The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection
- Luminal B BC: Epirubicin and cyclophosphamide (EC) or doxorubicin and cyclophosphamide (AC) for 16 patients; Epirubicin (EPI) + docetaxel for 33 patients.
- Luminal B + HER-2 BC: EC/AC + trastuzumab for 26 patients; EPI + docetaxel + Trastuzumab for 7 patients.
- TNBC: EC/AC for 11 patients; EPI + docetaxel for 21 patients.
2.2. Response Assessment and Follow-Up
2.3. Image Acquisition
2.4. Response Assessment and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. [18F]FDG PET/CT Results
3.2. [18F]FDG PET/CT Semiquantitative Data and Response to NAC
3.3. [18F]FDG PET/CT Semiquantitative Data and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Villegas, S.L.; Nekljudova, V.; Pfarr, N.; Engel, J.; Untch, M.; Schrodi, S.; Holms, F.; Ulmer, H.U.; Fasching, P.A.; Weber, K.E.; et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors—An analysis of 2765 patients from neoadjuvant clinical trials. Eur. J. Cancer 2021, 148, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Neo, S.Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393. [Google Scholar] [CrossRef] [PubMed]
- Tryfonidis, K.; Senkus, E.; Cardoso, M.J.; Cardoso, F. Management of locally advanced breast cancer—Perspectives and future directions. Nat. Rev. Clin. Oncol. 2015, 12, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Bar, Y.; Isakoff, S.J. The Evolving Role of Neoadjuvant Therapy for Operable Breast Cancer. J. Natl. Compr. Cancer Netw. 2022, 20, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 102375. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.G.M.; van de Velde, C.J.H. Neoadjuvant chemotherapy in breast cancer: More than just downsizing. Lancet Oncol. 2018, 19, 2–3. [Google Scholar] [CrossRef]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging neoadjuvant therapy response in breast cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef]
- Bahri, S.; Chen, J.H.; Mehta, R.S.; Carpenter, P.M.; Nie, K.; Kwon, S.Y.; Yu, H.J.; Nalcioglu, O.; Su, M.Y. Residual Breast Cancer Diagnosed by MRI in Patients Receiving Neoadjuvant Chemotherapy with and Without Bevacizumab. Ann. Surg. Oncol. 2009, 16, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, L.; Jin, P.; Hu, L.; Li, X.; Guo, T.; Yang, K. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast 2018, 40, 106–115. [Google Scholar] [CrossRef]
