Nectin-4 as Blood-Based Biomarker Enables Detection of Early Ovarian Cancer Stages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Patient Description
2.3. Control Cohort
2.4. Sera Samples
2.5. Detection of HB-EGF, Nectin-4 and AREG
2.6. Statistics
3. Results
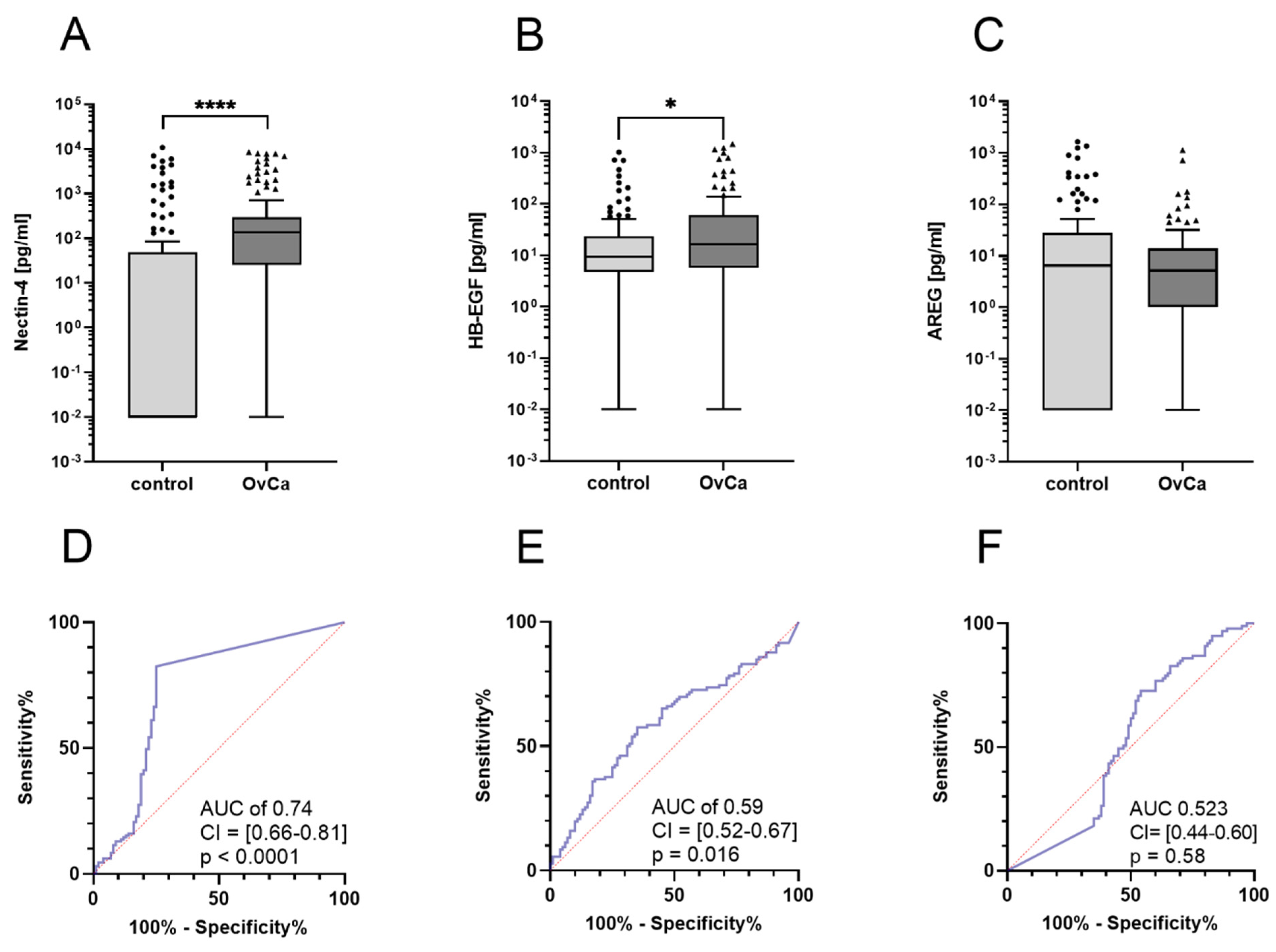
3.1. Nectin-4 and HB-EGF Levels Are Elevated in Ovarian Cancer Patients
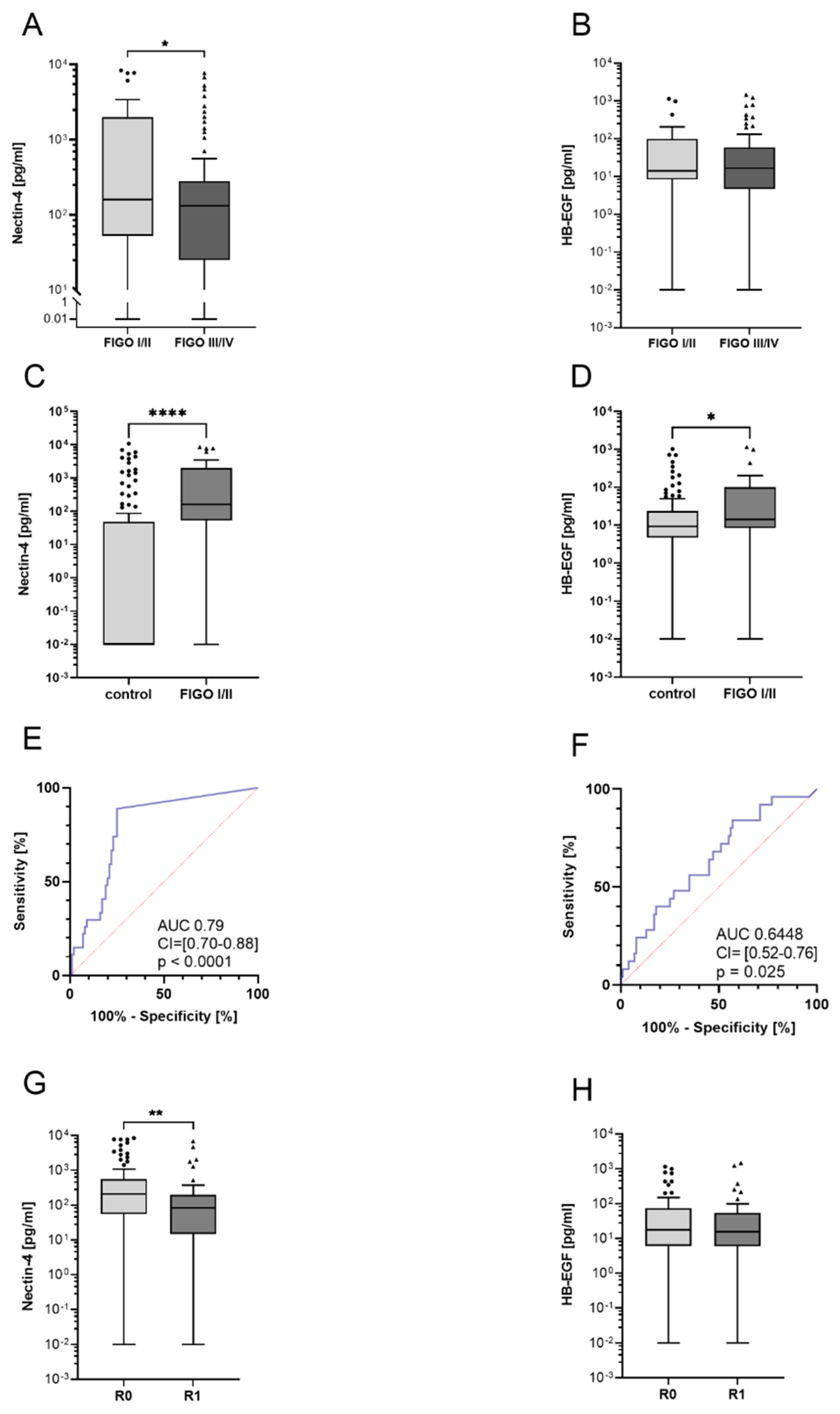
3.2. High Nectin-4 Levels Indicate Early-Stage Ovarian Cancer Patients
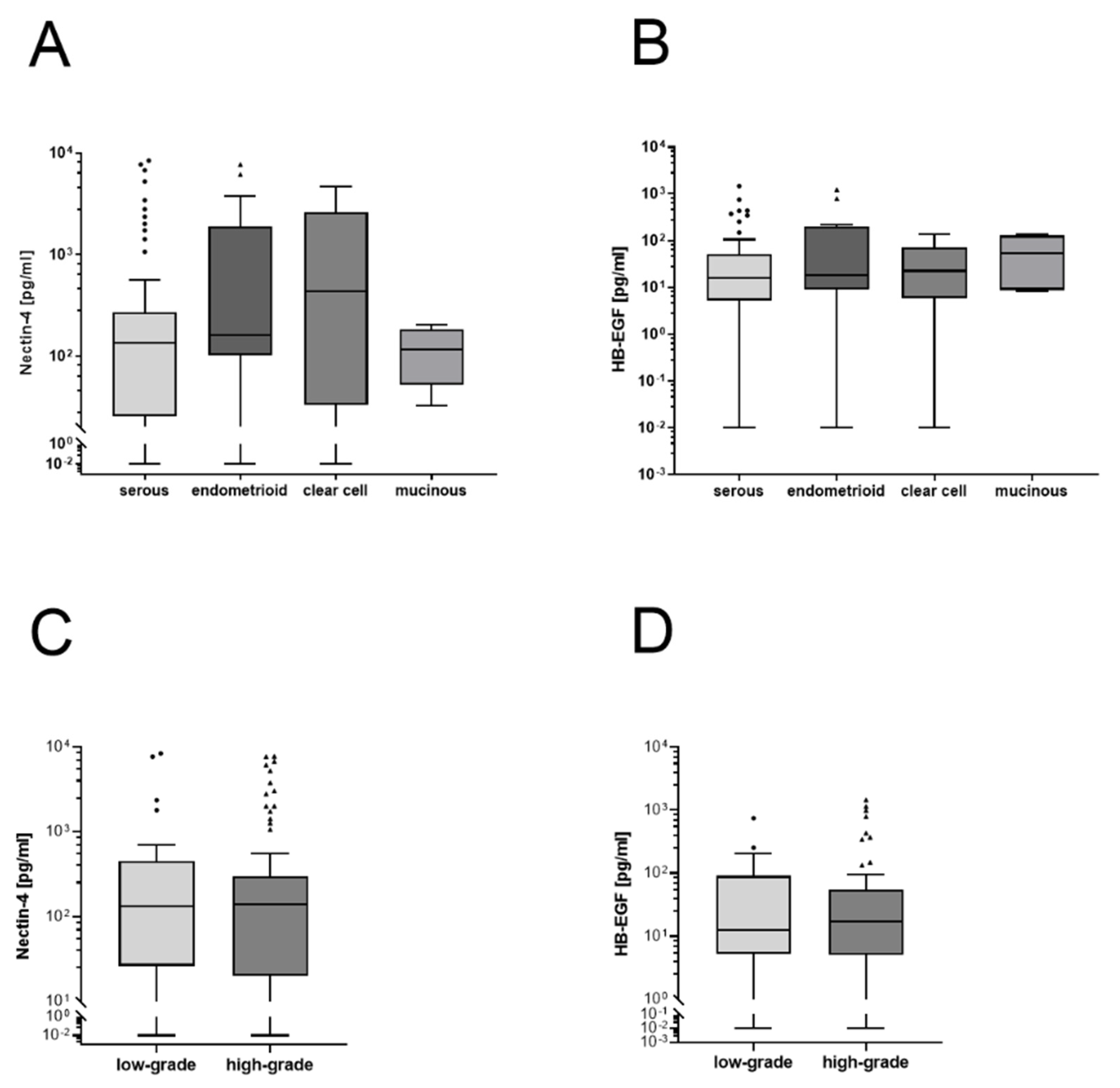
3.3. No Differences in Nectin-4 or HB-EGF Levels in Histological Subtypes or Tumor Grading
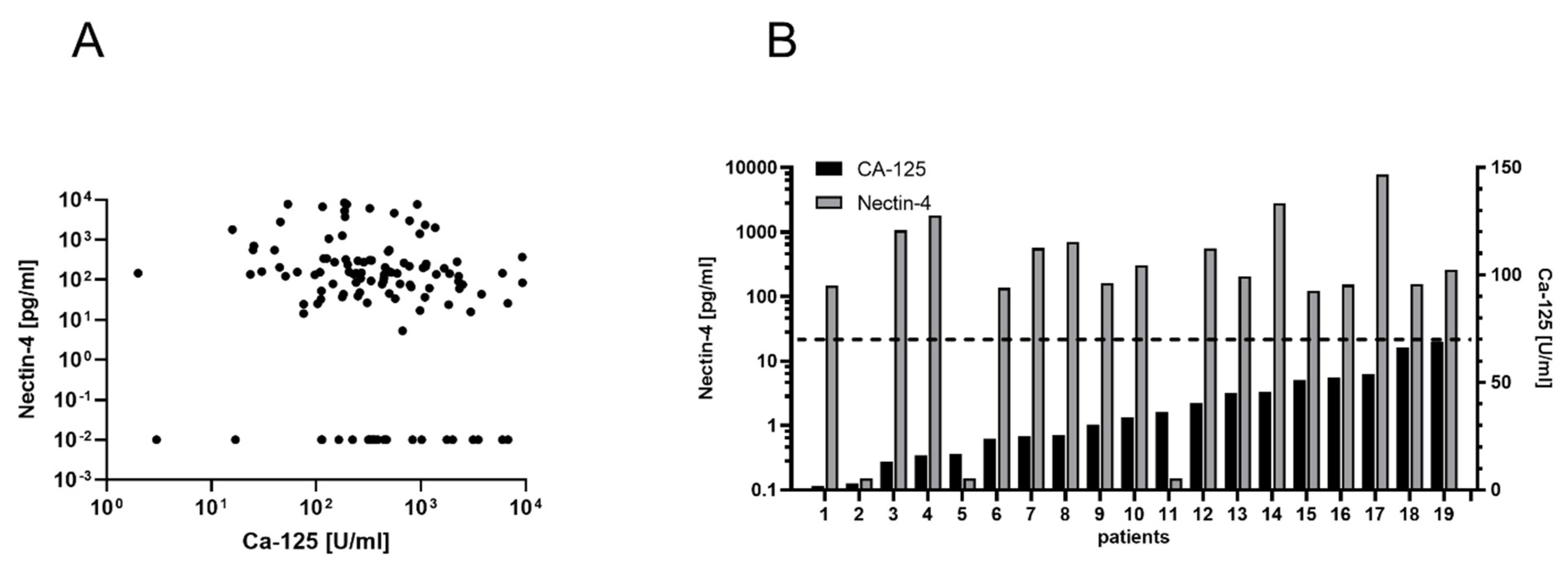
3.4. Ovarian Cancer Diagnostics When Ca-125 Fails
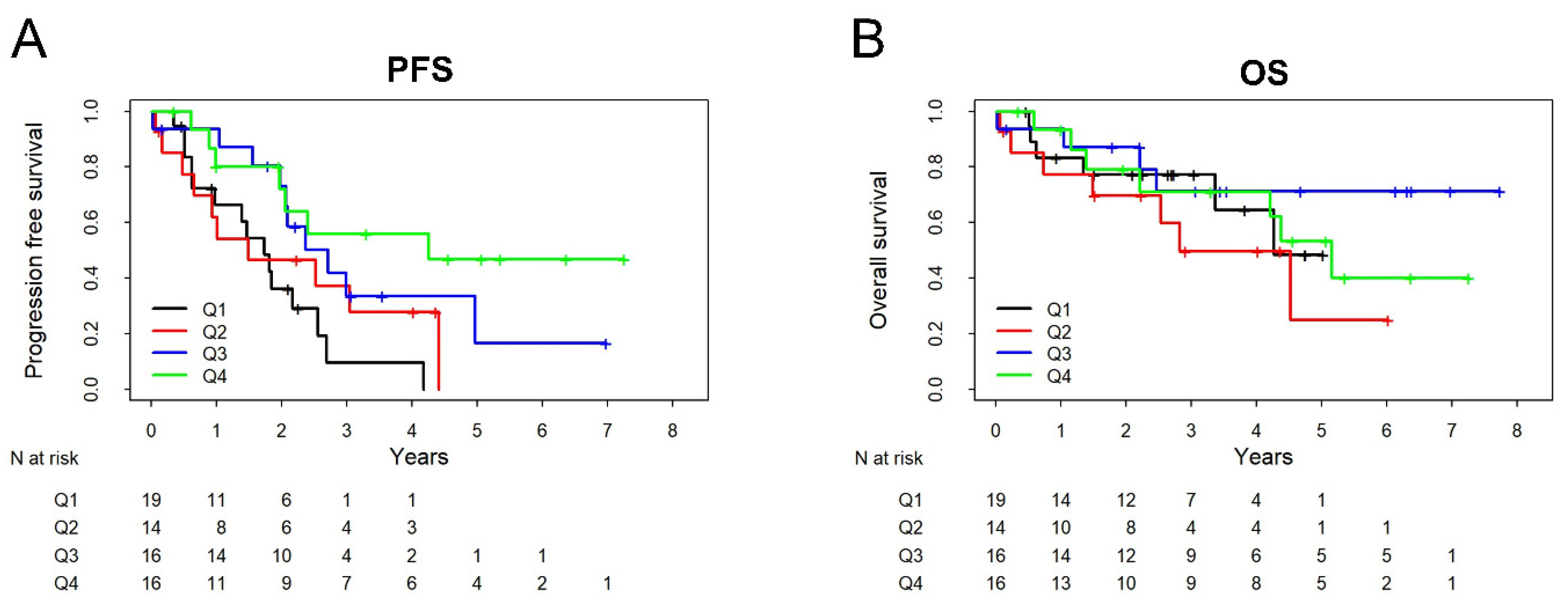
3.5. Prognostic Relevance of ADAM17 Substrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.N. Natural history of ovarian cancer. Ecancermedicalscience 2014, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Tropé, C.G. Ovarian cancer: Diagnostic, biological and prognostic aspects. Womens Health 2014, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Lück, H.-J.; Meier, W.; Adams, H.-P.; Möbus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, D.; Bast, R.C. Early detection of ovarian cancer. Dis. Markers 2007, 23, 397–410. [Google Scholar] [CrossRef]
- Kainz, C. Früherkennung und präoperative Diagnostik des Ovarialkarzinoms. Wien. Med. Wochenschr. 1996, 146, 2–7. [Google Scholar]
- Jacobs, I.; Bast, R.C. The CA 125 tumour-associated antigen: A review of the literature. Hum. Reprod. 1989, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Yetimalar, H.; Kasap, B.; Aydin, C.; Tatar, S.; Soylu, F.; Yildiz, F.S. Preoperative serum CA 125 level in the prediction of the stage of disease in endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Fiala, L.; Bob, P.; Raboch, J. Oncological markers CA-125, CA 19-9 and endometriosis. Medicine 2018, 97, e13759. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Kim, B.; Ahn, H.J.; Lee, K.-A.; Chung, J.E.; Han, S.W. Diagnostic performance of CA 125, HE4, and risk of Ovarian Malignancy Algorithm for ovarian cancer. J. Clin. Lab. Anal. 2019, 33, e22624. [Google Scholar] [CrossRef] [PubMed]
