; Hannula-Jouppi, K.; Hennekam, R.; Herzog, D.; et al. The Mutation Spectrum in RECQL4 Diseases. Eur. J. Hum. Genet. 2009, 17, 151–158. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Miller, R.W.; Machinami, R.; Sugano, H.; Goto, M. Atypical Osteosarcomas in Werner Syndrome (Adult Progeria). Jpn. J. Cancer Res. 2000, 91, 1345–1349. [Google Scholar] [CrossRef]
- German, J. Bloom’s Syndrome. XX. The First 100 Cancers. Cancer Genet. Cytogenet. 1997, 93, 100–106. [Google Scholar] [CrossRef]
- Piao, J.; Sakurai, N.; Iwamoto, S.; Nishioka, J.; Nakatani, K.; Komada, Y.; Mizutani, S.; Takagi, M. Functional Studies of a Novel Germline P53 Splicing Mutation Identified in a Patient with Li–F Raumeni-L Ike Syndrome. Mol. Carcinog. 2013, 52, 770–776. [Google Scholar] [CrossRef]
- Austin, F.; Oyarbide, U.; Massey, G.; Grimes, M.; Corey, S.J. Synonymous Mutation in TP53 Results in a Cryptic Splice Site Affecting Its DNA-binding Site in an Adolescent with Two Primary Sarcomas. Pediatr. Blood Cancer 2017, 64, e26584. [Google Scholar] [CrossRef]
- Sakurai, N.; Iwamoto, S.; Miura, Y.; Nakamura, T.; Matsumine, A.; Nishioka, J.; Nakatani, K.; Komada, Y. Novel P53 Splicing Site Mutation in Li-Fraumeni-like Syndrome with Osteosarcoma: Novel P53 Splicing Site Mutation in LFL. Pediatr. Int. 2013, 55, 107–111. [Google Scholar] [CrossRef]
- Renaux-Petel, M.; Charbonnier, F.; Théry, J.-C.; Fermey, P.; Lienard, G.; Bou, J.; Coutant, S.; Vezain, M.; Kasper, E.; Fourneaux, S.; et al. Contribution of de Novo and Mosaic TP53 Mutations to Li-Fraumeni Syndrome. J. Med. Genet. 2018, 55, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Lozano, G.; Gaff, C.L.; Gardner, R.J.M.; Strong, L.C.; Aittomäki, K.; Lindeman, G.J. Novel P53 Germline Mutation in a Patient with Li−Fraumeni Syndrome: Letters to the Editor. Int. Med. J. 2003, 33, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Mazoyer, S.; Lalle, P.; Moyret-Lalle, C.; Marçais, C.; Schraub, S.; Frappaz, D.; Sobol, H.; Ozturk, M. Two Germ-Line Mutations Affecting the Same Nucleotide at Codon 257 of P53 Gene, a Rare Site for Mutations. Oncogene 1994, 9, 1237–1239. [Google Scholar] [PubMed]
- Gillani, R.; Camp, S.Y.; Han, S.; Jones, J.K.; Chu, H.; O’Brien, S.; Young, E.L.; Hayes, L.; Mitchell, G.; Fowler, T.; et al. Germline Predisposition to Pediatric Ewing Sarcoma Is Characterized by Inherited Pathogenic Variants in DNA Damage Repair Genes. Am. J. Hum. Genet. 2022, 109, 1026–1037. [Google Scholar] [CrossRef]
- Wang, L.L.; Gannavarapu, A.; Kozinetz, C.A.; Levy, M.L.; Lewis, R.A.; Chintagumpala, M.M.; Ruiz-Maldanado, R.; Contreras-Ruiz, J.; Cunniff, C.; Erickson, R.P.; et al. Association Between Osteosarcoma and Deleterious Mutations in the RECQL4 Gene in Rothmund-Thomson Syndrome. JNCI J. Natl. Cancer Inst. 2003, 95, 669–674. [Google Scholar] [CrossRef]
- Lindor, N.M.; Furuichi, Y.; Kitao, S.; Shimamoto, A.; Arndt, C.; Jalal, S. Rothmund-Thomson Syndrome Due ToRECQ4 Helicase Mutations: Report and Clinical and Molecular Comparisons with Bloom Syndrome and Werner Syndrome. Am. J. Med. Genet. 2000, 90, 223–228. [Google Scholar] [CrossRef]
- Simon, T.; Kohlhase, J.; Wilhelm, C.; Kochanek, M.; De Carolis, B.; Berthold, F. Multiple Malignant Diseases in a Patient with Rothmund-Thomson Syndrome with RECQL4 Mutations: Case Report and Literature Review. Am. J. Med. Genet. 2010, 152A, 1575–1579. [Google Scholar] [CrossRef]
- Beghini, A.; Castorina, P.; Roversi, G.; Modiano, P.; Larizza, L. RNA Processing Defects of the Helicase GeneRECQL4 in a Compound Heterozygous Rothmund-Thomson Patient. Am. J. Med. Genet. 2003, 120, 395–399. [Google Scholar] [CrossRef]
- Van Hest, L.P.; Ruijs, M.W.G.; Wagner, A.; van der Meer, C.A.; Verhoef, S.; van‘t Veer, L.J.; Meijers-Heijboer, H. Two TP53 Germline Mutations in a Classical Li-Fraumeni Syndrome Family. Fam. Cancer 2007, 6, 311–316. [Google Scholar] [CrossRef]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Li, H.; Sisoudiya, S.D.; Martin-Giacalone, B.A.; Khayat, M.M.; Dugan-Perez, S.; Marquez-Do, D.A.; Scheurer, M.E.; Muzny, D.; Boerwinkle, E.; Gibbs, R.A.; et al. Germline Cancer Predisposition Variants in Pediatric Rhabdomyosarcoma: A Report from the Children’s Oncology Group. JNCI J. Natl. Cancer Inst. 2021, 113, 875–883. [Google Scholar] [CrossRef]
