CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
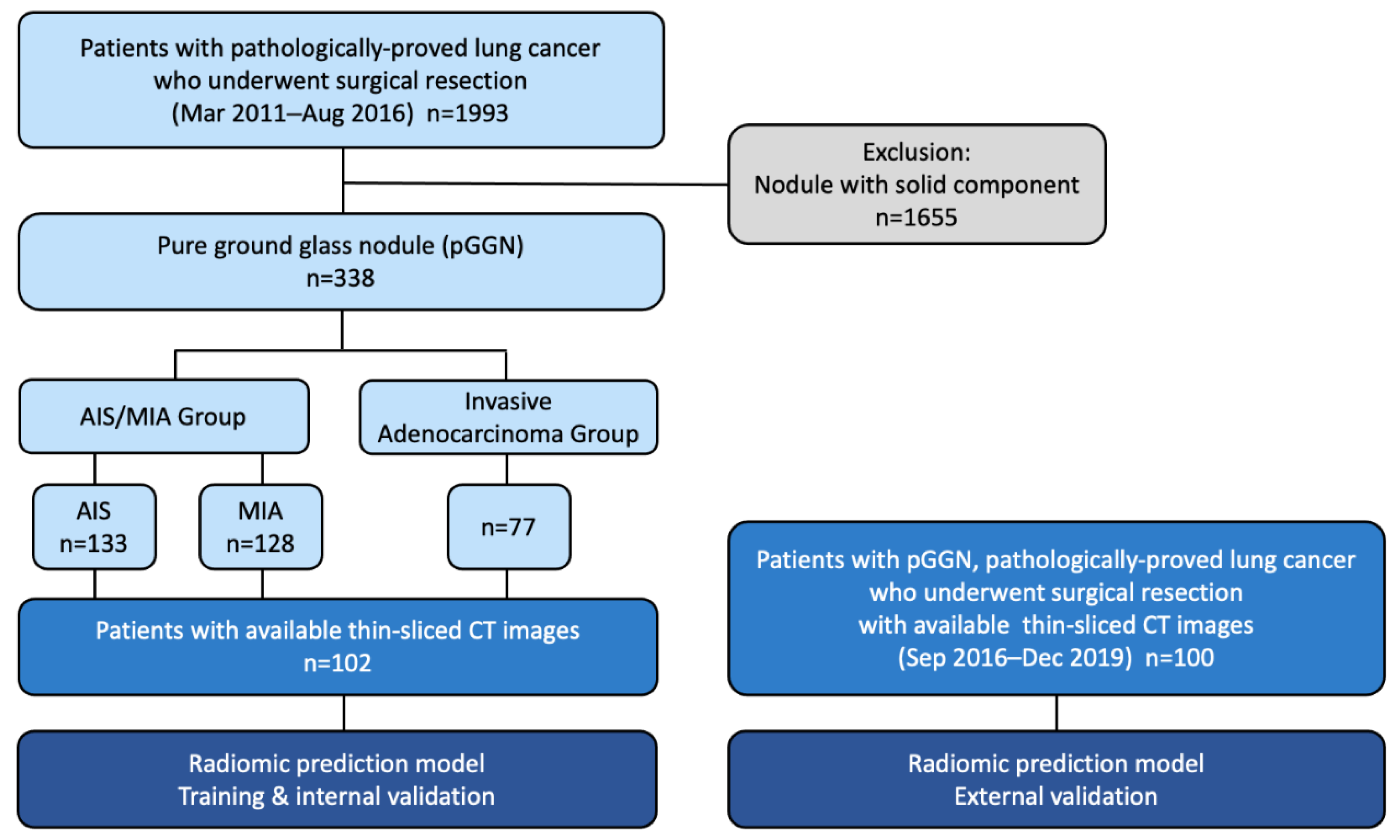
2.1. Study Population
2.2. Pathological Data Review
2.3. Radiomic Prediction Model Development
2.4. Image Acquisition
2.5. Segmentation
2.6. Statistical Feature Extraction and Prediction Model Construction
2.7. Statistical Analyses
3. Results
3.1. Patient Demographics and Clinicopathological Characteristics
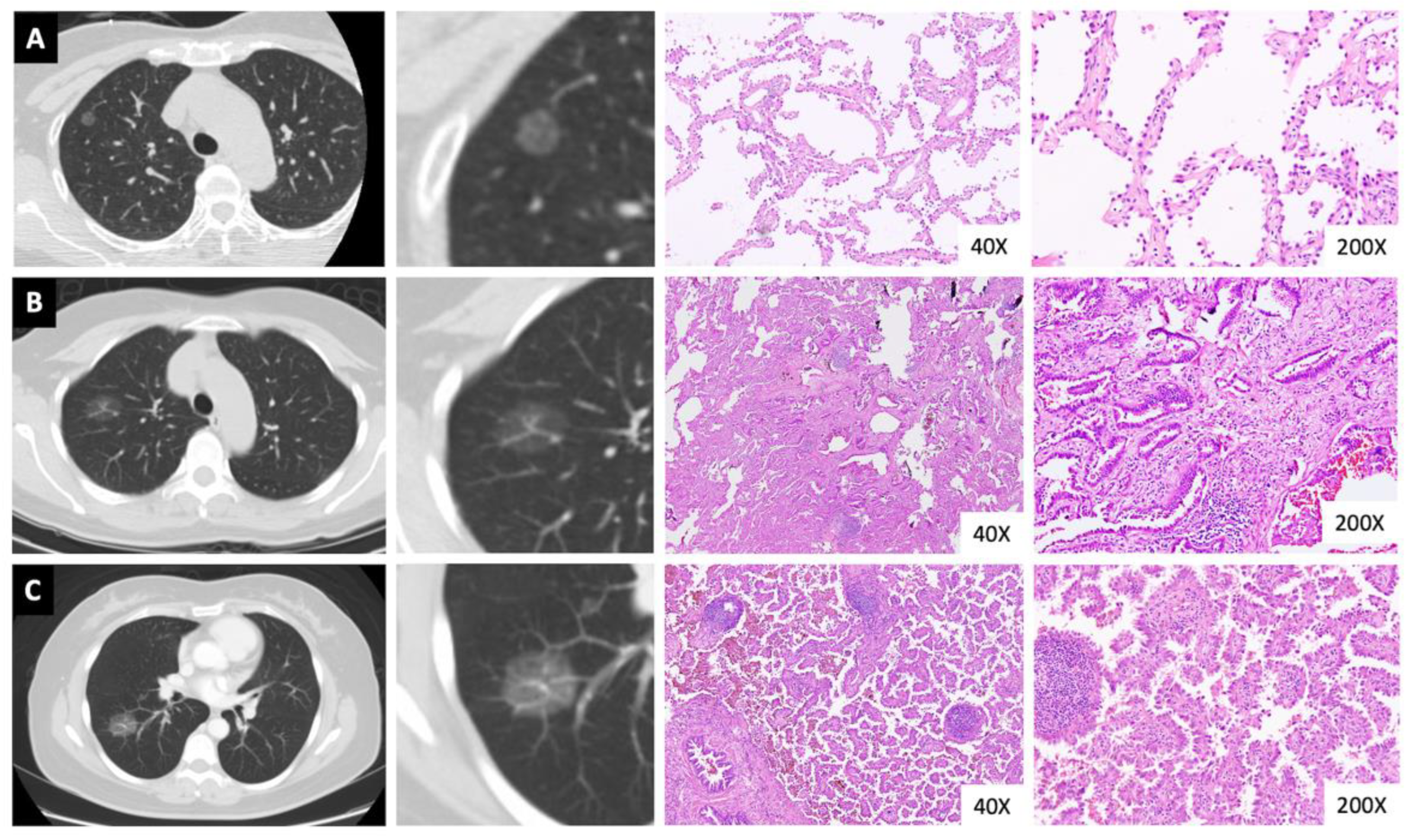
3.2. Pathological Outcomes
3.3. Perioperative Outcomes and Survival
3.4. Radiomic Feature Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Chiang, X.-H.; Hsu, H.-H.; Hsieh, M.-S.; Chang, C.-H.; Tsai, T.-M.; Liao, H.-C.; Tsou, K.-C.; Lin, M.-W.; Chen, J.-S. Propensity-matched analysis comparing survival after sublobar resection and lobectomy for cT1N0 lung adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 703–715. [Google Scholar] [CrossRef]
- Aoki, T.; Tomoda, Y.; Watanabe, H.; Nakata, H.; Kasai, T.; Hashimoto, H.; Kodate, M.; Osaki, T.; Yasumoto, K. Peripheral Lung Adenocarcinoma: Correlation of Thin-Section CT Findings with Histologic Prognostic Factors and Survival. Radiology 2001, 220, 803–809. [Google Scholar] [CrossRef]
- Suzuki, K.; Koike, T.; Asakawa, T.; Kusumoto, M.; Asamura, H.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; Shibata, T.; et al. A Prospective Radiological Study of Thin-Section Computed Tomography to Predict Pathological Noninvasiveness in Peripheral Clinical IA Lung Cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 2011, 6, 751–756. [Google Scholar] [CrossRef]
- Lin, M.W.; Su, K.Y.; Su, T.J.; Chang, C.C.; Lin, J.W.; Lee, Y.H.; Yu, S.L.; Chen, J.S.; Hsieh, M.S. Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer. Lung Cancer 2018, 125, 282–290. [Google Scholar] [CrossRef]
- Sundaram, V.; Gould, M.K.; Nair, V.S. A Comparison of the PanCAN Model and Lung-RADS to Assess Cancer Probability Among People with Screening-Detected, Solid Lung Nodules. Chest 2021, 159, 1273–1282. [Google Scholar] [CrossRef]
- IELCAP Investigators. International Early Lung Cancer Action Program Protocol 2021. Available online: www.IELCAP.org/protocols (accessed on 1 January 2022).
- Pedersen, J.H.; Rzyman, W.; Veronesi, G.; D’Amico, T.A.; Van Schil, P.; Molins, L.; Massard, G.; Rocco, G. Recommendations from the European Society of Thoracic Surgeons (ESTS) Regarding Computed Tomography Screening for Lung Cancer in Europe. Eur. J. Cardiothorac. Surg. 2017, 51, 411–420. [Google Scholar] [CrossRef] [PubMed]
