C-Reactive Protein Pretreatment-Level Evaluation for Ewing’s Sarcoma Prognosis Assessment—A 15-Year Retrospective Single-Centre Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Preevaluation
2.2. Statistical Analysis
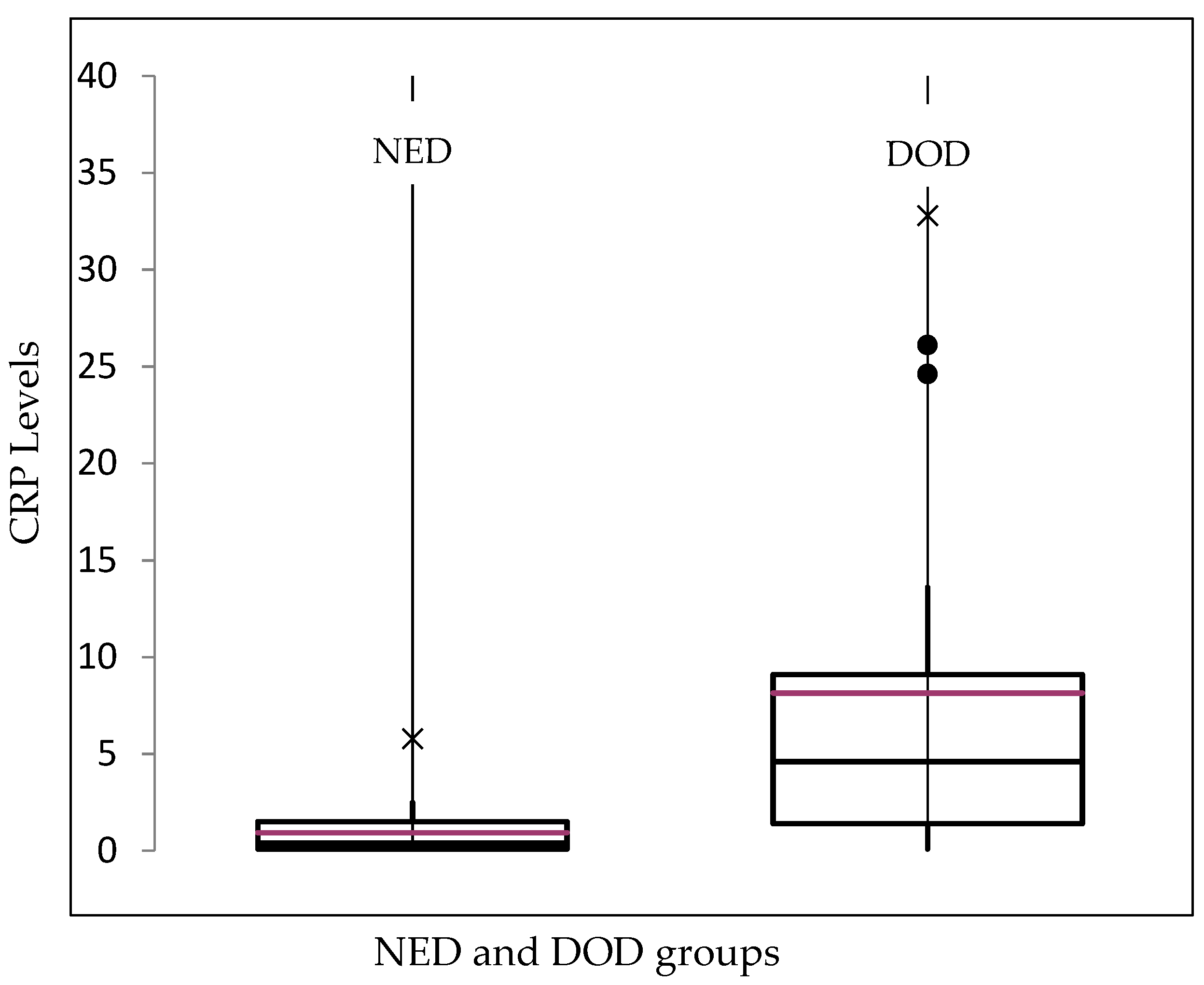
3. Results
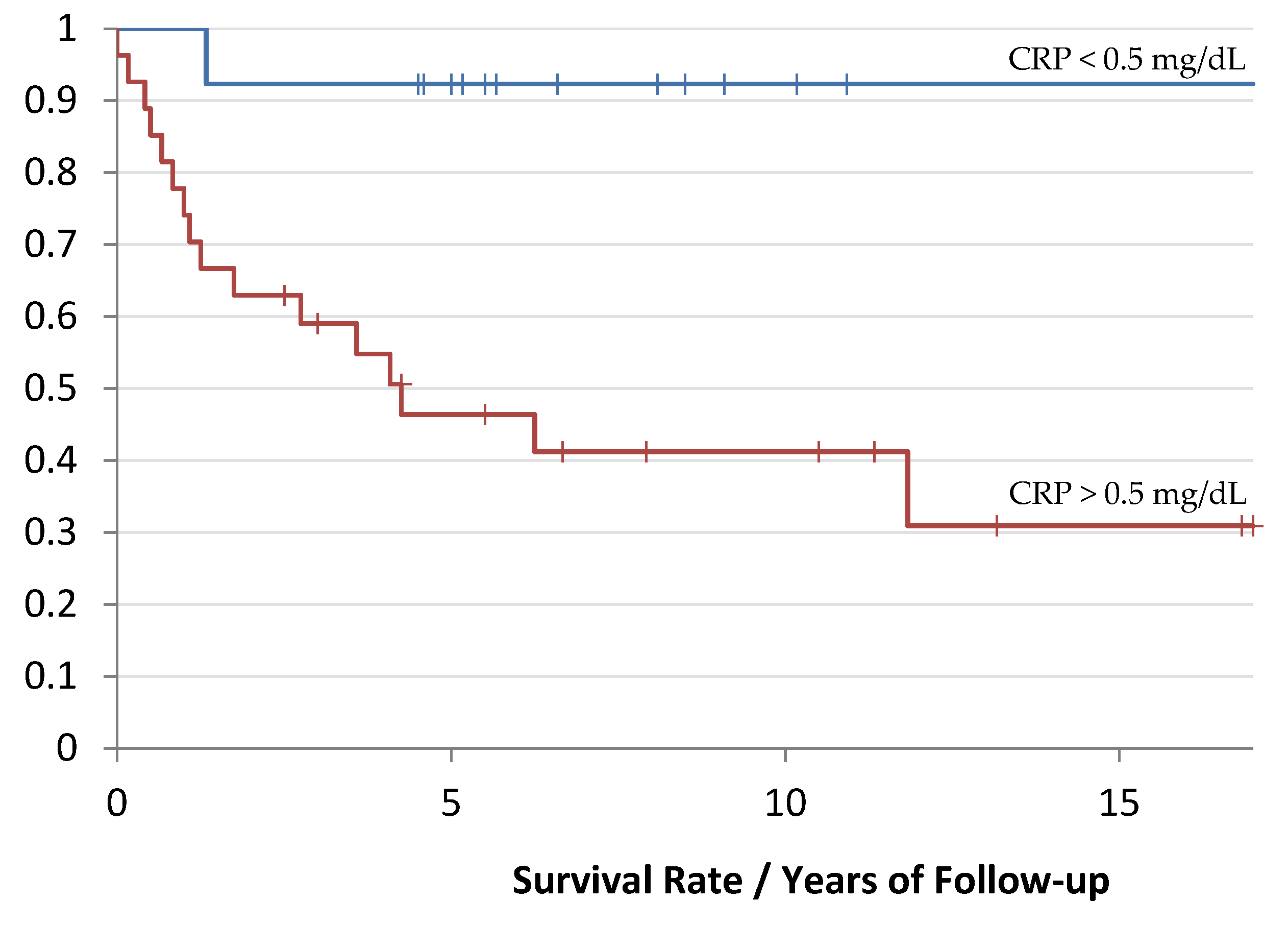
Survival Curves
4. Discussion
Mifamurtide
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Picci, P.; Manfrini, M.; Fabbri, N.; Gambarotti, M.; Vanel, D. Atlas of Musculoskeletal Tumors and Tumorlike Lesions: The Rizzoli Case Archive; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Ahmed, S.K.; Witten, B.G.; Harmsen, W.S.; Rose, P.S.; Krailo, M.; Marcus, K.J.; Randall, R.L.; DuBois, S.G.; Janeway, K.A.; Womer, R.B.; et al. Analysis of local control outcomes and clinical prognostic factors in localized pelvic Ewing sarcoma patients treated with radiation therapy: A Report from the Children’s Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2022. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Vega, R.B.M.; Viviers, E.; Morris, C.G.; Bradfield, S.M.; Gibbs, C.P.; Bradley, J.A. Modern Therapy for Chest Wall Ewing Sarcoma: An Update of the University of Florida Experience. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Vega, R.B.M.; Viviers, E.; Morris, C.G.; Bradfield, S.M.; Ranalli, N.J.; Bradley, J.A. Modern Therapy for Spinal and Paraspinal Ewing Sarcoma: An Update of the University of Florida Experience. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Durer, S.; Shaikh, H. Ewing Sarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shi, J.; Yang, J.; Ma, X.; Wang, X. Risk factors for metastasis and poor prognosis of Ewing sarcoma: A population based study. J. Orthop. Surg. Res. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Windsor, R.; Hamilton, A.; McTiernan, A.; Dileo, P.; Michelagnoli, M.; Seddon, B.; Strauss, S.J.; Whelan, J. Survival after high-dose chemotherapy for refractory and recurrent Ewing sarcoma. Eur. J. Cancer 2022, 170, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Anderton, J.; Moroz, V.; Marec-Berard, P.; Gaspar, N.; Laurence, V.; Martin-Broto, J.; Sastre, A.; Gelderblom, H.; Owens, C.; Kaiser, S.; et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours—EURO