- Nashed, L.M.; Mayhew, A.; Gomez-Lobo, V.; Lawlor, C. DICER1 Mutation Detected in an Infant Guides Accurate Diagnosis of Auto-Amputated Embryonal Rhabdomyosarcoma. J. Pediatr. Adolesc. Gynecol. 2021, 34, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Dommering, C.J.; Marees, T.; van der Hout, A.H.; Imhof, S.M.; Meijers-Heijboer, H.; Ringens, P.J.; van Leeuwen, F.E.; Moll, A.C. RB1 Mutations and Second Primary Malignancies after Hereditary Retinoblastoma. Fam. Cancer 2012, 11, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Bouteille, M.; Gauthier-Villars, M.; Leroux, D.; Meunier, I.; Aerts, I.; Lumbroso-Le Rouic, L.; Lejeune, S.; Delnatte, C.; Abadie, C.; Pujol, P.; et al. Osteosarcoma without Prior Retinoblastoma Related to RB1 Low-penetrance Germline Pathogenic Variants: A Novel Type of RB1 -related Hereditary Predisposition Syndrome? Mol. Genet. Genom. Med. 2019, 7, e913. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Larizza, L.; Roversi, G.; Volpi, L. Rothmund-Thomson Syndrome. Orphanet. J. Rare Dis. 2010, 5, 2. [Google Scholar] [CrossRef]
- Esiashvili, N.; Goodman, M.; Marcus, R.B. Changes in Incidence and Survival of Ewing Sarcoma Patients Over the Past 3 Decades: Surveillance Epidemiology and End Results Data. J. Pediatr. Hematol./Oncol. 2008, 30, 425–430. [Google Scholar] [CrossRef]
- Choi, E.-Y.K.; Gardner, J.M.; Lucas, D.R.; McHugh, J.B.; Patel, R.M. Ewing Sarcoma. Semin. Diagn. Pathol. 2014, 31, 39–47. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef]
- Crompton, B.D.; Stewart, C.; Taylor-Weiner, A.; Alexe, G.; Kurek, K.C.; Calicchio, M.L.; Kiezun, A.; Carter, S.L.; Shukla, S.A.; Mehta, S.S.; et al. The Genomic Landscape of Pediatric Ewing Sarcoma. Cancer Discov. 2014, 4, 1326–1341. [Google Scholar] [CrossRef] [PubMed]
- Machiela, M.J.; Grünewald, T.G.P.; Surdez, D.; Reynaud, S.; Mirabeau, O.; Karlins, E.; Rubio, R.A.; Zaidi, S.; Grossetete-Lalami, S.; Ballet, S.; et al. Genome-Wide Association Study Identifies Multiple New Loci Associated with Ewing Sarcoma Susceptibility. Nat. Commun. 2018, 9, 3184. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Kim, S.; Rangel Rivera, G.O.; Sen, N.; Haddock, S.; Harlow, M.L.; Maloney, N.K.; Zhu, J.; O’Neill, M.; Jones, T.L.; et al. Functional Genomic Screening Reveals Splicing of the EWS-FLI1 Fusion Transcript as a Vulnerability in Ewing Sarcoma. Cell Rep. 2016, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Tan, K.; Dong, Y.; Lu, W.; Liu, F.; Mei, Y.; Huang, H.; Zhao, K.; Lv, Z.; Ye, Y.; et al. Therapeutic Targeting the Oncogenic Driver EWSR1::FLI1 in Ewing Sarcoma through Inhibition of the FACT Complex. Oncogene 2022, 38, 307–3092. [Google Scholar] [CrossRef] [PubMed]
- Vibert, J.; Saulnier, O.; Collin, C.; Petit, F.; Borgman, K.J.E.; Vigneau, J.; Gautier, M.; Zaidi, S.; Pierron, G.; Watson, S.; et al. Oncogenic Chimeric Transcription Factors Drive Tumor-Specific Transcription, Processing, and Translation of Silent Genomic Regions. Mol. Cell 2022, 82, 2458–2471.e9. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Wallace, S.S.; Pederson, D.S. Initiation of Base Excision Repair of Oxidative Lesions in Nucleosomes by the Human, Bifunctional DNA Glycosylase NTH1. Mol. Cell. Biol. 2007, 27, 8442–8453. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Nuclease Delivery: Versatile Functions of SLX4/FANCP in Genome Maintenance. Mol. Cells 2014, 37, 569–574. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. Chk1 and Chk2 Kinases in Checkpoint Control and Cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Ito, S.; Kuraoka, I.; Chymkowitch, P.; Compe, E.; Takedachi, A.; Ishigami, C.; Coin, F.; Egly, J.-M.; Tanaka, K. XPG Stabilizes TFIIH, Allowing Transactivation of Nuclear Receptors: Implications for Cockayne Syndrome in XP-G/CS Patients. Mol. Cell 2007, 26, 231–243. [Google Scholar] [CrossRef]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous Recombination and Human Health: The Roles of BRCA1, BRCA2, and Associated Proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef]
- Castella, M.; Pujol, R.; Callén, E.; Trujillo, J.P.; Casado, J.A.; Gille, H.; Lach, F.P.; Auerbach, A.D.; Schindler, D.; Benítez, J.; et al. Origin, Functional Role, and Clinical Impact of Fanconi Anemia FANCA Mutations. Blood 2011, 117, 3759–3769. [Google Scholar] [CrossRef]
- Kitao, H.; Yamamoto, K.; Matsushita, N.; Ohzeki, M.; Ishiai, M.; Takata, M. Functional Interplay between BRCA2/FancD1 and FancC in DNA Repair. J. Biol. Chem. 2006, 281, 21312–21320. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewska, A.; Matsuoka, S.; Vinciguerra, P.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Ballif, B.A.; Gygi, S.P.; Hofmann, K.; D’Andrea, A.D.; et al. Identification of the FANCI Protein, a Monoubiquitinated FANCD2 Paralog Required for DNA Repair. Cell 2007, 129, 289–301. [Google Scholar] [CrossRef]
- Nalepa, G.; Clapp, D.W. Fanconi Anaemia and Cancer: An Intricate Relationship. Nat. Rev. Cancer 2018, 18, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Sarangi, P.; D’Andrea, A.D. The Fanconi Anaemia Pathway: New Players and New Functions. Nat. Rev. Mol. Cell Biol. 2016, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Prim. 2019, 5, 1. [Google Scholar] [CrossRef]