- NCC Network. Lung Cancer Screening 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf (accessed on 1 January 2022).
- Callister, M.E.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society Guidelines for the Investigation and Management of Pulmonary Nodules. Thorax 2015, 70 (Suppl. S2), ii1–ii54. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- ACoRCo Lung-RADS®. Lung-RADS Assessment Categories Version 1.1. 2019. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf (accessed on 1 January 2022).
- She, Y.; Zhao, L.; Dai, C.; Ren, Y.; Zha, J.; Xie, H.; Jiang, S.; Shi, J.; Shi, S.; Shi, W.; et al. Preoperative Nomogram for Identifying Invasive Pulmonary Adenocarcinoma in Patients with Pure Ground-Glass Nodule: A Multi-institutional Study. Oncotarget 2017, 8, 17229–17238. [Google Scholar] [CrossRef]
- Qi, L.; Xue, K.; Li, C.; He, W.; Mao, D.; Xiao, L.; Hua, Y.; Li, M. Analysis of CT Morphologic Features and Attenuation for Differentiating Among Transient Lesions, Atypical Adenomatous Hyperplasia, Adenocarcinoma In Situ, Minimally Invasive and Invasive Adenocarcinoma Presenting as Pure Ground-Glass Nodules. Sci. Rep. 2019, 9, 14586. [Google Scholar] [CrossRef]
- Heidinger, B.H.; Anderson, K.R.; Nemec, U.; Costa, D.B.; Gangadharan, S.P.; VanderLaan, P.A.; Bankier, A.A. Lung Adenocarcinoma Manifesting as Pure Ground-Glass Nodules: Correlating CT Size, Volume, Density, and Roundness with Histopathologic Invasion and Size. J. Thorac. Oncol. 2017, 12, 1288–1298. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Powell, C.A.; Beer, D.; Riely, G.; Garg, K.; et al. International Association for the study of lung cancer/american thoracic society/european respiratory society: International multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc. Am. Thorac. Soc. 2011, 8, 381–385. [Google Scholar]
- Suzuki, K.; Watanabe, S.; Wakabayashi, M.; Moriya, Y.; Yoshino, I.; Tsuboi, M.; Mitsudomi, T.; Asamura, H. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J. Clin. Oncol. 2017, 35, 8561. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, S.-I.; Wakabayashi, M.; Saji, H.; Aokage, K.; Moriya, Y.; Yoshino, I.