Atezolizumab Plus Bevacizumab in Patients with Advanced and Progressing Hepatocellular Carcinoma: Retrospective Multicenter Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Selection of Patients
2.2. Statistical Analysis
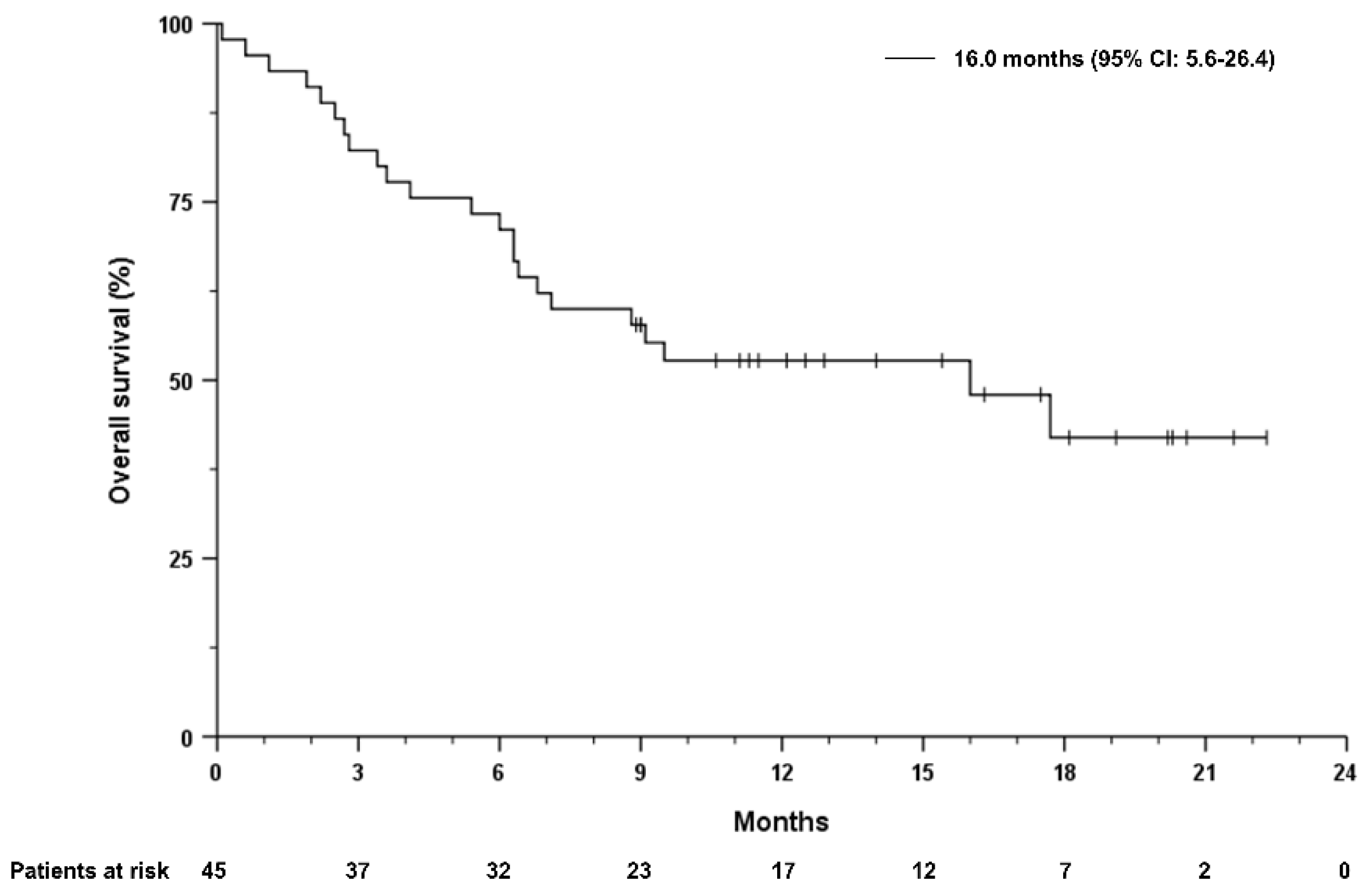
3. Results
3.1. Patients
3.2. Treatment Response
3.3. Safety
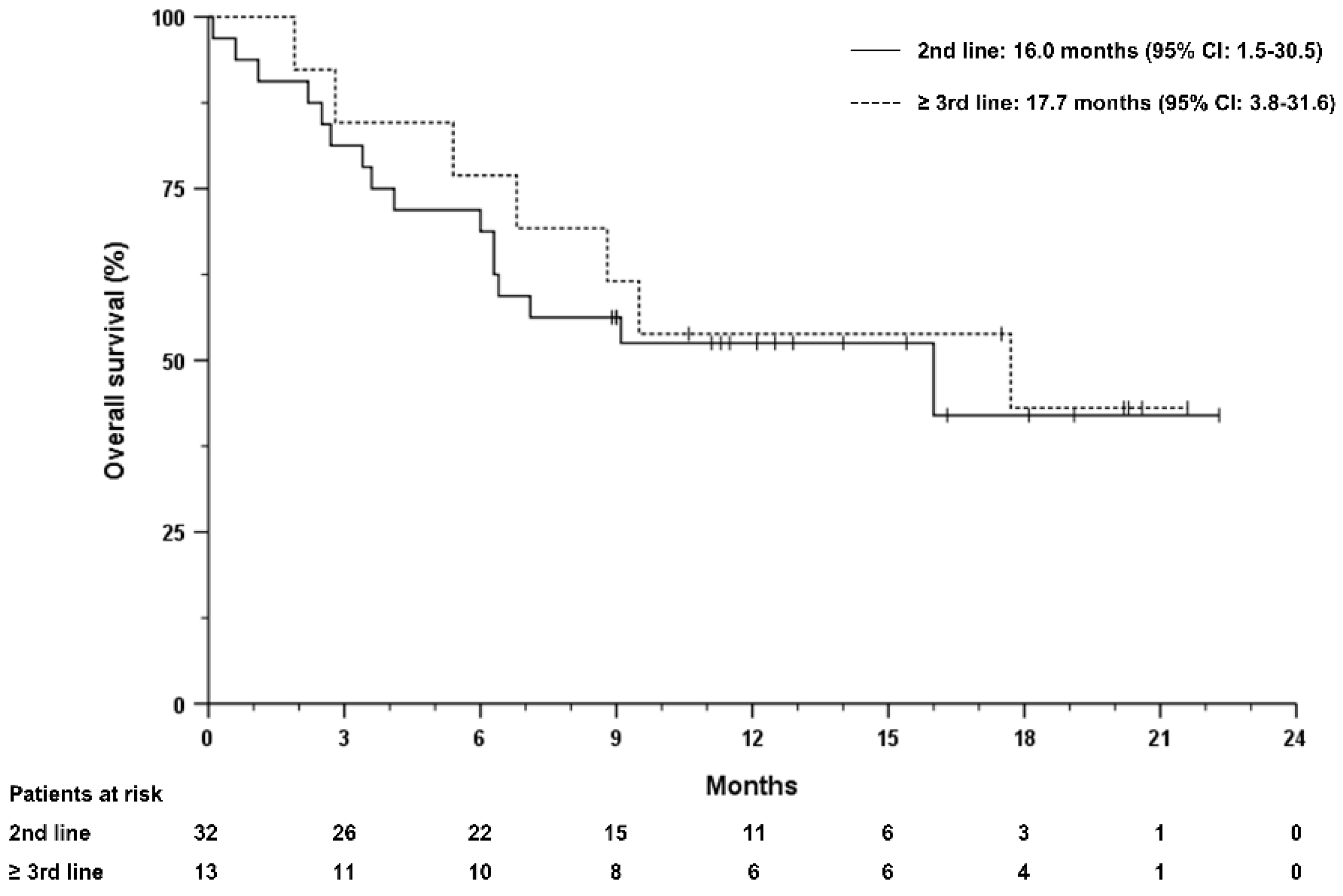
3.4. Prognostic Markers Associated with Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kim, E.; Viatour, P. Hepatocellular Carcinoma: Old Friends and New Tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging Cancer Trends among Young Adults in the USA: Analysis of a Population-Based Cancer Registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Voesch, A.S.; Bitzer, M.; Blödt, S.; Follmann, M.; Freudenberger, P.; Langer, T.; Lorenz, P.; Jansen, P.L.; Steubesand, N.; Galle, P.; et al. S3-Leitlinie: Diagnostik Und Therapie Des Hepatozellulären Karzinoms Und Biliärer Karzinome. Z. Gastroenterol. 2022, 60, 131–185. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet. Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.H.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Lim, H.Y.; Pracht, M.; et al. REACH-2: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of Ramucirumab versus Placebo as Second-Line Treatment in Patients with Advanced Hepatocellular Carcinoma (HCC) and Elevated Baseline Alpha-Fetoprotein (AFP) Following First-Line Sorafe. J. Clin. Oncol. 2018, 36, 4003. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A Randomized, Multi-Center Phase III Study of Nivolumab (NIVO) vs Sorafenib (SOR) as First-Line (1L) Treatment in Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Finn, R.S.; Chan, S.L.; Zhu, A.X.; Knox, J.J.; Cheng, A.-L.; Siegel, A.B.; Bautista, O.; Watson, P.; Kudo, M. KEYNOTE-240: Randomized Phase III Study of Pembrolizumab versus Best Supportive Care for Second-Line Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2017, 35, TPS503. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Tecentriq. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecentriq (accessed on 7 May 2022).