- Kondalsamy-Chennakesavan, S.; Hackethal, A.; Bowtell, D.; Obermair, A. Differentiating stage 1 epithelial ovarian cancer from benign ovarian tumours using a combination of tumour markers HE4, CA125, and CEA and patient’s age. Gynecol. Oncol. 2013, 129, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Rogmans, C.; Kuhlmann, J.D.; Hugendieck, G.; Link, T.; Arnold, N.; Weimer, J.P.; Flörkemeier, I.; Rambow, A.-C.; Lieb, W.; Maass, N.; et al. ADAM17-A Potential Blood-Based Biomarker for Detection of Early-Stage Ovarian Cancer. Cancers 2021, 13, 5563. [Google Scholar] [CrossRef]
- Sahin, U.; Blobel, C.P. Ectodomain shedding of the EGF-receptor ligand epigen is mediated by ADAM17. FEBS Lett. 2007, 581, 41–44. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.M.; McKiernan, E.; Bolster, F.; Ryan, B.M.; Hill, A.D.K.; McDermott, E.W.; Evoy, D.; O’Higgins, N.; Crown, J.; Duffy, M.J. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann. Oncol. 2008, 19, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.I.; Rose-John, S.; Jenkins, B.J. ADAM17: An Emerging Therapeutic Target for Lung Cancer. Cancers 2019, 11, 1218. [Google Scholar] [CrossRef]
- Richards, F.M.; Tape, C.J.; Jodrell, D.I.; Murphy, G. Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS ONE 2012, 7, e40597. [Google Scholar] [CrossRef]
- Blobel, C.P. ADAMs: Key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005, 6, 32–43. [Google Scholar] [CrossRef]
- Fukuhara, A.; Irie, K.; Yamada, A.; Katata, T.; Honda, T.; Shimizu, K.; Nakanishi, H.; Takai, Y. Role of nectin in organization of tight junctions in epithelial cells. Genes Cells 2002, 7, 1059–1072. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.H.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2013, 2, e00358. [Google Scholar] [CrossRef] [PubMed]
- Ogita, H.; Ikeda, W.; Takai, Y. Roles of cell adhesion molecules nectin and nectin-like molecule-5 in the regulation of cell movement and proliferation. J. Microsc. 2008, 231, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, R.; Ghasemi, R.; Brancati, F.; Sorda, R.L.; Tinari, N.; Perracchio, L.; Iacobelli, S.; Mottolese, M.; Natali, P.G.; Piantelli, M. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis 2014, 3, e118. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hu, H.; Pan, Y.; Gao, S. The Prognostic Role of Expression of Nectin-4 in Esophageal Cancer. Med. Sci. Monit. 2019, 25, 10089–10094. [Google Scholar] [CrossRef] [PubMed]