- Parham, D.M.; Barr, F.G. Classification of Rhabdomyosarcoma and Its Molecular Basis. Adv. Anat. Pathol. 2013, 20, 387–397. [Google Scholar] [CrossRef]
- Shern, J.F.; Selfe, J.; Izquierdo, E.; Patidar, R.; Chou, H.-C.; Song, Y.K.; Yohe, M.E.; Sindiri, S.; Wei, J.; Wen, X.; et al. Genomic Classification and Clinical Outcome in Rhabdomyosarcoma: A Report From an International Consortium. J. Clin. Oncol. 2021, 39, 2859–2871. [Google Scholar] [CrossRef]
- Diller, L.; Sexsmith, E.; Gottlieb, A.; Li, F.P.; Malkin, D. Germline P53 Mutations Are Frequently Detected in Young Children with Rhabdomyosarcoma. J. Clin. Investig. 1995, 95, 1606–1611. [Google Scholar] [CrossRef]
- Doros, L.; Yang, J.; Dehner, L.; Rossi, C.T.; Skiver, K.; Jarzembowski, J.A.; Messinger, Y.; Schultz, K.A.; Williams, G.; André, N.; et al. DICER1 Mutations in Embryonal Rhabdomyosarcomas from Children with and without Familial PPB-Tumor Predisposition Syndrome. Pediatr. Blood Cancer 2012, 59, 558–560. [Google Scholar] [CrossRef]
- Capasso, M.; Montella, A.; Tirelli, M.; Maiorino, T.; Cantalupo, S.; Iolascon, A. Genetic Predisposition to Solid Pediatric Cancers. Front. Oncol. 2020, 10, 590033. [Google Scholar] [CrossRef]
- Ney, G.M.; McKay, L.; Koschmann, C.; Mody, R.; Li, Q. The Emerging Role of Ras Pathway Signaling in Pediatric Cancer. Cancer Res. 2020, 80, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Niihori, T.; Inoue, S.; Matsubara, Y. Recent Advances in RASopathies. J. Hum. Genet. 2016, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.L.; Birch, J.M.; Marsden, H.B.; Harris, M.; Blair, V. Neurofibromatosis in Children with Soft Tissue Sarcoma. Pediatr. Hematol. Oncol. 1988, 5, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kratz, C.P.; Rapisuwon, S.; Reed, H.; Hasle, H.; Rosenberg, P.S. Cancer in Noonan, Costello, Cardiofaciocutaneous and LEOPARD Syndromes. Am. J. Med. Genet. 2011, 157, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Priest, J.R.; Duchaine, T.F. DICER1: Mutations, MicroRNAs and Mechanisms. Nat. Rev. Cancer 2014, 14, 662–672. [Google Scholar] [CrossRef]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-Risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018, 24, 2251–2261. [Google Scholar] [CrossRef]
- Capasso, M.; Diskin, S.J. Genetics and Genomics of Neuroblastoma. In Cancer Genetics; Pasche, B., Ed.; Cancer Treatment and Research; Springer US: Boston, MA, USA, 2010; Volume 155, pp. 65–84. ISBN 978-1-4419-6032-0. [Google Scholar]
- Chitayat, D.; Friedman, J.M.; Dimmick, J.E. Neuroblastoma in a Child with Wiedemann-Beckwith Syndrome. Am. J. Med. Genet. 1990, 35, 433–436. [Google Scholar] [CrossRef]
- Maas, S.M.; Vansenne, F.; Kadouch, D.J.M.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.M.; Maher, E.R.; Hennekam, R.C. Phenotype, Cancer Risk, and Surveillance in Beckwith-Wiedemann Syndrome Depending on Molecular Genetic Subgroups. Am. J. Med. Genet. 2016, 170, 2248–2260. [Google Scholar] [CrossRef]
- Trochet, D.; Bourdeaut, F.; Janoueix-Lerosey, I.; Deville, A.; de Pontual, L.; Schleiermacher, G.; Coze, C.; Philip, N.; Frébourg, T.; Munnich, A.; et al. Germline Mutations of the Paired–Like Homeobox 2B (PHOX2B) Gene in Neuroblastoma. Am. J. Hum. Genet. 2004, 74, 761–764. [Google Scholar] [CrossRef]
- Rohrer, T.; Trachsel, D.; Engelcke, G.; Hammer, J. Congenital Central Hypoventilation Syndrome Associated with Hirschsprung’s Disease and Neuroblastoma: Case of Multiple Neurocristopathies. Pediatr. Pulmonol. 2002, 33, 71–76. [Google Scholar] [CrossRef]
- Barr, E.; Applebaum, M. Genetic Predisposition to Neuroblastoma. Children 2018, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Lin, A.E. Costello Syndrome: A Ras/Mitogen Activated Protein Kinase Pathway Syndrome (Rasopathy) Resulting from HRAS Germline Mutations. Genet. Med. 2012, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Schimke, R.N.; Collins, D.L.; Stolle, C.A. Paraganglioma, Neuroblastoma, and a SDHB Mutation: Resolution of a 30-Year-Old Mystery. Am. J. Med. Genet. 2010, 152A, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.M.; Alston, R.D.; McNally, R.J.; Evans, D.G.R.; Kelsey, A.M.; Harris, M.; Eden, O.B.; Varley, J.M. Relative Frequency and Morphology of Cancers in Carriers of Germline TP53 Mutations. Oncogene 2001, 20, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, T.; Bielack, S.S.; Borkhardt, A.; Brecht, I.B.; Burkhardt, B.; Calaminus, G.; Debatin, K.-M.; Deubzer, H.; Dirksen, U.; Eckert, C.; et al. Childhood Cancer Predisposition Syndromes-A Concise Review and Recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am. J. Med. Genet. 2017, 173, 1017–1037. [Google Scholar] [CrossRef]
- Cotton, J.L. Noonan Syndrome and Neuroblastoma. Arch. Pediatr. Adolesc. Med. 1995, 149, 1280. [Google Scholar] [CrossRef]