- FDA. FDA Approves Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma (accessed on 7 May 2022).
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellula Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G. Reply: Recent EUpdates to the ESMO Clinical Practice Guidelines on Hepatocellular Carcinoma, Cancer of the Pancreas, Soft Tissue and Visceral Sarcomas, Cancer of the Prostate and Gastric Cancer. Ann. Oncol. 2019, 30, 1396–1397. [Google Scholar] [CrossRef]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef]
- Yau, T.; Tai, D.; Chan, S.L.; Huang, Y.-H.; Choo, S.P.; Hsu, C.; Cheung, T.T.; Lin, S.-M.; Yong, W.P.; Lee, J.; et al. Systemic Treatment of Advanced Unresectable Hepatocellular Carcinoma After First-Line Therapy: Expert Recommendations from Hong Kong, Singapore and Taiwan. Liver Cancer 2022, 11, 426–439. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary Evidence of Safety and Tolerability of Atezolizumab plus Bevacizumab in Patients with Hepatocellular Carcinoma and Child-Pugh A and B Cirrhosis: A Real-World Study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef]
- Welland, S.; Leyh, C.; Finkelmeier, F.; Jefremow, A.; Shmanko, K.; Gonzalez-Carmona, M.A.; Kandulski, A.; Jeliazkova, P.; Best, J.; Fründt, T.W.; et al. Real-World Data for Lenvatinib in Hepatocellular Carcinoma (ELEVATOR): A Retrospective Multicenter Study. Liver Cancer 2022, 11, 219–232. [Google Scholar] [CrossRef]
- Himmelsbach, V.; Pinter, M.; Scheiner, B.; Venerito, M.; Sinner, F.; Zimpel, C.; Marquardt, J.U.; Trojan, J.; Waidmann, O.; Finkelmeier, F. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: Experience from Four Tertiary Centers. Cancers 2022, 14, 1722. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T. Articles Cabozantinib Plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co. Merck and Eisai Provide Update on Phase 3 LEAP-002 Trial Evaluating KEYTRUDA® (Pembrolizumab) Plus LENVIMA® (Lenvatinib) versus LENVIMA Monotherapy in Patients With Unresectable Hepatocellular Carcinoma. Available online: https://www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-leap-002-trial-evaluating-keytruda-pembrolizumab-plus-lenvima-lenvatinib-versus-lenvima-monotherapy-in-patients-with-unresectable-hepatocellul/ (accessed on 7 August 2022).

| Parameters | Patients, n = 50 (%) * |
|---|---|
| Median age, years (range) | 65 (50–80) |
| Gender, male/female | 41 (82)/9 (18) |
| ECOG, 0/1/2 | 27 (54)/17 (34)/6 (12) |
| Liver cirrhosis present | 41 (82) |
| Child-Pugh class, A/B/C | 30 (73)/9 (22)/2 (5) |
| BCLC stage, B/C/D | 7 (17)/23 (56)/11 (27) |
| ALBI score, grade 1/2/3 | 19 (38)/28 (56)/3 (6) |
| Risk factors for HCC: HCV/HBV/alcohol/NAFLD | 11 (21)/6 (12)/13 (25)/12 (23) |
| /other **/none | /5 (10)/5 (10) |
| AFP ≥ 400 ng/mL | 32 (64) |
| EHS | 27 (54) |
| Portal invasion | 17 (34) |
| EHS and/or portal invasion | 37 (74) |
| Prior surgical treatment | 20 (40) |
| Prior loco-regional treatment | 35 (70) |
| Prior loco-regional and/or surgical treatment | 40 (80) |
| Systemic treatment in first-line, lenvatinib/sorafenib/other *** | 26 (52)/16 (32)/11 (22) |
| Received atezolizumab plus bevacizumab in line, 2/3/4/5 | 34 (68)/9 (18)/6 (12)/1 (2) |