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 289–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Shen, X.; Wang, S.; Li, Y.; Hu, H.; Chen, H. Surgery for pre- and minimally invasive lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2022, 163, 456–464. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A Multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Shi, Z.; Deng, J.; She, Y.; Zhang, L.; Ren, Y.; Sun, W.; Su, H.; Dai, C.; Jiang, G.; Sun, X.; et al. Quantitative Features Can Predict Further Growth of Persistent Pure Ground-Glass Nodule. Quant. Imaging. Med. Surg. 2019, 9, 283–291. [Google Scholar] [CrossRef]
- Gu, Y.; She, Y.; Xie, D.; Dai, C.; Ren, Y.; Fan, Z.; Zhu, H.; Sun, X.; Xie, H.; Jiang, G.; et al. A Texture Analysis-Based Prediction Model for Lymph Node Metastasis in Stage IA Lung Adenocarcinoma. Ann. Thorac. Surg. 2018, 106, 214–220. [Google Scholar] [CrossRef]
- Wang, H.J.; Lin, M.W.; Chen, Y.C.; Chen, L.W.; Hsieh, M.S.; Yang, S.M.; Chen, H.F.; Wang, C.W.; Chen, J.S.; Chang, Y.C.; et al. A Radiomics Model Can Distinguish Solitary Pulmonary Capillary Haemangioma from Lung Adenocarcinoma. Interact. Cardiovasc. Thorac. Surg. 2021, 34, 369–377. [Google Scholar] [CrossRef]
- Chen, L.W.; Lin, M.W.; Hsieh, M.S.; Yang, S.M.; Wang, H.J.; Chen, Y.C.; Chen, H.Y.; Hu, Y.H.; Lee, C.E.; Chen, J.S.; et al. Radiomic Values from High-Grade Subtypes to Predict Spread Through Air Spaces in Lung Adenocarcinoma. Ann. Thorac. Surg. 2021, 114, 999–1006. [Google Scholar] [CrossRef]
- Chen, L.W.; Yang, S.M.; Wang, H.J.; Chen, Y.C.; Lin, M.W.; Hsieh, M.S.; Song, H.L.; Ko, H.J.; Chen, C.M.; Chang, Y.C. Prediction of Micropapillary and Solid Pattern in Lung Adenocarcinoma Using Radiomic Values Extracted from near-Pure Histopathological Subtypes. Eur. Radiol. 2021, 31, 5127–5138. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fang, M.; Li, Z.; Tu, W.; Wang, S.; Chen, W.; Tian, J.; Dong, D.; Liu, S. Radiomics Signature: A Biomarker for the Preoperative Discrimination of Lung Invasive Adenocarcinoma Manifesting as a Ground-Glass Nodule. Eur. Radiol. 2019, 29, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours: Thoracic Tumors, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 5. [Google Scholar]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Hsieh, M.S.; Hsu, H.H.; Tsai, T.M.; Chiang, X.H.; Tsou, K.C.; Liao, H.C.; Lin, M.W.; Chen, J.S. Correlation of Tumor Spread Through Air Spaces and Clinicopathological Characteristics in Surgically Resected Lung Adenocarcinomas. Lung Cancer 2018, 126, 189–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Matuszewski, B.J.; Shark, L.