- Origone, P.; Defferrari, R.; Mazzocco, K.; Cunsolo, C.L.; Bernardi, B.D.; Tonini, G.P. Homozygous Inactivation of NF1 Gene in a Patient with Familial NF1 and Disseminated Neuroblastoma. Am. J. Med. Genet. 2003, 118A, 309–313. [Google Scholar] [CrossRef]
- Crucis, A.; Richer, W.; Brugières, L.; Bergeron, C.; Marie-Cardine, A.; Stephan, J.-L.; Girard, P.; Corradini, N.; Munzer, M.; Lacour, B.; et al. Rhabdomyosarcomas in Children with Neurofibromatosis Type I: A National Historical Cohort: Rhabdomyosarcomas and NF1. Pediatr. Blood Cancer 2015, 62, 1733–1738. [Google Scholar] [CrossRef]
- De Kort, E.; Conneman, N.; Diderich, K. A Case of Rubinstein-Taybi Syndrome and Congenital Neuroblastoma. Am. J. Med. Genet. 2014, 164, 1332–1333. [Google Scholar] [CrossRef]
- Berdasco, M.; Ropero, S.; Setien, F.; Fraga, M.F.; Lapunzina, P.; Losson, R.; Alaminos, M.; Cheung, N.-K.; Rahman, N.; Esteller, M. Epigenetic Inactivation of the Sotos Overgrowth Syndrome Gene Histone Methyltransferase NSD1 in Human Neuroblastoma and Glioma. Proc. Natl. Acad. Sci. USA 2009, 106, 21830–21835. [Google Scholar] [CrossRef]
- Nance, M.A.; Neglia, J.P.; Talwar, D.; Berry, S.A. Neuroblastoma in a Patient with Sotos’ Syndrome. J. Med. Genet. 1990, 27, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Murray, A.; Hanks, S.; Douglas, J.; Armstrong, R.; Banka, S.; Bird, L.M.; Clericuzio, C.L.; Cormier-Daire, V.; Cushing, T.; et al. Weaver Syndrome and EZH2 Mutations: Clarifying the Clinical Phenotype. Am. J. Med. Genet. 2013, 161, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Coulter, D.; Powell, C.M.; Gold, S. Weaver Syndrome and Neuroblastoma. J. Pediatr. Hematol./Oncol. 2008, 30, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Acer, H.; Ciraci, S.; Gumus, H.; Karakukcu, M.; Patiroglu, T.; Ozdemir, M.A.; Unal, E. Neuroblastoma in a Child With Wolf-Hirschhorn Syndrome. J. Pediatr. Hematol./Oncol. 2017, 39, e224–e226. [Google Scholar] [CrossRef]
- Bachetti, T.; Ceccherini, I. Causative and Common PHOX2B Variants Define a Broad Phenotypic Spectrum. Clin. Genet. 2020, 97, 103–113. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a Major Familial Neuroblastoma Predisposition Gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children with Solid Tumors. JAMA Oncol. 2016, 2, 616. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The Genetic Landscape of High-Risk Neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Lasorsa, V.A.; Formicola, D.; Pignataro, P.; Cimmino, F.; Calabrese, F.M.; Mora, J.; Esposito, M.R.; Pantile, M.; Zanon, C.; De Mariano, M.; et al. Exome and Deep Sequencing of Clinically Aggressive Neuroblastoma Reveal Somatic Mutations That Affect Key Pathways Involved in Cancer Progression. Oncotarget 2016, 7, 21840–21852. [Google Scholar] [CrossRef]
- Mody, R.J.; Wu, Y.-M.; Lonigro, R.J.; Cao, X.; Roychowdhury, S.; Vats, P.; Frank, K.M.; Prensner, J.R.; Asangani, I.; Palanisamy, N.; et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 2015, 314, 913. [Google Scholar] [CrossRef]
- Trubicka, J.; Grajkowska, W.; Dembowska-Bagińska, B. Molecular Markers of Pediatric Solid Tumors—Diagnosis, Optimizing Treatments, and Determining Susceptibility: Current State and Future Directions. Cells 2022, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, J.; Wang, Y.; Zhao, M.; Zhang, X.; Yang, L.; Ni, X.; Zhao, J.; Gallie, B.L. RB1 Germline Mutation Spectrum and Clinical Features in Patients with Unilateral Retinoblastomas. Ophthalmic Genet. 2021, 42, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Cygan, K.J.; Soemedi, R.; Rhine, C.L.; Profeta, A.; Murphy, E.L.; Murray, M.F.; Fairbrother, W.G. Defective Splicing of the RB1 Transcript Is the Dominant Cause of Retinoblastomas. Hum. Genet. 2017, 136, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; García-Miguel, P.; Abelairas, J.; Mendiola, M.; Sarret, E.; Vendrell, M.T.; Navajas, A.; Pestaña, A. Spectrum of Germline RB1 Gene Mutations in Spanish Retinoblastoma Patients: Phenotypic and Molecular Epidemiological Implications: Novel RB1 Mutations in Retinoblastoma. Hum. Mutat. 2001, 17, 412–422. [Google Scholar] [CrossRef]
- Parsam, V.L.; Ali, M.J.; Honavar, S.G.; Vemuganti, G.K.; Kannabiran, C. Splicing Aberrations Caused by Constitutional RB1 Gene Mutations in Retinoblastoma. J. Biosci. 2011, 36, 281–287. [Google Scholar] [CrossRef]
- Lohmann, D.R.; Brandt, B.; Höpping, W.; Passarge, E.; Horsthemke, B. The Spectrum of RB1 Germ-Line Mutations in Hereditary Retinoblastoma. Am. J. Hum. Genet. 1996, 58, 940–949. [Google Scholar]
- Lan, X.; Xu, W.; Tang, X.; Ye, H.; Song, X.; Lin, L.; Ren, X.; Yu, G.; Zhang, H.; Wu, S. Spectrum of RB1 Germline Mutations and Clinical Features in Unrelated Chinese Patients With Retinoblastoma. Front. Genet. 2020, 11, 142. [Google Scholar] [CrossRef]