| Vital status at last follow-up, dead/alive/unknown | 23 (46), 22 (44), 5 (10) |
| Median observation period, months (range) | 10.1 (0.1–25.3) |
| Best Documented Response | Patients, n = 50 (%) | 2nd Line, n = 34 (%) | ≥3rd Line, n = 16 (%) |
|---|---|---|---|
| Complete response (CR) | 1 (2) | 1 (3) | 0 (0) |
| Partial response (PR) | 15 (30) | 10 (29) | 5 (31) |
| Stable disease (SD) | 18 (36) | 12 (35) | 6 (38) |
| Progressive disease | 9 (18) | 5 (15) | 4 (25) |
| Not evaluable | 7 (14) | 6 (18) | 1 (6) |
| Objective response rate (ORR) | 16 (32) | 11 (32) | 5 (31) |
| Disease control rate (DCR) | 34 (68) | 23 (68) | 11 (69) |
| TRAE | Any Grade, n (%) | Grade 1–2, n (%) | Grade 3–4, n (%) | Death, n (%) |
|---|---|---|---|---|
| Rash/exanthema | 6 (12) | 6 (12) | 0 | 0 |
| Esophageal variceal bleeding | 4 (8) | 0 | 3 (6) | 1 (2) |
| Fatigue | 4 (8) | 4 (8) | 0 | 0 |
| Thyroid toxicity | 3 (6) | 3 (6) | 0 | 0 |
| Hepatotoxicity/hepatitis | 2 (4) | 0 | 1 (2) | 1 (2) |
| Epistaxis | 2 (4) | 2 (4) | 0 | 0 |
| Hyponatremia | 2 (4) | 2 (4) | 0 | 0 |
| Hypertension | 2 (4) | 2 (4) | 0 | 0 |
| Pruritus | 2 (4) | 2 (4) | 0 | 0 |
| Hypoglycemia | 1 (2) | 0 | 0 | 1 (2) |
| Retroperitoneal bleeding | 1 (2) | 0 | 1 (2) | 0 |
| Hyperglycemia | 1 (2) | 0 | 1 (2) | 0 |
| Worsening asthma | 1 (2) | 0 | 1 (2) | 0 |
| Hepatic encephalopathy | 1 (2) | 1 (2) | 0 | 0 |
| Dyspnea | 1 (2) | 1 (2) | 0 | 0 |
| Dysphonia | 1 (2) | 1 (2) | 0 | 0 |
| Infusion reaction | 1 (2) | 1 (2) | 0 | 0 |
| Appetite loss | 1 (2) | 1 (2) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinner, F.; Pinter, M.; Scheiner, B.; Ettrich, T.J.; Sturm, N.; Gonzalez-Carmona, M.A.; Waidmann, O.; Finkelmeier, F.; Himmelsbach, V.; De Toni, E.N.; et al. Atezolizumab Plus Bevacizumab in Patients with Advanced and Progressing Hepatocellular Carcinoma: Retrospective Multicenter Experience. Cancers 2022, 14, 5966. https://doi.org/10.3390/cancers14235966
Sinner F, Pinter M, Scheiner B, Ettrich TJ, Sturm N, Gonzalez-Carmona MA, Waidmann O, Finkelmeier F, Himmelsbach V, De Toni EN, et al. Atezolizumab Plus Bevacizumab in Patients with Advanced and Progressing Hepatocellular Carcinoma: Retrospective Multicenter Experience. Cancers. 2022; 14(23):5966. https://doi.org/10.3390/cancers14235966
Chicago/Turabian StyleSinner, Friedrich, Matthias Pinter, Bernhard Scheiner, Thomas Jens Ettrich, Niklas Sturm, Maria A. Gonzalez-Carmona, Oliver Waidmann, Fabian Finkelmeier, Vera Himmelsbach, Enrico N. De Toni, and et al. 2022. "Atezolizumab Plus Bevacizumab in Patients with Advanced and Progressing Hepatocellular Carcinoma: Retrospective Multicenter Experience" Cancers 14, no. 23: 5966. https://doi.org/10.3390/cancers14235966
APA StyleSinner, F., Pinter, M., Scheiner, B., Ettrich, T. J., Sturm, N., Gonzalez-Carmona, M. A., Waidmann, O., Finkelmeier, F., Himmelsbach, V., De Toni, E. N., Ben Khaled, N., Mohr, R., Fründt, T. W., Kütting, F., Bömmel, F. v., Lieb, S., Krug, S., Bettinger, D., Schultheiß, M., ... Venerito, M. (2022). Atezolizumab Plus Bevacizumab in Patients with Advanced and Progressing Hepatocellular Carcinoma: Retrospective Multicenter Experience. Cancers, 14(23), 5966. https://doi.org/10.3390/cancers14235966