EWING 2012 Protocol. Trials 2020, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, U.; Brennan, B.; Le Deley, M.C.; Cozic, N.; van den Berg, H.; Bhadri, V.; Brichard, B.; Claude, L.; Craft, A.; Amler, S.; et al. High-Dose Chemotherapy Compared with Standard Chemotherapy and Lung Radiation in Ewing Sarcoma with Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. J. Clin. Oncol. 2019, 37, 3192–3202. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Aggerholm-Pedersen, N.; Sandfeld-Paulsen, B. Inflammation-scores as prognostic markers of overall survival in lung cancer: A register-based study of 6210 Danish lung cancer patients. BMC Cancer 2022, 22, 63. [Google Scholar] [CrossRef]
- Crassini, K.; Mulligan, S.P.; Best, O.G. Targeting chronic lymphocytic leukemia cells in the tumor microenviroment: A review of the in vitro and clinical trials to date. World J. Clin. Cases 2015, 3, 694–704. [Google Scholar] [CrossRef]
- Noruzinia, M.; Tavakkoly-Bazzaz, J.; Ahmadvand, M.; Azimi, F.; Dehghanifard, A.; Khakpour, G. Young Breast Cancer: Novel Gene Methylation in WBC. Asian Pac. J. Cancer Prev. 2021, 22, 2371–2375. [Google Scholar] [CrossRef]
- Danilova, A.B.; Baldueva, I.A. Neutrophils as tumor microenviroment member. Vopr. Onkol. 2016, 62, 35–44. [Google Scholar] [PubMed]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; Wrenger, S.; Gunzel, S.; Grunding, A.R.; Golpon, H.; Welte, T. Potential Roles of Acute Phase Proteins in Cancer: Why Do Cancer Cells Produce or Take up Exogenous Acute Phase Protein Alpha1-Antitrypsin? Front. Oncol. 2021, 11, 622076. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Eder, P.; Kolodziejczak, B.; Sobieska, M.; Krela-Kazmierczak, I.; Grzymislawski, M. Long-term prognostic utility of selected acute phase proteins in colorectal cancer. Pol. Arch. Intern. Med. 2019, 129, 292–294. [Google Scholar] [CrossRef]
- Biswas, B.; Rastogi, S.; Khan, S.A.; Mohanti, B.K.; Sharma, D.N.; Sharma, M.C.; Mridha, A.R.; Bakhshi, S. Outcomes and prognostic factors for Ewing-family tumors of the extremities. J. Bone Jt. Surg. Am. 2014, 96, 841–849. [Google Scholar] [CrossRef]