- Dommering, C.J.; Mol, B.M.; Moll, A.C.; Burton, M.; Cloos, J.; Dorsman, J.C.; Meijers-Heijboer, H.; van der Hout, A.H. RB1 Mutation Spectrum in a Comprehensive Nationwide Cohort of Retinoblastoma Patients. J. Med. Genet. 2014, 51, 366–374. [Google Scholar] [CrossRef]
- Mahamdallie, S.; Yost, S.; Poyastro-Pearson, E.; Holt, E.; Zachariou, A.; Seal, S.; Elliott, A.; Clarke, M.; Warren-Perry, M.; Hanks, S.; et al. Identification of New Wilms Tumour Predisposition Genes: An Exome Sequencing Study. Lancet Child Adolesc. Health 2019, 3, 322–331. [Google Scholar] [CrossRef]
- Treger, T.D.; Chowdhury, T.; Pritchard-Jones, K.; Behjati, S. The Genetic Changes of Wilms Tumour. Nat. Rev. Nephrol. 2019, 15, 240–251. [Google Scholar] [CrossRef]
- Cooper, W.N.; Luharia, A.; Evans, G.A.; Raza, H.; Haire, A.C.; Grundy, R.; Bowdin, S.C.; Riccio, A.; Sebastio, G.; Bliek, J.; et al. Molecular Subtypes and Phenotypic Expression of Beckwith–Wiedemann Syndrome. Eur. J. Hum. Genet. 2005, 13, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Cairney, A.E.L.; Andrews, M.; Greenberg, M.; Smith, D.; Weksberg, R. Wilms Tumor in Three Patients with Bloom Syndrome. J. Pediatr. 1987, 111, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Johnston, J.J.; Biesecker, L.G.; Kramer, N.; Pickart, A.; Rhead, W.; Tan, W.-H.; Brownstein, C.A.; Kate Clarkson, L.; Dobson, A.; et al. Clinical Management of Patients with ASXL1 Mutations and Bohring-Opitz Syndrome, Emphasizing the Need for Wilms Tumor Surveillance. Am. J. Med. Genet. 2015, 167, 2122–2131. [Google Scholar] [CrossRef]
- Pelletier, J.; Bruening, W.; Kashtan, C.E.; Mauer, S.M.; Manivel, J.C.; Striegel, J.E.; Houghton, D.C.; Junien, C.; Habib, R.; Fouser, L. Germline Mutations in the Wilms’ Tumor Suppressor Gene Are Associated with Abnormal Urogenital Development in Denys-Drash Syndrome. Cell 1991, 67, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Palculict, T.B.; Ruteshouser, E.C.; Fan, Y.; Wang, W.; Strong, L.; Huff, V. Identification of Germline DICER1 Mutations and Loss of Heterozygosity in Familial Wilms Tumour. J. Med. Genet. 2016, 53, 385–388. [Google Scholar] [CrossRef]
- Scollon, S.; Anglin, A.K.; Thomas, M.; Turner, J.T.; Wolfe Schneider, K. A Comprehensive Review of Pediatric Tumors and Associated Cancer Predisposition Syndromes. J. Genet. Couns. 2017, 26, 387–434. [Google Scholar] [CrossRef]
- Scott, R.H. Syndromes and Constitutional Chromosomal Abnormalities Associated with Wilms Tumour. J. Med. Genet. 2006, 43, 705–715. [Google Scholar] [CrossRef]
- Reid, S. Biallelic BRCA2 Mutations Are Associated with Multiple Malignancies in Childhood Including Familial Wilms Tumour. J. Med. Genet. 2005, 42, 147–151. [Google Scholar] [CrossRef][Green Version]
- Reid, S.; Schindler, D.; Hanenberg, H.; Barker, K.; Hanks, S.; Kalb, R.; Neveling, K.; Kelly, P.; Seal, S.; Freund, M.; et al. Biallelic Mutations in PALB2 Cause Fanconi Anemia Subtype FA-N and Predispose to Childhood Cancer. Nat. Genet. 2007, 39, 162–164. [Google Scholar] [CrossRef]
- Barbaux, S.; Niaudet, P.; Gubler, M.-C.; Grünfeld, J.-P.; Jaubert, F.; Kuttenn, F.; Fékété, C.N.; Souleyreau-Therville, N.; Thibaud, E.; Fellous, M.; et al. Donor Splice-Site Mutations in WT1 Are Responsible for Frasier Syndrome. Nat. Genet. 1997, 17, 467–470. [Google Scholar] [CrossRef]
- Cajaiba, M.M.; Bale, A.E.; Alvarez-Franco, M.; McNamara, J.; Reyes-Múgica, M. Rhabdomyosarcoma, Wilms Tumor, and Deletion of the Patched Gene in Gorlin Syndrome. Nat. Rev. Clin. Oncol. 2006, 3, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Isidor, B.; Bourdeaut, F.; Lafon, D.; Plessis, G.; Lacaze, E.; Kannengiesser, C.; Rossignol, S.; Pichon, O.; Briand, A.; Martin-Coignard, D.; et al. Wilms’ Tumor in Patients with 9q22.3 Microdeletion Syndrome Suggests a Role for PTCH1 in Nephroblastomas. Eur. J. Hum. Genet. 2013, 21, 784–787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuman, C.; Smith, A.C.; Steele, L.; Ray, P.N.; Clericuzio, C.; Zackai, E.; Parisi, M.A.; Meadows, A.T.; Kelly, T.; Tichauer, D.; et al. Constitutional UPD for Chromosome 11p15 in Individuals with Isolated Hemihyperplasia Is Associated with High Tumor Risk and Occurs Following Assisted Reproductive Technologies. Am. J. Med. Genet. 2006, 140A, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.L.; Birch, J.M.; Tricker, K.; Wallace, S.A.; Kelsey, A.M.; Harris, M.; Morris Jones, P.H. Wilms’ Tumor in the Li-Fraumeni Cancer Family Syndrome. Cancer Genet. Cytogenet. 1993, 67, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.; Coleman, K.; Reid, S.; Plaja, A.; Firth, H.; FitzPatrick, D.; Kidd, A.; Méhes, K.; Nash, R.; Robin, N.; et al. Constitutional Aneuploidy and Cancer Predisposition Caused by Biallelic Mutations in BUB1B. Nat. Genet. 2004, 36, 1159–1161. [Google Scholar] [CrossRef]