- Boylan, K.L.M.; Buchanan, P.C.; Manion, R.D.; Shukla, D.M.; Braumberger, K.; Bruggemeyer, C.; Skubitz, A.P.N. The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 2017, 8, 9717–9738. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, P.C.; Boylan, K.L.M.; Walcheck, B.; Heinze, R.; Geller, M.A.; Argenta, P.A.; Skubitz, A.P.N. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J. Biol. Chem. 2017, 292, 6339–6351. [Google Scholar] [CrossRef]
- Nabih, E.S.; Abdel Motaleb, F.I.; Salama, F.A. The diagnostic efficacy of nectin 4 expression in ovarian cancer patients. Biomarkers 2014, 19, 498–504. [Google Scholar] [CrossRef]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef]
- Kim, S.; Subramanian, V.; Abdel-Latif, A.; Lee, S. Role of Heparin-Binding Epidermal Growth Factor-Like Growth Factor in Oxidative Stress-Associated Metabolic Diseases. Metab. Syndr. Relat. Disord. 2020, 18, 186–196. [Google Scholar] [CrossRef]
- Tokumaru, S.; Higashiyama, S.; Endo, T.; Nakagawa, T.; Miyagawa, J.I.; Yamamori, K.; Hanakawa, Y.; Ohmoto, H.; Yoshino, K.; Shirakata, Y.; et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 2000, 151, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, R.; Yamazaki, S.; Asakura, M.; Takashima, S.; Hasuwa, H.; Miyado, K.; Adachi, S.; Kitakaze, M.; Hashimoto, K.; Raab, G.; et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc. Natl. Acad. Sci. USA 2003, 100, 3221–3226. [Google Scholar] [CrossRef]
- Lee, S.-W.; Commisso, C. Metabolic regulation of EGFR effector and feedback signaling in pancreatic cancer cells requires K-Ras. Biochem. Biophys. Res. Commun. 2020, 533, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; de Vos, J.; Jourdan, M.; Couderc, G.; Lu, Z.-Y.; Rossi, J.-F.; Klein, B. Cooperation between heparin-binding EGF-like growth factor and interleukin-6 in promoting the growth of human myeloma cells. Oncogene 2002, 21, 2584–2592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kjær, I.M.; Olsen, D.A.; Brandslund, I.; Bechmann, T.; Jakobsen, E.H.; Bogh, S.B.; Madsen, J.S. Prognostic impact of serum levels of EGFR and EGFR ligands in early-stage breast cancer. Sci. Rep. 2020, 10, 16558. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Tong, D.; Wang, X.; Guo, C.; Guo, B.; Yang, Y.; Zhao, L.; Zhang, J.; Yang, J.; et al. MeCP2 drives hepatocellular carcinoma progression via enforcing HOXD3 promoter methylation and expression through the HB-EGF/EGFR pathway. Mol. Oncol. 2021, 15, 3147–3163. [Google Scholar] [CrossRef]