-K.; Moore, C.J. Medical image segmentation using new hybrid level-set method. In Proceedings of the 2008 Fifth International Conference BioMedical Visualization: Information Visualization in Medical and Biomedical Informatics, London, UK, 9–11 July 2008. [Google Scholar]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI’98, Cambridge, MA, USA, 11–13 October 1998; pp. 130–137. [Google Scholar]
- Xu, F.; Zhu, W.; Shen, Y.; Wang, J.; Xu, R.; Outesh, C.; Song, L.; Gan, Y.; Pu, C.; Hu, H. Corrigendum: Radiomic-Based Quantitative CT Analysis of Pure Ground-Glass Nodules to Predict the Invasiveness of Lung Adenocarcinoma. Front. Oncol. 2020, 10, 608365. [Google Scholar] [CrossRef]
- Henschke, C.I.; Yip, R.; Shaham, D.; Zulueta, J.J.; Aguayo, S.M.; Reeves, A.P.; Jirapatnakul, A.; Avila, R.; Moghanaki, D.; Yankelevitz, D.F.; et al. The Regimen of Computed Tomography Screening for Lung Cancer: Lessons Learned Over 25 Years from the International Early Lung Cancer Action Program. J. Thorac. Imaging. 2021, 36, 6–23. [Google Scholar] [CrossRef]
- Yanagawa, M.; Tanaka, Y.; Kusumoto, M.; Watanabe, S.; Tsuchiya, R.; Honda, O.; Sumikawa, H.; Inoue, A.; Inoue, M.; Okumura, M.; et al. Automated Assessment of Malignant Degree of Small Peripheral Adenocarcinomas Using Volumetric CT Data: Correlation with Pathologic Prognostic Factors. Lung Cancer 2010, 70, 286–294. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–623. [Google Scholar] [CrossRef] [PubMed]



| Variables | All (n = 338) | AIS/MIA (n = 261) | Invasive Adenocarcinoma (n = 77) | p-Value |
|---|---|---|---|---|
| Age > 65 years old | 67 (19.8) | 51 (19.5) | 16 (20.8) | 0.224 |
| Sex | 0.405 | |||
| Female | 241 (71.3) | 191 (73.2) | 50 (64.9) | |
| Male | 97 (28.7) | 70 (26.8) | 27 (35.1) | |
| ECOG | 0.329 | |||
| 0 | 277 (82.0) | 204 (78.2) | 73 (94.8) | |
| 1 | 61 (18.1) | 57 (21.8) | 4 (5.2) | |
| ≥2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| FVC (%) | 109.2 ± 13.0 | 110.0 ± 13.2 | 107.9 ± 12.5 | 0.939 |
| FEV1 (%) | 109.4 ± 14.8 | 109.7 ± 15.5 | 108.8 ± 13.8 | 0.308 |
| Smoker | 25 (7.4) | 20 (7.7) | 5 (6.5) | 0.102 |
| Family history of lung cancer | 92 (27.2) | 75 (28.7) | 17 (22.1) | 0.568 |
| Comorbidities | 129 (38.2) | 95 (36.4) | 34 (44.2) | 0.314 |