- Scott, R.H.; Walker, L.; Olsen, O.E.; Levitt, G.; Kenney, I.; Maher, E.; Owens, C.M.; Pritchard-Jones, K.; Craft, A.; Rahman, N. Surveillance for Wilms Tumour in At-Risk Children: Pragmatic Recommendations for Best Practice. Arch. Dis. Child. 2006, 91, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Yost, S.; de Wolf, B.; Hanks, S.; Zachariou, A.; Marcozzi, C.; Clarke, M.; de Voer, R.M.; Etemad, B.; Uijttewaal, E.; Ramsay, E.; et al. Biallelic TRIP13 Mutations Predispose to Wilms Tumor and Chromosome Missegregation. Nat. Genet. 2017, 49, 1148–1151. [Google Scholar] [CrossRef]
- Karlberg, N.; Karlberg, S.; Karikoski, R.; Mikkola, S.; Lipsanen-Nyman, M.; Jalanko, H. High Frequency of Tumours in Mulibrey Nanism. J. Pathol. 2009, 218, 163–171. [Google Scholar] [CrossRef]
- Astuti, D.; Morris, M.R.; Cooper, W.N.; Staals, R.H.J.; Wake, N.C.; Fews, G.A.; Gill, H.; Gentle, D.; Shuib, S.; Ricketts, C.J.; et al. Germline Mutations in DIS3L2 Cause the Perlman Syndrome of Overgrowth and Wilms Tumor Susceptibility. Nat. Genet. 2012, 44, 277–284. [Google Scholar] [CrossRef]
- Gripp, K.W.; Baker, L.; Kandula, V.; Conard, K.; Scavina, M.; Napoli, J.A.; Griffin, G.C.; Thacker, M.; Knox, R.G.; Clark, G.R.; et al. Nephroblastomatosis or Wilms Tumor in a Fourth Patient with a Somatic PIK3CA Mutation. Am. J. Med. Genet. 2016, 170, 2559–2569. [Google Scholar] [CrossRef]
- Cottereau, E.; Mortemousque, I.; Moizard, M.-P.; Bürglen, L.; Lacombe, D.; Gilbert-Dussardier, B.; Sigaudy, S.; Boute, O.; David, A.; Faivre, L.; et al. Phenotypic Spectrum of Simpson-Golabi-Behmel Syndrome in a Series of 42 Cases With a Mutation in GPC 3 and Review of the Literature: American Journal of Medical Genetics Part C (Seminars in Medical Genetics). Am. J. Med. Genet. 2013, 163, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Fagali, C.; Kok, F.; Nicola, P.; Kim, C.; Bertola, D.; Albano, L.; Koiffmann, C.P. MLPA Analysis in 30 Sotos Syndrome Patients Revealed One Total NSD1 Deletion and Two Partial Deletions Not Previously Reported. Eur. J. Med. Genet. 2009, 52, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E.; Norris, R.; Norkool, P.A.; Kang, T.; Beckwith, J.B.; Perlman, E.J.; Ritchey, M.L.; Green, D.M.; Nichols, K.E. Characteristics and Outcomes of Children With the Wilms Tumor-Aniridia Syndrome: A Report from the National Wilms Tumor Study Group. J. Clin. Oncol. 2003, 21, 4579–4585. [Google Scholar] [CrossRef]
- Ciceri, S.; Gamba, B.; Corbetta, P.; Mondini, P.; Terenziani, M.; Catania, S.; Nantron, M.; Bianchi, M.; D’Angelo, P.; Torri, F.; et al. Genetic and Epigenetic Analyses Guided by High Resolution Whole-Genome SNP Array Reveals a Possible Role of CHEK2 in Wilms Tumour Susceptibility. Oncotarget 2018, 9, 34079–34089. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Yamamura, T.; Nagano, C.; Horinouchi, T.; Sakakibara, N.; Ishiko, S.; Aoto, Y.; Rossanti, R.; Okada, E.; Tanaka, E.; et al. Systematic Review of Genotype-Phenotype Correlations in Frasier Syndrome. Kidney Int. Rep. 2021, 6, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, R.T.; Cransberg, K.; Loos, W.J.; Wagner, A.; Alders, M.; van den Heuvel-Eibrink, M.M. Topotecan Distribution in an Anephric Infant with Therapy Resistant Bilateral Wilms Tumor with a Novel Germline WT1 Gene Mutation. Cancer Chemother. Pharmacol. 2008, 62, 1039–1044. [Google Scholar] [CrossRef][Green Version]
- Martins, A.G.; Pinto, A.T.; Domingues, R.; Cavaco, B.M. Identification of a Novel CTR9 Germline Mutation in a Family with Wilms Tumor. Eur. J. Med. Genet. 2018, 61, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.; Perdeaux, E.R.; Seal, S.; Ruark, E.; Mahamdallie, S.S.; Murray, A.; Ramsay, E.; Del Vecchio Duarte, S.; Zachariou, A.; de Souza, B.; et al. Germline Mutations in the PAF1 Complex Gene CTR9 Predispose to Wilms Tumour. Nat. Commun. 2014, 5, 4398. [Google Scholar] [CrossRef]
- Hol, J.A.; Diets, I.J.; Krijger, R.R.; Heuvel-Eibrink, M.M.; Jongmans, M.C.; Kuiper, R.P. TRIM28 Variants and Wilms’ Tumour Predisposition. J. Pathol. 2021, 254, 494–504. [Google Scholar] [CrossRef]
- Bessa, C.; Matos, P.; Jordan, P.; Gonçalves, V. Alternative Splicing: Expanding the Landscape of Cancer Biomarkers and Therapeutics. Int. J. Mol. Sci. 2020, 21, 9032. [Google Scholar] [CrossRef]
- Desterro, J.; Bak-Gordon, P.; Carmo-Fonseca, M. Targeting MRNA Processing as an Anticancer Strategy. Nat. Rev. Drug Discov. 2020, 19, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.A.; Urabe, V.K.; Jurica, M.S. Modulating Splicing with Small Molecular Inhibitors of the Spliceosome: Modulating Splicing with Small Molecular Inhibitors. WIREs RNA 2017, 8, e1381. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M. The Powerful World of Antisense Oligonucleotides: From Bench to Bedside. WIREs RNA 2020, 11, e1594. [Google Scholar] [CrossRef]