- Kramer, C.; Klasmeyer, K.; Bojar, H.; Schulz, W.A.; Ackermann, R.; Grimm, M.-O. Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer 2007, 109, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Yotsumoto, F.; Fukagawa, S.; Kiyoshima, C.; Ouk, N.S.; Urushiyama, D.; Ito, T.; Katsuda, T.; Kurakazu, M.; Araki, R.; et al. Serum Heparin-binding Epidermal Growth Factor-like Growth Factor (HB-EGF) as a Biomarker for Primary Ovarian Cancer. Anticancer Res. 2017, 37, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.M.; Warunek, J.; Wohlfert, E.A. Therapeutic administration of IL-10 and amphiregulin alleviates chronic skeletal muscle inflammation and damage induced by infection. Immunohorizons 2018, 2, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nishioka, Y.; Yokoyama, Y.; Higashiyama, S.; Matsuura, N.; Matsuura, S.; Hieda, M. Nuclear envelope-localized EGF family protein amphiregulin activates breast cancer cell migration in an EGF-like domain independent manner. Biochem. Biophys. Res. Commun. 2012, 420, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Weskamp, G.; Kelly, K.; Zhou, H.-M.; Higashiyama, S.; Peschon, J.; Hartmann, D.; Saftig, P.; Blobel, C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004, 164, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Addison, C.L.; Ding, K.; Zhao, H.; Le Maître, A.; Goss, G.D.; Seymour, L.; Tsao, M.-S.; Shepherd, F.A.; Bradbury, P.A. Plasma transforming growth factor alpha and amphiregulin protein levels in NCIC Clinical Trials Group BR.21. J. Clin. Oncol. 2010, 28, 5247–5256. [Google Scholar] [CrossRef]
- Jing, C.; Jin, Y.H.; You, Z.; Qiong, Q.; Jun, Z. Prognostic value of amphiregulin and epiregulin mRNA expression in metastatic colorectal cancer patients. Oncotarget 2016, 7, 55890–55899. [Google Scholar] [CrossRef]
- McBryan, J.; Howlin, J.; Kenny, P.A.; Shioda, T.; Martin, F. ERalpha-CITED1 co-regulated genes expressed during pubertal mammary gland development: Implications for breast cancer prognosis. Oncogene 2007, 26, 6406–6419. [Google Scholar] [CrossRef] [PubMed]
- Lieb, W.; Jacobs, G.; Wolf, A.; Richter, G.; Gaede, K.I.; Schwarz, J.; Arnold, N.; Böhm, R.; Buyx, A.; Cascorbi, I.; et al. Linking pre-existing biorepositories for medical research: The PopGen 2.0 Network. J. Community Genet. 2019, 10, 523–530. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Klug, T.L.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Crimi, S.; Lavoro, A.; Rizzo, R.; Musumarra, G.; Gallo, S.; Facciponte, F.; Paratore, S.; Russo, A.; Bordonaro, R.; et al. Liquid Biopsy and Circulating Biomarkers for the Diagnosis of Precancerous and Cancerous Oral Lesions. Noncoding RNA 2022, 8, 60. [Google Scholar] [CrossRef]