- Han, S.; Choi, J.Y. Prognostic value of 18F-FDG PET and PET/CT for assessment of treatment response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2020, 22, 1–15. [Google Scholar] [CrossRef]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, D.M.; Mason, B.A.; Prestipino, A.J.; Seinige, U.L.; Lieber, C.P.; Baloch, Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: A determinant of outcome. J. Am. Coll. Surg. 1995, 180, 297–306. [Google Scholar] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Van der Hoeven, J.J.M.; Van der Wall, E.; Van der Groep, P.; Van Diest, P.J.; Comans, E.F.I.; Joshi, U.; Semenza, G.L.; Hoekstra, O.S.; Lammertsma, A.A.; et al. Biologic correlates of 18fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J. Clin. Oncol. 2002, 20, 379–387. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; De Roquancourt, A.; Hamy, A.S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Urso, L.; Quartuccio, N.; Caracciolo, M.; Evangelista, L.; Schirone, A.; Frassoldati, A.; Arnone, G.; Panareo, S.; Bartolomei, M. Impact on the long-term prognosis of FDG PET/CT in luminal-A and luminal-B breast cancer. Nucl. Med. Commun. 2022, 43, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Lee, M.; Jeon, T.J.; Han, K.; Lee, H.M.; Lee, S.A.; Ryu, Y.H.; Son, E.J.; Jeong, J. [18F]-fluorodeoxyglucose positron emission tomography can contribute to discriminate patients with poor prognosis in hormone receptor-positive breast cancer. PLoS ONE 2014, 9, e105905. [Google Scholar] [CrossRef]
- Evangelista, L.; Urso, L.; Caracciolo, M.; Stracuzzi, F.; Panareo, S.; Cistaro, A.; Catalano, O. FDG PET/CT Volume-Based Quantitative Data and Survival Analysis in Breast Cancer Patients: A Systematic Review of the Literature. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2022, 18. [Google Scholar] [CrossRef]
- Urso, L.; Rocca, G.C.; Borgia, F.; Lancia, F.; Malorgio, A.; Gagliano, M.; Zanetto, M.; Uccelli, L.; Cittanti, C.; Ippolito, C.; et al. The Role of [18F]F-Choline PET/CT in the Initial Management and Outcome Prediction of Prostate Cancer: A Real-World Experience from a Multidisciplinary Approach. Biomedicines 2022, 10, 2463. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Tello Galán, M.J.; García Vicente, A.M.; Pérez Beteta, J.; Amo Salas, M.; Jiménez Londoño, G.A.; Pena Pardo, F.J.; Soriano Castrejón, M.; Pérez García, V.M. Medidas de heterogeneidad global y esfericidad con 18F-FDG PET/TC en el cáncer de mama: Relación con la biología tumoral, valor predictivo y pronóstico. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 290–297. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Saibene, T.; Michieletto, S.; Fernando, B.; Orvieto, E.; Guarneri, V.; Conte, P. Could semiquantitative FDG analysis add information to the prognosis in patients with stage II/III breast cancer undergoing neoadjuvant treatment? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1648–1655. [Google Scholar] [CrossRef]
- Hyun, S.H.; Ahn, H.K.; Park, Y.H.; Im, Y.H.; Kil, W.H.; Lee, J.E.; Nam, S.J.; Cho, E.Y.; Choi, J.Y. Volume-based metabolic tumor response to neoadjuvant chemotherapy is associated with an increased risk of recurrence in breast cancer. Radiology 2015, 275, 235–244. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Umutlu, L.; Kirchner, J.; Bruckmann, N.M.; Morawitz, J.; Antoch, G.; Ting, S.; Bittner, A.K.; Hoffmann, O.; Häberle, L.; Ruckhäberle, E.; et al. Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2022, 14, 1727. [Google Scholar] [CrossRef]
- Castello, A.; Castellani, M.; Florimonte, L.; Urso, L.; Mansi, L.; Lopci, E. The Role of Radiomics in the Era of Immune Checkpoint Inhibitors: A New Protagonist in the Jungle of Response Criteria. J. Clin. Med. 2022, 11, 1740. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, H.A.; Kim, W.; Lim, I.; Lee, I.; Byun, B.H.; Noh, W.C.; Seong, M.K.; Lee, S.S.; Kim, B.I.; et al. Early prediction of neoadjuvant chemotherapy response for advanced breast cancer using PET/MRI image deep learning. Sci. Rep. 2020, 10, 21149. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.L.; Hindié, E.; Mankoff, D. 18F-FDG PET/CT for staging and restaging of breast cancer. J. Nucl. Med. 2016, 57, 17S–26S. [Google Scholar] [CrossRef] [PubMed]
- Vadi, S.K.; Mittal, B.R.; Sood, A.; Singh, G.; Bal, A.; Parihar, A.S.; Bhattacharya, A.; Basher, R.K.; Kapoor, R. Diagnostic and prognostic value of 18 F-FDG PET/CT imaging in suspected recurrence of male breast cancer. Nucl. Med. Commun. 2019, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Laas, E.; Labrosse, J.; Hamy, A.S.; Benchimol, G.; de Croze, D.; Feron, J.G.; Coussy, F.; Balezeau, T.; Guerin, J.; Lae, M.; et al. Determination of breast cancer prognosis after neoadjuvant chemotherapy: Comparison of Residual Cancer Burden (RCB) and Neo-Bioscore. Br. J. Cancer 2021, 124, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929. [Google Scholar]


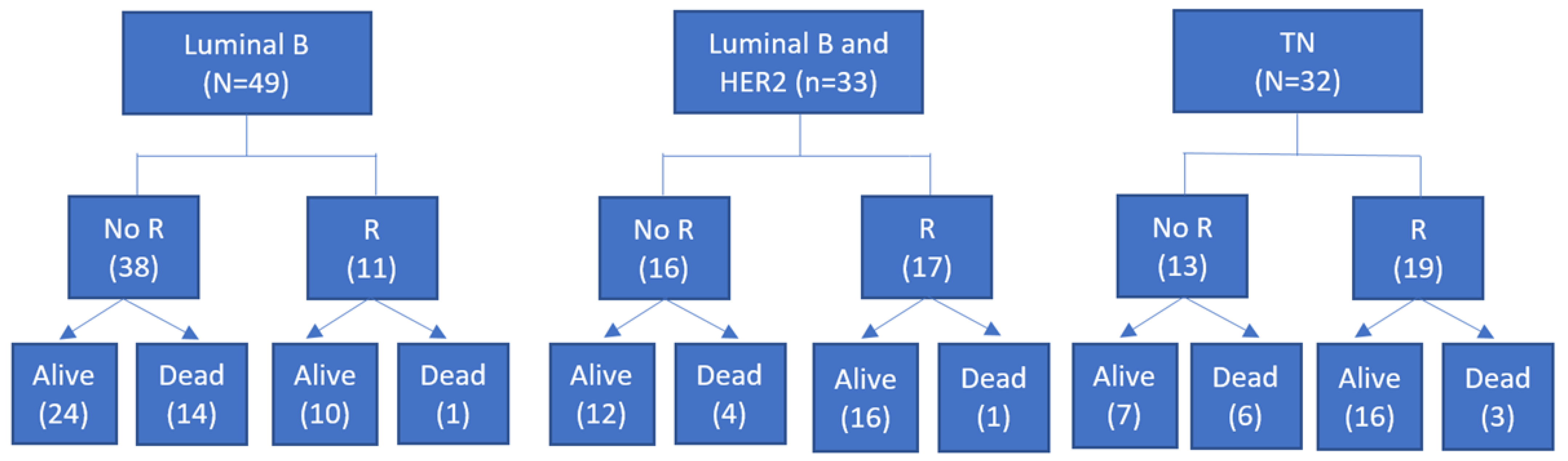