- Inoue, D.; Chew, G.-L.; Liu, B.; Michel, B.C.; Pangallo, J.; D’Avino, A.R.; Hitchman, T.; North, K.; Lee, S.C.-W.; Bitner, L.; et al. Spliceosomal Disruption of the Non-Canonical BAF Complex in Cancer. Nature 2019, 574, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The Delivery of Therapeutic Oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Boisguérin, P.; Deshayes, S.; Gait, M.J.; O’Donovan, L.; Godfrey, C.; Betts, C.A.; Wood, M.J.A.; Lebleu, B. Delivery of Therapeutic Oligonucleotides with Cell Penetrating Peptides. Adv. Drug Deliv. Rev. 2015, 87, 52–67. [Google Scholar] [CrossRef]
- Schneider, E.K.; Huang, J.X.; Carbone, V.; Baker, M.; Azad, M.A.K.; Cooper, M.A.; Li, J.; Velkov, T. Drug-Drug Plasma Protein Binding Interactions of Ivacaftor: Ivacaftor Plasma Protein Binding. J. Mol. Recognit. 2015, 28, 339–348. [Google Scholar] [CrossRef]
- Mogilevsky, M.; Shimshon, O.; Kumar, S.; Mogilevsky, A.; Keshet, E.; Yavin, E.; Heyd, F.; Karni, R. Modulation of MKNK2 Alternative Splicing by Splice-Switching Oligonucleotides as a Novel Approach for Glioblastoma Treatment. Nucleic Acids Res. 2018, 46, 11396–11404. [Google Scholar] [CrossRef]
- Kaehler, M.; Cascorbi, I. Germline Variants in Cancer Therapy. Cancer Drug Resist. 2019, 2, 18–30. [Google Scholar] [CrossRef]
- Ng, K.P.; Hillmer, A.M.; Chuah, C.T.H.; Juan, W.C.; Ko, T.K.; Teo, A.S.M.; Ariyaratne, P.N.; Takahashi, N.; Sawada, K.; Fei, Y.; et al. A Common BIM Deletion Polymorphism Mediates Intrinsic Resistance and Inferior Responses to Tyrosine Kinase Inhibitors in Cancer. Nat. Med. 2012, 18, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Pan, Z.; Zhang, Z.; Lin, L.; Xing, Y. The Expanding Landscape of Alternative Splicing Variation in Human Populations. Am. J. Hum. Genet. 2018, 102, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.M.; Swanson, M.S. RNA Mis-Splicing in Disease. Nat. Rev. Genet. 2016, 17, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Karam, R.; Conner, B.; LaDuca, H.; McGoldrick, K.; Krempely, K.; Richardson, M.E.; Zimmermann, H.; Gutierrez, S.; Reineke, P.; Hoang, L.; et al. Assessment of Diagnostic Outcomes of RNA Genetic Testing for Hereditary Cancer. JAMA Netw. Open 2019, 2, e1913900. [Google Scholar] [CrossRef] [PubMed]
- Landrith, T.; Li, B.; Cass, A.A.; Conner, B.R.; LaDuca, H.; McKenna, D.B.; Maxwell, K.N.; Domchek, S.; Morman, N.A.; Heinlen, C.; et al. Splicing Profile by Capture RNA-Seq Identifies Pathogenic Germline Variants in Tumor Suppressor Genes. NPJ Precis. Oncol. 2020, 4, 4. [Google Scholar] [CrossRef]

| Gene a | Pathway/Function | Associated Cancer Predisposition Syndrome |
|---|---|---|
| APC | Tumor suppressor | Familial adenomatous polyposis |
| ATM | Tumor suppressor | Ataxia telangiectasia |
| BRCA1 | Tumor suppressor | Hereditary breast and ovarian cancer syndrome |
| BRCA2 | Tumor suppressor | Hereditary breast and ovarian cancer syndrome |
| COL1A1 | Pro-alpha1 chains of type I collagen | - |
| COL2A1 | Pro-alpha1 chains of type II collagen | - |
| ELN | Elastic fiber formation | - |
| EXT1 | Tumor suppressor | Hereditary multiple exostoses, Langer–Giedion syndrome |
| FANCA | Fanconi anemia complementation group A | Fanconi anemia |
| FANCD2 | Fanconi anemia complementation group D2 | Fanconi anemia |
| FANCG | Fanconi anemia complementation Group G | Fanconi anemia |
| MLH1 | Tumor suppressor | Lynch syndrome |
| MSH2 | Tumor suppressor | Lynch syndrome |
| NF1 | Tumor suppressor | Neurofibromatosis type 1 |
| NF2 | Tumor suppressor | Neurofibromatosis type 2 |
| PMS2 | Tumor suppressor | Lynch syndrome |
| PRKAR1A | Protein Kinase CAMP-Dependent Type I Regulatory Subunit Alpha/tumor suppressor | Carney complex |
| RB1 | Tumor suppressor | Retinoblastoma |
| TSC2 | Tumor suppressor | Tuberous sclerosis type 2 |
| WAS | Effector protein for Rho-type GTPases | Wiskott–Aldrich syndrome gene |
| Gene | Diagnosis | Variant | Type of Mutation | Reference |
|---|---|---|---|---|
| ATM | Medulloblastoma | c.6095G>A | I | [15] |
| ATM | Medulloblastoma | c.2921+1G>C | I | [15] |
| BRCA2 | Medulloblastoma | c.631+2T>G | I | [15] |
| BRCA2 | Medulloblastoma | c.-39-1_-39delGA | II | [15] |
| ELP1 | Medulloblastoma | c.3700+1G>A | I | [40] |
| ELP1 | Medulloblastoma | c.3572+1G>A | I | [40] |
| ELP1 | Medulloblastoma | c.2959-1G>T | II | [40] |
| ELP1 | Medulloblastoma | c.741-1G>T | II | [40] |
| ELP1 | Medulloblastoma | c.649G>A | I | [40] |
| ELP1 | Medulloblastoma | c.2959-1G>T | II | [40] |
| FANCA | Medulloblastoma | c.2778+1G>A | I | [15] |
| FANCC | Medulloblastoma | c.996+1G>T | I | [15] |