- Peres, L.C.; Townsend, M.K.; Birmann, B.M.; Conejo-Garcia, J.R.; Kim, Y.; Kubzansky, L.D.; Magpantay, L.I.; Martinez-Maza, O.; Tworoger, S.S. Circulating Biomarkers of Inflammation and Ovarian Cancer Risk in the Nurses’ Health Studies. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Pfeiffer, R.M.; Hildesheim, A.; Engels, E.A.; Kemp, T.J.; Park, J.-H.; Katki, H.A.; Koshiol, J.; Shelton, G.; Caporaso, N.E.; et al. Circulating inflammation markers and prospective risk for lung cancer. J. Natl. Cancer Inst. 2013, 105, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Makgoeng, S.B.; Bolanos, R.S.; Jeon, C.Y.; Weiss, R.E.; Arah, O.A.; Breen, E.C.; Martínez-Maza, O.; Hussain, S.K. Markers of Immune Activation and Inflammation, and Non-Hodgkin Lymphoma: A Meta-Analysis of Prospective Studies. JNCI Cancer Spectr. 2018, 2, pky082. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, J.; Zhao, Z.; Zhao, C.; Xiang, Y. Circulating tumor DNA: A noninvasive biomarker for tracking ovarian cancer. Reprod. Biol. Endocrinol. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Sassu, C.M.; Palaia, I.; Boccia, S.M.; Caruso, G.; Perniola, G.; Tomao, F.; Di Donato, V.; Musella, A.; Muzii, L. Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 13650. [Google Scholar] [CrossRef]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2017, 8, 4008–4042. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.M.; Ryan, B.M.; Hill, A.D.K.; McDermott, E.; O’Higgins, N.; Duffy, M.J. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin. Cancer Res. 2007, 13, 2335–2343. [Google Scholar] [CrossRef]
- Saftig, P.; Reiss, K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: Novel drug targets with therapeutic potential? Eur. J. Cell Biol. 2011, 90, 527–535. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Z.; Wang, Y.; Dang, Y.-Z.; Meng, B.-X.; Wang, G.-D.; Zhang, J.; Wu, J.; Wen, N. ADAM17 promotes cell migration and invasion through the integrin β1 pathway in hepatocellular carcinoma. Exp. Cell Res. 2018, 370, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, H.; Nakajima, G.; Kaneko, Y.; Nakamura, A.; Inoue, Y.; Yamamoto, M.; Hayashi, K. Amphiregulin and Epiregulin mRNA expression in primary colorectal cancer and