- Whelan, J.; Le Deley, M.C.; Dirksen, U.; Le Teuff, G.; Brennan, B.; Gaspar, N.; Hawkins, D.S.; Amler, S.; Bauer, S.; Bielack, S.; et al. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared with Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J. Clin. Oncol. 2018, 36, JCO2018782516. [Google Scholar] [CrossRef] [PubMed]
- Aggerholm-Pedersen, N.; Maretty-Kongstad, K.; Keller, J.; Baerentzen, S.; Safwat, A. The Prognostic Value of Serum Biomarkers in Localized Bone Sarcoma. Transl. Oncol. 2016, 9, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Grimer, R.J.; Gaston, C.L.; Watanuki, M.; Sudo, A.; Jeys, L. The prognostic value of the serum level of C-reactive protein for the survival of patients with a primary sarcoma of bone. Bone Jt. J. 2013, 95-B, 411–418. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, X.; Zhang, W.B.; Yi, C.; Wang, F.; Li, P. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag. Res. 2017, 9, 443–451. [Google Scholar] [CrossRef]
- Xu, K.; Lou, Y.; Sun, R.; Liu, Y.; Li, B.; Li, J.; Huang, Q.; Wan, W.; Xiao, J. Establishment of a Nomogram-Based Model for Predicting the Prognostic Value of Inflammatory Biomarkers and Preoperative D-Dimer Level in Spinal Ewing’s Sarcoma Family Tumors: A Retrospective Study of 83 Patients. World Neurosurg. 2019, 121, e104–e112. [Google Scholar] [CrossRef]
- Biswas, B.; Shukla, N.K.; Deo, S.V.; Agarwala, S.; Sharma, D.N.; Vishnubhatla, S.; Bakhshi, S. Evaluation of outcome and prognostic factors in extraosseous Ewing sarcoma. Pediatr. Blood Cancer 2014, 61, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Rastogi, S.; Khan, S.A.; Shukla, N.K.; Deo, S.V.; Agarwala, S.; Mohanti, B.K.; Sharma, M.C.; Vishnubhatla, S.; Bakhshi, S. Developing a prognostic model for localized Ewing sarcoma family of tumors: A single institutional experience of 224 cases treated with uniform chemotherapy protocol. J. Surg. Oncol. 2015, 111, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Thakar, A.; Mohanti, B.K.; Vishnubhatla, S.; Bakhshi, S. Prognostic factors in head and neck Ewing sarcoma family of tumors. Laryngoscope 2015, 125, E112–E117. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Fukushi, J.; Yamamoto, S.; Matsumoto, Y.; Setsu, N.; Oda, Y.; Yamada, H.; Okada, S.; Watari, K.; Ono, M.; et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 2011, 179, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.; Kwappenberg, K.M.; Varypataki, E.M.; Santos, S.J.; Kuijjer, M.L.; Mohamed, S.; Wijnen, J.T.; van Tol, M.J.; Cleton-Jansen, A.M.; Egeler, R.M.; et al. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-gamma. J. Exp. Clin. Cancer Res. 2014, 33, 27. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A. Muramyl Tripeptide-Phosphatidyl Ethanolamine Encapsulated in Liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 133–139. [Google Scholar] [PubMed]
- Kokkali, S.; Kotsantis, I.; Magou, E.; Sophia, T.; Kormas, T.; Diakoumis, G.; Spathas, N.; Psyrri, A.; Ardavanis, A. The addition of the immunomodulator mifamurtide to adjuvant chemotherapy for early osteosarcoma: A retrospective analysis. Investig. New Drugs 2022, 40, 668–675. [Google Scholar] [CrossRef]
- Punzo, F.; Bellini, G.; Tortora, C.; Pinto, D.D.; Argenziano, M.; Pota, E.; Paola, A.D.; Martino, M.D.; Rossi, F. Mifamurtide and TAM-like macrophages: Effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget 2020, 11, 687–698. [Google Scholar] [CrossRef]


| Spalte1 | n | Percentage | Mean | Range | SD |
|---|---|---|---|---|---|
| Age | 40 | 21.5 years | 4–62 years | 13.54 years | |
| Sex | |||||
| Male | 28 | 70% | - | - | - |
| Female | 12 | 30% | - | - | - |
| Site | |||||
| Femur | 11 | 27.50% | - | - | - |
| Tibia | 8 | 20% | - | - | - |
| Humerus | 2 | 5% | - | - | - |
| Clavicula | 2 | 5% | - | - | - |
| Radius | 2 | 5% | - | - | - |
| Hip | 8 | 20% | - | - | - |
| Foot | 2 | 5% | - | - | - |
| Spine | 1 | 2.50% | - | - | - |
| Multifocal | 4 | 10% | - | - | - |
| Follow-up | |||||
| DOD | 17 | 42.50% | - | - | - |
| NED | 21 | 52.50% | - | - | - |
| AWD | 2 | 5% | - | - | - |
| Metastasis | |||||
| Pulmonary | 7 | 17.50% | - | - | - |
| Skeletal | 2 | 5% | - | - | - |