| Type 2 diabetes mellitus | 17 (5.0) | 12 (4.6) | 5 (6.5) | |
| Hypertension | 65 (19.2) | 51 (19.5) | 14 (18.2) | |
| Cardiac diseases | 29 (8.6) | 25 (9.6) | 4 (5.2) | |
| End-stage renal disease | 4 (1.2) | 4 (1.5) | 0 (0.0) | |
| History of other malignancies | 53 (15.7) | 48 (18.4) | 5 (6.5) | |
| Abnormal serum CEA level | 7 (2.1) | 3 (1.2) | 4 (5.2) | 0.033 |
| Thin-sliced CT images | 102 (30.2) | 90 (34.5) | 12 (15.6) | 0.002 |
| Tumor size on CT images (cm) | 1.1 ± 0.5 | 1.1 ± 0.4 | 1.3 ± 0.6 | <0.001 |
| Tumor density on CT images (HU) | −722.7 ± 47.3 | −727.0 ± 46.3 | −691.0 ± 45.1 | 0.013 |
| Variables | All (n = 338) | AIS/MIA (n = 261) | Invasive Adenocarcinoma (n = 77) | p-Value |
|---|---|---|---|---|
| LVI | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| VPI | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| STAS | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Grading | <0.001 | |||
| 1 | 287 (84.9) | 261 (100.0) | 26 (33.8) | |
| 2 | 51 (15.1) | 0 (0.0) | 51 (66.2) | |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Histological type | <0.001 | |||
| AIS | 133 (39.4) | 133 (51.0) | 0 (0.0) | |
| MIA | 128 (37.9) | 128 (49.0) | 0 (0.0) | |
| IA | 77 (22.8) | 0 (0.0) | 77 (100.0) | |
| Predominant subtype | <0.001 | |||
| Lepidic | 287 (84.9) | 261 (100.0) | 26 (33.8) | |
| Acinar | 43 (12.7) | 0 (0.0) | 43 (55.8) | |
| Papillary | 8 (2.4) | 0 (0.0) | 8 (10.4) | |
| Micropapillary | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Solid | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Presence of micropapillary orsolid components | 2 (0.6) | 0 (0.00) | 2 (2.6) | 0.009 |
| T stage | <0.001 | |||
| Tis | 133 (39.4) | 133 (51.0) | 0 (0.0) | |
| T1mi | 128 (37.9) | 128 (49.0) | 0 (0.0) | |
| T1a | 42 (12.4) | 0 (0.0) | 42 (54.6) | |
| T1b | 32 (9.5) | 0 (0.0) | 32 (41.6) | |
| T1c | 6 (1.8) | 0 (0.0) | 6 (7.8) | |
| LN metastasis | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Distant metastasis | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| TNM stage | <0.001 | |||
| AIS | 133 (39.4) | 133 (51.0) | 0 (0.0) | |
| IA1 | 170 (50.3) | 128 (49.0) | 42 (54.6) | |
| IA2 | 32 (9.5) | 0 (0.0) | 32 (41.6) | |
| IA3 | 6 (1.8) | 0 (0.0) | 6 (7.8) |
| Variables | All (n = 338) | AIS/MIA (n = 261) | Invasive Adenocarcinoma (n = 77) | p-Value |
|---|---|---|---|---|
| Surgery method | <0.001 | |||