corresponding liver metastases. BMC Cancer 2012, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Khambata-Ford, S.; Garrett, C.R.; Meropol, N.J.; Basik, M.; Harbison, C.T.; Wu, S.; Wong, T.W.; Huang, X.; Takimoto, C.H.; Godwin, A.K.; et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 2007, 25, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Daigo, Y.; Takano, A.; Taniwaki, M.; Kato, T.; Hayama, S.; Murakami, H.; Takeshima, Y.; Inai, K.; Nishimura, H.; et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005, 65, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.E.; Ebrahim, M.A.; Eissa, L.A.; El-Shishtawy, M.M. Dickkopf-1 and Amphiregulin as Novel Biomarkers and Potential Therapeutic Targets in Hepatocellular Carcinoma. Int. J. Hematol. Oncol. Stem Cell Res. 2019, 13, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.A.; Pectasides, E.; Shabbeer, S.; Wiechmann, L.; Sparano, J.A.; Kenny, P.A. Evaluation of serum Amphiregulin levels in breast cancer patients and cancer-free controls. Exp. Hematol. Oncol. 2013, 2, 25. [Google Scholar] [CrossRef]
- Hanata, N.; Nagafuchi, Y.; Sugimori, Y.; Kobayashi, S.; Tsuchida, Y.; Iwasaki, Y.; Shoda, H.; Fujio, K. Serum Amphiregulin and Heparin-Binding Epidermal Growth Factor as Biomarkers in Patients with Idiopathic Inflammatory Myopathy. J. Clin. Med. 2021, 10, 3730. [Google Scholar] [CrossRef] [PubMed]
- Holtan, S.G.; Hoeschen, A.L.; Cao, Q.; Arora, M.; Bachanova, V.; Brunstein, C.G.; Miller, J.S.; Rashidi, A.; Slungaard, A.; Ustun, C.; et al. Facilitating resolution of life-threatening acute GVHD with human chorionic gonadotropin and epidermal growth factor. Blood Adv. 2020, 4, 1284–1295. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Elemam, N.M.; Ramakrishnan, R.K.; Salameh, L.; Olivenstein, R.; Hachim, I.Y.; Venkatachalam, T.; Mahboub, B.; Al Heialy, S.; Halwani, R.; et al. Blood and Salivary Amphiregulin Levels as Biomarkers for Asthma. Front. Med. 2020, 7, 561866. [Google Scholar] [CrossRef]
- Das, S.K.; Chakraborty, I.; Paria, B.C.; Wang, X.N.; Plowman, G.; Dey, S.K. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol. Endocrinol. 1995, 9, 691–705. [Google Scholar] [CrossRef]
- Ciarloni, L.; Mallepell, S.; Brisken, C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc. Natl. Acad. Sci. USA 2007, 104, 5455–5460. [Google Scholar] [CrossRef]