| Multifocal | 10 | 25% | - | - | - |
| At diagnosis | 15 | 37.50% | - | - | - |
| None | 6 | 15% | |||
| Local recurrence | 6 | 15% | - | - | - |
| DFS | 40 | - | 3.5 years | 0–17 years | 54.5 years |
| Follow-up | 40 | - | 4.79 years | 0–17 years | 53.6 years |
| CRP | |||||
| >0.5 mg/dL | 27 | 67.50% | 1.6 mg/dL | 0.6–32.8 mg/dL | 9 mg/dL |
| <0.5 md/dL | 13 | 32. 50% | 0.1 mg/dL | 0.1–0.2 mg/dL | 0.05 mg/dL |
| CRP Levels | CRP Levels | p-Value | |||
|---|---|---|---|---|---|
| n (%) 95% CI | n (%) 95% CI | ||||
| Presence of | Absence of | ||||
| Metastasis | 19 (47.5%) | 2.3 to 11.9 | 21 (52.5%) | 0.3 to 1.8 | 0.009 |
| LR | 9 (22.5%) | −0.2 to 17.7 | 31 (77.5%) | 0.6 to 4.5 | 0.02 |
| Prognosis | DOD | NED/AWD | |||
| 17 (42.5%) | 2.9 to 13.3 | 23 (57.5%) | 0.3 to 1.4 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consalvo, S.; Hinterwimmer, F.; Harrasser, N.; Lenze, U.; Matziolis, G.; von Eisenhart-Rothe, R.; Knebel, C. C-Reactive Protein Pretreatment-Level Evaluation for Ewing’s Sarcoma Prognosis Assessment—A 15-Year Retrospective Single-Centre Study. Cancers 2022, 14, 5898. https://doi.org/10.3390/cancers14235898
Consalvo S, Hinterwimmer F, Harrasser N, Lenze U, Matziolis G, von Eisenhart-Rothe R, Knebel C. C-Reactive Protein Pretreatment-Level Evaluation for Ewing’s Sarcoma Prognosis Assessment—A 15-Year Retrospective Single-Centre Study. Cancers. 2022; 14(23):5898. https://doi.org/10.3390/cancers14235898
Chicago/Turabian StyleConsalvo, Sarah, Florian Hinterwimmer, Norbert Harrasser, Ulrich Lenze, Georg Matziolis, Rüdiger von Eisenhart-Rothe, and Carolin Knebel. 2022. "C-Reactive Protein Pretreatment-Level Evaluation for Ewing’s Sarcoma Prognosis Assessment—A 15-Year Retrospective Single-Centre Study" Cancers 14, no. 23: 5898. https://doi.org/10.3390/cancers14235898
APA StyleConsalvo, S., Hinterwimmer, F., Harrasser, N., Lenze, U., Matziolis, G., von Eisenhart-Rothe, R., & Knebel, C. (2022). C-Reactive Protein Pretreatment-Level Evaluation for Ewing’s Sarcoma Prognosis Assessment—A 15-Year Retrospective Single-Centre Study. Cancers, 14(23), 5898. https://doi.org/10.3390/cancers14235898