| MSH6 | Medulloblastoma | c.(4002-31_4002-8delins24) + (4002-31_4002-8delins24) | IV | [41] |
| MUTYH | Medulloblastoma | c.925-2A>G | II | [22] |
| PALB2 | Medulloblastoma | c.3201+1G>C | I | [15] |
| PTCH1 | Medulloblastoma | c.1729-2A>G | II | [15] |
| PTCH1 | Medulloblastoma | c.584 +2T>G | I | [42] |
| RAD51C | Medulloblastoma | c.904+5G>T | I | [15] |
| SUFU | Medulloblastoma | c.1022 +1 G>A | I | [43] |
| SUFU | Medulloblastoma | c. 1365+2T>A | I | [44] |
| SUFU | Medulloblastoma | c.182+3A>T | I | [45] |
| SUFU | Medulloblastoma | c.318-10delT | IV | [45] |
| SUFU | Medulloblastoma | c.1297-1G>C | II | [45] |
| SUFU | Medulloblastoma | c.183-1G>T | II | [46] |
| SUFU | Medulloblastoma | c.684-2A>G | II | [15] |
| SUFU | Medulloblastoma | c.455-1G>A | II | [15] |
| TP53 | Medulloblastoma | c.376-2A>G | II | [47] |
| WRN | Medulloblastoma | c.3139-1G>C | II | [15] |
| WT1 | Medulloblastoma | c.769+1G>C | I | [15] |
| XPC | Medulloblastoma | c.2251-1G>C | II | [15] |
| CHEK2 | Astrocytoma | c.444+1G>A | I | [48] |
| NF1 | Pilocytic astrocytoma | c.205_205insTC | III | [49] |
| NF1 | Pilocytic astrocytoma | c.1185+1G>A | I | [49] |
| NF1 | Pilocytic astrocytoma | c.889-2A>G | II | [49] |
| NF1 | Optic pathway glioma | c.2325+1G>A | I | [50] |
| NF1 | Optic pathway glioma | c.1260+1G>T | I | [49] |
| NF1 | Low-grade glioma | c.6641+1G>A | I | [22] |
| NF2 | Ependymoma | c.447+1G>A | I | [22] |
| ERCC2 | Diffuse astrocytoma | Not available | [51] | |
| MUTYH | Highly infiltrative astrocytoma | Not available | [51] | |
| ATM | High-grade glioma | c.7630-2A>C | II | [22] |
| MUTYH | High-grade midline glioma | c.892-2A>G | II | [52] |
| MSH6 | Glioblastoma | c.(4002-31_4002-8delins24) + (4002-31_4002-8delins24) | IV | [41] |
| NF1 | Glioblastoma | c.1641+2T>A | I | [49] |
| NF1 | Anaplastic astrocytoma | c.4174-2>AG | II | [49] |
| TP53 | Glioblastoma | c.919+1G>A | I | [49] |
| SMARCB1 | AT/RT | c.501-2A>G | II | [53] |
| DICER 1 | Pinealoblastoma | c.4050+1G>A | I | [54] |
| TP53 | Choroid plexus carcinoma | c.560-2A>C | II | [55] |
| Gene | Diagnosis | Variant | Type of Mutation | Reference |
|---|---|---|---|---|
| TP53 | Osteosarcoma | c.671+1G>A | I | [101] |
| TP53 | Osteosarcoma | c.672+1G>A | I | [103] |
| TP53 | Osteosarcoma | c.375+1G>A | I | [104] |
| TP53 | Osteosarcoma | c.559+2T>G | I | [105] |
| TP53 | Osteosarcoma | c.672+1G>A | I | [101] |
| TP53 | Osteosarcoma | c.770T>A | III | [106] |
| TP53 | Telangiectatic osteosarcoma | c.672G>A | I | [102] |
| TP53 | Osteosarcoma | c.258+1G>T | I | [107] |
| RECQL4 | Osteosarcoma | g.2746del11 | IV | [108] |
| RECQL4 | Osteosarcoma | g.3685G>A | I | [108] |
| RECQL4 | Osteosarcoma | g.2626G>A | II | [108] |
| RECQL4 | Osteosarcoma | g.3712del24 | IV | [108] |
| RECQL4 | Osteosarcoma | c.1391-1G>A | II | [109] |
| RECQL4 | Osteosarcoma | c.1704-1G>A | II | [110] |
| RECQL4 | Osteosarcoma | c.2059-1G>C | II | [111] |
| RB1 | Osteosarcoma | c.940-1G>A | II | [22] |
| RB1 | Osteosarcoma | c.2106+2_2106+5del | I | [107] |
| NTHL1 | Ewing sarcoma | c.116-1G>A | II | [107] |
| SLX4 | Ewing sarcoma | c.1684-1G>A | II | [107] |
| FANCA | Ewing sarcoma | c.523-1G>C | II | [107] |
| FANCA | Ewing sarcoma | c.3828+1G>C | I | [107] |
| RAD51C | Ewing sarcoma | c.905-3_906del | II | [107] |
| RAD51C | Ewing sarcoma | c.1026+5_1026+7del | I | [107] |
| CHEK2 | Ewing sarcoma | c.812+1G>T | I | [107] |
| FANCC | Ewing sarcoma | c.456+4A>T | I | [107] |
| EXT2 | Ewing sarcoma | c.69+2insAGGG | I | [19] |
| FANCD2 | Ewing sarcoma | c.2715+1G>A | I | [19] |
| TP53 | Rhabdomyosarcoma | c.560-1G>A | II | [112] |
| TP53 | Rhabdomyosarcoma | c.376-1G>A | II | [113] |
| TP53 | Embryonal rhabdomyosarcoma | c.783-2A>G | II | [113] |
| TP53 | Spindle cell rhabdomyosarcoma | c.560-1G>C | II | [114] |
| NF1 | Embryonal rhabdomyosarcoma | c.6704+1G>T | I | [114] |
| DICER1 | Embryonal rhabdomyosarcoma | c.1907+1G>A | I | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba-Pavón, P.; Alaña, L.; Astigarraga, I.; Villate, O. Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer. Cancers 2022, 14, 5967. https://doi.org/10.3390/cancers14235967
Alba-Pavón P, Alaña L, Astigarraga I, Villate O. Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer. Cancers. 2022; 14(23):5967. https://doi.org/10.3390/cancers14235967
Chicago/Turabian StyleAlba-Pavón, Piedad, Lide Alaña, Itziar Astigarraga, and Olatz Villate. 2022. "Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer" Cancers 14, no. 23: 5967. https://doi.org/10.3390/cancers14235967
APA StyleAlba-Pavón, P., Alaña, L., Astigarraga, I., & Villate, O. (2022). Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer. Cancers, 14(23), 5967. https://doi.org/10.3390/cancers14235967