- Kjær, I.M.; Olsen, D.A.; Alnor, A.; Brandslund, I.; Bechmann, T.; Madsen, J.S. EGFR and EGFR ligands in serum in healthy women; reference intervals and age dependency. Clin. Chem. Lab. Med. 2019, 57, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Kasai, N.; Kobayashi, K.; Shioya, S.; Yoshikawa, Y.; Yotsumoto, F.; Miyamoto, S.; Mekada, E.; Enokizono, J. Soluble heparin-binding EGF-like growth factor (HB-EGF) detected by newly developed immuno-PCR method is a clear-cut serological biomarker for ovarian cancer. Am. J. Transl. Res. 2012, 4, 415–421. [Google Scholar] [PubMed]
- Higashiyama, S.; Nanba, D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim. Biophys. Acta 2005, 1751, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, N.; Herz, A.; Schiepanski, J.H.; Dittrich, J.; Sebens, S.; Dempfle, A.; Feuerborn, J.; Rogmans, C.; Tribian, N.; Flörkemeier, I.; et al. ADAM17 Inhibition Increases the Impact of Cisplatin Treatment in Ovarian Cancer Spheroids. Cancers 2021, 13, 2039. [Google Scholar] [CrossRef]
- Siddharth, S.; Nayak, A.; Das, S.; Nayak, D.; Panda, J.; Wyatt, M.D.; Kundu, C.N. The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-β4. Int. J. Biochem. Cell Biol. 2018, 102, 151–160. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Cheng, N.; Chytil, A.; Shyr, Y.; Joly, A.; Moses, H.L. Transforming growth factor-beta signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol. Cancer Res. 2008, 6, 1521–1533. [Google Scholar] [CrossRef]
- Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2059–2070. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Cui, R.; Wang, Y.; Li, Y.; Li, Y. Clinical value of ROMA index in diagnosis of ovarian cancer: Meta-analysis. Cancer Manag. Res. 2019, 11, 2545–2551. [Google Scholar] [CrossRef]
- Bekos, C.; Muqaku, B.; Dekan, S.; Horvat, R.; Polterauer, S.; Gerner, C.; Aust, S.; Pils, D. NECTIN4 (PVRL4) as Putative Therapeutic Target for a Specific Subtype of High Grade Serous Ovarian Cancer-An Integrative Multi-Omics Approach. Cancers 2019, 11, 698. [Google Scholar] [CrossRef]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef]
- Khella, C.A.; Mehta, G.A.; Mehta, R.N.; Gatza, M.L. Recent Advances in Integrative Multi-Omics Research in Breast and Ovarian Cancer. J. Pers. Med. 2021, 11, 149. [Google Scholar] [CrossRef]
- Yan, T.; Ma, X.; Hu, H.; Gong, Z.; Zheng, H.; Xie, S.; Guo, L.; Lu, R. Serology-Based Model for Personalized Epithelial Ovarian Cancer Risk Evaluation. Curr. Oncol. 2022, 29, 2695–2705. [Google Scholar] [CrossRef]