| Wedge resection | 230 (68.1) | 187 (71.7) | 43 (55.8) | |
| Segmentectomy | 53 (15.7) | 43 (16.5) | 10 (13.0) | |
| Lobectomy | 55 (16.3) | 31 (11.9) | 24 (31.2) | |
| Surgery approach | >0.999 | |||
| VATS | 338 (100.0) | 261 (100.0) | 77 (100.0) | |
| Thoracotomy | 0 | 0 | 0 | |
| Single Port VATS | 169 (50.0) | 133 (51.0) | 36 (46.8) | 0.401 |
| Nonintubated VATS | 160 (47.3) | 130 (49.8) | 30 (39.0) | 0.886 |
| Mean no. of dissected lymph node stations | 3.0 (0–7) | 2.9 (0–7) | 3.4 (0–7) | 0.043 |
| Mean no. of dissected lymph nodes | 7.2 (0–41) | 6.9 (0–41) | 8.3 (0–37) | 0.050 |
| Operation time (minute) | 101.0 ± 40.5 | 99.2 ± 39.5 | 107.3 ± 43.3 | 0.079 |
| Blood loss (mL) | 4.3 (0–300) | 4.5 (0–300) | 3.8 (0–100) | 0.623 |
| Post-operative hospital stay (day) | 3 (1) | 3 (1) | 4 (1) | 0.077 |
| ICU stay (day) | 0 (0) | 0 (0) | 0 (0) | 0.521 |
| Chest tube drainage (day) | 1.7 (0–16) | 1.7 (0–11) | 1.8 (0–16) | 0.933 |
| Morbidities | 0.648 | |||
| Prolonged air leak > 5 days | 8 (2.4) | 5 (1.9) | 3 (3.9) | |
| Chylothorax | 2 (0.6) | 2 (0.8) | 0 | |
| Wound infection | 1 (0.30) | 1 (0.4) | 0 | |
| Hemothorax for re-open | 0 | 0 | 0 | |
| Vocal cord palsy | 0 | 0 | 0 | |
| 30-day mortality | 0 | 0 | 0 | >0.999 |
| Recurrence | 0 | 0 | 0 | >0.999 |
| Mean ± Standard Deviation | p-Value | ||
|---|---|---|---|
| Invasive Adenocarcinoma Group (n = 12) | AIS/MIA Group (n = 90) | ||
| Morphological features | |||
| Elongation | 0.83 ± 0.07 | 0.81 ± 0.11 | 0.532 |
| Flatness | 0.67 ± 0.13 | 0.63 ± 0.12 | 0.363 |
| MeshVolume | 745.59 ± 901.85 | 556.76 ± 436.62 | 0.489 |
| Sphericity | 0.62 ± 0.08 | 0.59 ± 0.11 | 0.281 |
| SurfaceArea | 615.02 ± 521.77 | 576.76 ± 402.49 | 0.811 |
| Histogram features | |||
| Skewness | 0.83 ± 0.32 | 1.04 ± 0.32 | 0.049 |
| Kurtosis | 2.85 ± 0.86 | 3.39 ± 1.04 | 0.064 |
| Uniformity | 0.003 ± 0.001 | 0.004 ± 0.001 | <0.005 |
| Entropy | 5.87 ± 0.19 | 5.63 ± 0.22 | <0.005 |
| 75th percentile (HU) | −615.33 ± 56.09 | −667.44 ± 59.21 | 0.009 |
| GLCM | |||
| Autocorrelation | 9648.29 ± 1620.72 | 8427.60 ± 1571.13 | 0.028 |
| Contrast | 136.19 ± 108.84 | 109.99 ± 87.14 | 0.438 |
| Correlation | 0.77 ± 0.11 | 0.74 ± 0.13 | 0.467 |
| ClusterProminence | 2,762,101.27 ± 1,823,084.98 | 1,684,816.56 ± 1,551,971.846 | 0.072 |
| ClusterShade | 4343.83 ± 8476.11 | 6282.64 ± 9819.01 | 0.477 |
| MaximumProbability | 0.004 ± 0.001 | 0.01 ± 0.00 | <0.005 |
| homogenity | 0.11 ± 0.02 | 0.14 ± 0.03 | <0.005 |
| GLRLM | |||
| ShortRunEmphasis | 0.92 ± 0.02 | 0.91 ± 0.03 | 0.259 |
| LongRunEmphasis | 1.48 ± 0.17 | 1.57 ± 0.29 | 0.159 |
| LowGrayLevelRunEmphasis | 0.001 ± 0.00 | 0.002 ± 0.00 | 0.046 |
| HighGrayLevelRunEmphasis | 632.07 ± 97.21 | 554.47 ± 97.40 | 0.021 |
| RunLengthVariance | 0.00002 ± 0.00 | 0.0003 ± 0.00 | <0.005 |
| GLSZM | |||
| SmallAreaEmphasis | 0.42 ± 0.08 | 0.39 ± 0.13 | 0.213 |
| LargeAreaEmphasis | 327,774.4 ± 335,630.8 | 282,469.3 ± 440,822.3 | 0.679 |
| LowGrayLevelZoneEmphasis | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.223 |
| HighGrayLevelZoneEmphasis | 18.01 ± 5.58 | 14.94 ± 4.37 | 0.090 |
| Variables | All (n = 100) | AIS/MIA (n = 58) | Invasive Adenocarcinoma (n = 42) | p-Value |
|---|---|---|---|---|
| Age > 65 years old | 23 (23.0) | 13 (22.4) | 10 (23.8) | 0.870 |
| Sex | 0.312 | |||
| Female | 72 (72.0) | 44 (75.9) | 28 (66.7) | |
| Male | 28 (28.0) | 14 (24.1) | 14 (33.3) | |
| ECOG | 0.123 | |||
| 0 | 83 (83.0) | 51 (87.9) | 32 (76.2) | |
| 1 | 17 (17.0) | 7 (12.1) | 10 (23.8) | |
| ≥2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| FVC (%) | 108.5 ± 14.4 | 110.1 ± 14.3 | 106.3 ± 14.5 | 0.211 |
| FEV1 (%) | 107.9 ± 14.1 | 109.4 ± 13.9 | 105.8 ± 14.2 | 0.209 |
| Smoker | 7 (7.0) | 3 (5.2) | 4 (9.5) | 0.400 |
| Family history of lung cancer | 24 (24.0) | 14 (24.1) | 10 (23.8) | 0.970 |
| Comorbidities | 33 (33.0) | 21 (36.2) | 12 (28.6) | 0.423 |
| Type 2 diabetes mellitus | 6 (6.0) | 4 (6.9) | 2 (4.8) | |
| Hypertension | 16 (16.0) | 9 (15.5) | 7 (16.7) | |
| Cardiac diseases | 8 (8.0) | 4 (6.9) | 4 (9.5) | |
| End-stage renal disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| History of other malignancies | 14 (14.0) | 9 (15.5) | 5 (11.9) | |
| Abnormal serum CEA level | 2 (2.0) | 1 (1.7) | 1 (2.4) | 0.909 |
| Tumor size on CT images (cm) | 1.9 ± 0.4 | 1.8 ± 0.4 | 2.0 ± 0.4 | 0.019 |
| Tumor density on CT images (HU) | −717.1 ± 51.0 | −731.1 ± 50.7 | −697.8 ± 45.2 | 0.001 |
| Variables | All (n = 100) | AIS/MIA (n = 58) | Invasive Adenocarcinoma (n = 42) | p-Value |
|---|---|---|---|---|
| LVI | 3 (3.0) | 0 (0.0) | 3 (7.1) | 0.039 |
| VPI | 1 (1.0) | 0 (0.0) | 1 (2.4) | 0.238 |
| STAS | 7 (7.0) | 0 (0.0) | 7 (16.7) | 0.001 |
| Grading | <0.001 | |||
| 1 | 73 (73.0) | 58 (100.0) | 15 (35.7) | |