- Bast, R.C.; Lu, Z.; Han, C.Y.; Lu, K.H.; Anderson, K.S.; Drescher, C.W.; Skates, S.J. Biomarkers and Strategies for Early Detection of Ovarian Cancer. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 2504–2512. [Google Scholar] [CrossRef]
| Clinical Parameter | Characteristics | N | Percentage |
|---|---|---|---|
| Histology | Serous | 97 | 74.0% |
| Endometrioid | 17 | 13.0% | |
| Clear-cell | 8 | 6.1% | |
| Mucinous | 4 | 3.0% | |
| Others | 5 | 3.8% | |
| Grading | Low | 29 | 22.1% |
| High | 90 | 68.7% | |
| n.a. | 12 | 9.1% | |
| FIGO | I | 20 | 15.3% |
| II | 6 | 4.5% | |
| III | 81 | 61.8% | |
| IV | 23 | 17.5% | |
| n.a. | 1 | 0.9% | |
| Resection of the tumor | R0 | 72 | 54.9% |
| R1 | 56 | 42.7% | |
| n.a. | 3 | 2.3% | |
| Response to chemotherapy | Sensitive | 86 | 65.6% |
| Resistant | 24 | 18.3% | |
| n.a. | 21 | 16.0% | |
| Progression-free survival | Median 23.0/Min.: 0/Max.: 87/25.P.: 10.5/75.P.: 35.5 (in month) | ||
| Overall survival | Median 33.8/Min.: 0.2/Max.: 94/25.P.: 15.7/75.P.: 55.7 (in month) | ||
| Age | Mean 60.3 +/− 10.4 /Min.: 29/Max.: 89 (in years) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogmans, C.; Feuerborn, J.; Treeck, L.; Tribian, N.; Flörkemeier, I.; Arnold, N.; Weimer, J.P.; Maass, N.; Jansen, P.; Lieb, W.; et al. Nectin-4 as Blood-Based Biomarker Enables Detection of Early Ovarian Cancer Stages. Cancers 2022, 14, 5867. https://doi.org/10.3390/cancers14235867
Rogmans C, Feuerborn J, Treeck L, Tribian N, Flörkemeier I, Arnold N, Weimer JP, Maass N, Jansen P, Lieb W, et al. Nectin-4 as Blood-Based Biomarker Enables Detection of Early Ovarian Cancer Stages. Cancers. 2022; 14(23):5867. https://doi.org/10.3390/cancers14235867
Chicago/Turabian StyleRogmans, Christoph, Julia Feuerborn, Leonie Treeck, Nils Tribian, Inken Flörkemeier, Norbert Arnold, Jörg Paul Weimer, Nicolai Maass, Peer Jansen, Wolfgang Lieb, and et al. 2022. "Nectin-4 as Blood-Based Biomarker Enables Detection of Early Ovarian Cancer Stages" Cancers 14, no. 23: 5867. https://doi.org/10.3390/cancers14235867
APA StyleRogmans, C., Feuerborn, J., Treeck, L., Tribian, N., Flörkemeier, I., Arnold, N., Weimer, J. P., Maass, N., Jansen, P., Lieb, W., Dempfle, A., Bauerschlag, D. O., & Hedemann, N. (2022). Nectin-4 as Blood-Based Biomarker Enables Detection of Early Ovarian Cancer Stages. Cancers, 14(23), 5867. https://doi.org/10.3390/cancers14235867