| 2 | 27 (27.0) | 0 (0.0) | 27 (64.3) | |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Histological type | <0.001 | |||
| AIS | 24 (24.0) | 24 (41.4) | 0 (0.0) | |
| MIA | 34 (34.0) | 34 (58.6) | 0 (0.0) | |
| IA | 42 (42.0) | 0 (0.0) | 42 (100.0) | |
| Predominant subtype | <0.001 | |||
| Lepidic | 71 (71.0) | 58 (100.0) | 13 (31.0) | |
| Acinar | 27 (27.0) | 0 (0.0) | 27 (64.3) | |
| Papillary | 1 (1.0) | 0 (0.0) | 1 (2.4) | |
| Micropapillary | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Solid | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Presence of micropapillary orsolid components | 0 (0.0) | 0 (0.00) | 0 (0.0) | >0.999 |
| T stage | <0.001 | |||
| Tis | 24 (24.0) | 24 (41.4) | 0 (0.0) | |
| T1mi | 34 (34.0) | 34 (58.6) | 0 (0.0) | |
| T1a | 24 (24.0) | 0 (0.0) | 24 (57.1) | |
| T1b | 17 (17.0) | 0 (0.0) | 17 (16.7) | |
| T1c | 1 (1.0) | 0 (0.0) | 1 (2.4) | |
| LN metastasis | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Distant metastasis | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| TNM stage | <0.001 | |||
| AIS | 24 (24.0) | 24 (41.4) | 0 (0.0) | |
| IA1 | 58 (58.0) | 34 (58.6) | 24 (57.1) | |
| IA2 | 17 (17.0) | 0 (0.0) | 17 (16.7) | |
| IA3 | 1 (1.0) | 0 (0.0) | 1 (2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, T.-N.; Hsieh, M.-S.; Chen, L.-W.; Yang, C.-F.J.; Chuang, C.-C.; Chiang, X.-H.; Chen, Y.-C.; Lee, Y.-H.; Hsu, H.-H.; Chen, C.-M.; et al. CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule. Cancers 2022, 14, 5888. https://doi.org/10.3390/cancers14235888
Kao T-N, Hsieh M-S, Chen L-W, Yang C-FJ, Chuang C-C, Chiang X-H, Chen Y-C, Lee Y-H, Hsu H-H, Chen C-M, et al. CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule. Cancers. 2022; 14(23):5888. https://doi.org/10.3390/cancers14235888
Chicago/Turabian StyleKao, Tzu-Ning, Min-Shu Hsieh, Li-Wei Chen, Chi-Fu Jeffrey Yang, Ching-Chia Chuang, Xu-Heng Chiang, Yi-Chang Chen, Yi-Hsuan Lee, Hsao-Hsun Hsu, Chung-Ming Chen, and et al. 2022. "CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule" Cancers 14, no. 23: 5888. https://doi.org/10.3390/cancers14235888
APA StyleKao, T.-N., Hsieh, M.-S., Chen, L.-W., Yang, C.-F. J., Chuang, C.-C., Chiang, X.-H., Chen, Y.-C., Lee, Y.-H., Hsu, H.-H., Chen, C.-M., Lin, M.-W., & Chen, J.-S. (2022). CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule. Cancers, 14(23), 5888. https://doi.org/10.3390/cancers14235888