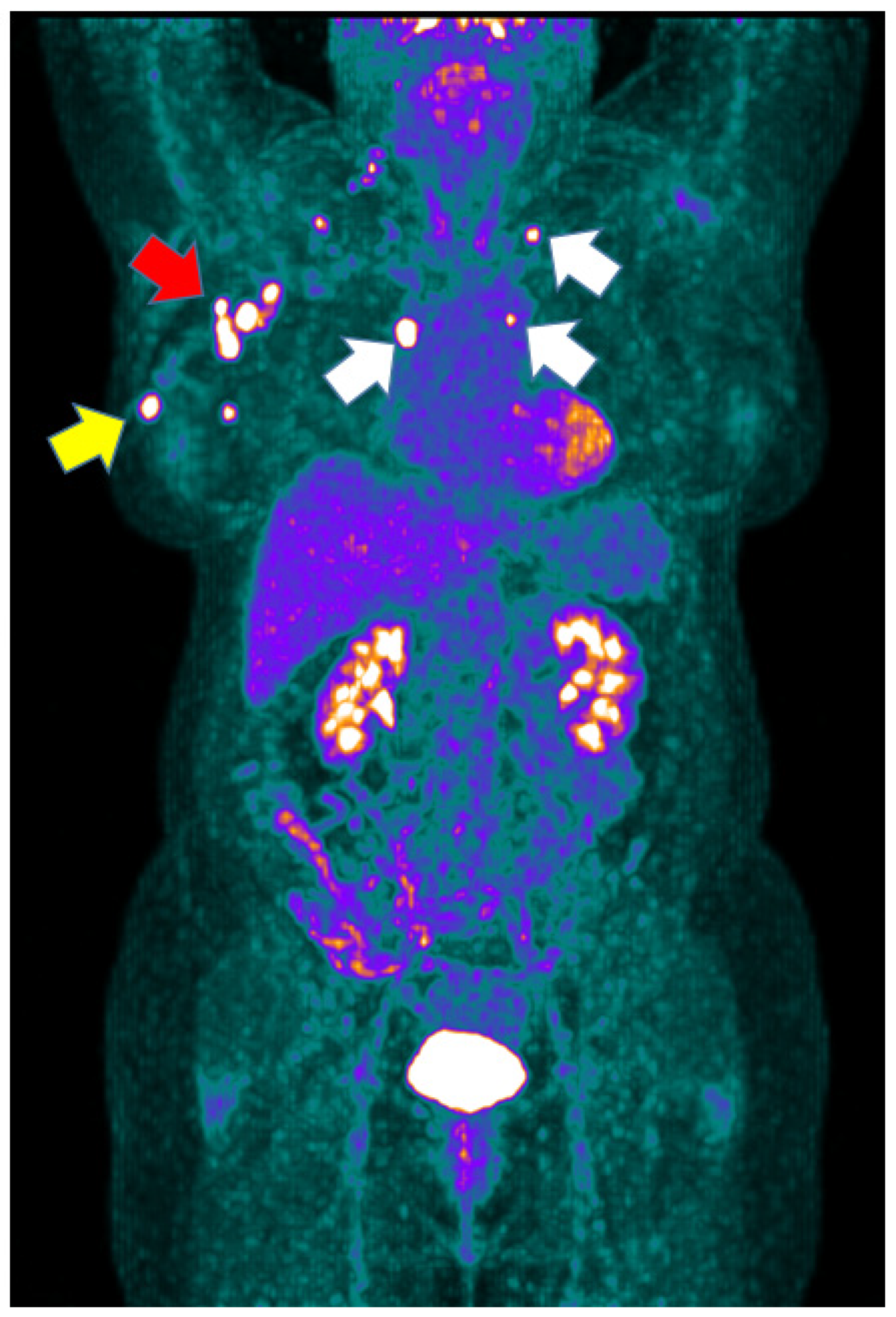
| Variables | Luminal B | Luminal B + HER-2 | TNBC |
|---|---|---|---|
| N | 49 | 33 | 32 |
| Median age (range), years | 50 (30–73) | 48 (27–77) | 51 (32–73) |
| Clinical stage I II IIIA IIIB IV NA | 4 (8.2%) 11 (22.4%) 10 (20.4%) 12 (24.5%) 9 (18.4%) 3 (6.1%) | 5 (15.2%) 9 (27.3%) 12 (36.4%) 2 (6.1%) 1 (3%) 4 (12.1%) | 8 (25%) 9 (28.1%) 9 (28.1%) 1 (3.1%) 3 (9.4%) 2 (6.3%) |
| Histology ILC IDC Mixed NA | 7 (14.3%) 41 (83.7%) 1 (2%) 0 | 7 (21.2%) 25 (75.8%) 0 1 (3%) | 4 (12.5%) 28 (87.5%) 0 0 |
| Grade G1 G2 G3 Unknown | 1 (2%) 12 (24.5%) 34 (69.4%) 2 (4.1%) | 1 (3%) 5 (15.2%) 22 (66.7%) 5 (15.2%) | 0 2 (6.3%) 27 (84.4%) 3 (9.4%) |
| ER expression No Yes | 5 (10.2%) 44 (89.8%) | 9 (27.3%) 24 (72.7%) | 32 (100%) 0 |
| PR expression No Yes | 13 (26.5%) 36 (73.5%) | 11 (33.3%) 22 (66.7%) | 32 (100%) 0 |
| Ki67 median (range), % | 35 (14–90) | 38 (10–80) | 63 (5–90) |
| HER-2 expression No Yes | 49 (100%) 0 | 0 33 (100%) | 32 (100%) 0 |
| Trastuzumab No Yes | 49 (100%) 0 | 0 33 (100%) | 32 (100%) 0 |
| pCR after NAC No Yes | 38 (77.6%) 11 (22.4%) | 16 (48.5%) 17 (51.5%) | 13 (40.6%) 19 (59.4%) |
| Luminal B (n = 49) | Luminal B + HER-2 (n = 33) | TNBC (n = 32) | p Value | |
|---|---|---|---|---|
| Breast PET No Yes | 0 49 (100%) | 1 (3%) 32 (97%) | 1 (3.1%) 31 (96.9%) | 0.464 |
| Axillary LN PET No Yes | 17 (34.7%) 32 (65.3%) | 18 (54.5%) 15 (45.5%) | 15 (46.9%) 17 (53.1%) | 0.190 |
| Distant LN PET No Yes | 36 (75.3%) 13 (26.5%) | 26 (78.8%) 7 (21.2%) | 26 (81.3%) 6 (18.8%) | 0.693 |
| Luminal B (n = 49) | Luminal B + HER-2 (n = 33) | TNBC (n = 32) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Response to NAC | Response to NAC | p Value | No Response to NAC | Response to NAC | p Value | No Response to NAC | Response to NAC | p Value | |
| SUVmax_B | 10.3 ± 6.4 | 11.1 ± 9.2 | 0.732 | 9.3 ± 5.1 | 9.5 ± 4.9 | 0.917 | 17.6 ± 12.1 | 14.9 ± 7.7 | 0.443 |
| SUVmean_B | 4.4 ± 2.1 | 7.06 ± 5.9 | 0.027 | 4.9 ± 2.1 | 5.1 ± 2.4 | 0.808 | 7.1 ± 3.9 | 10.5 ± 9.9 | 0.240 |
| MTV_B | 67.9 ± 135 | 8.8 ± 12.9 | 0.160 | 7.3 ± 4.2 | 3.5 ± 2.5 | 0.003 | 38.1 ± 63 | 11.9 ± 17.9 | 0.095 |
| TLG_B | 296.4 ± 584 | 115.8 ± 292.9 | 0.330 | 36.5 ± 24.9 | 18.9 ± 17.7 | 0.025 | 447.7 ± 1055 | 121.7 ± 182.3 | 0.194 |
| SUVmax_N | 10.4 ± 8.3 | 10.3 ± 9.9 | 0.977 | 8.9 ± 7.8 | 12.2 ± 8.1 | 0.478 | 4.9 ± 3.5 | 9.1 ± 4.8 | 0.063 |
| SUVmean_N | 4.1 ± 1.8 | 6.3 ± 5.9 | 0.105 | 3.9 ± 1.9 | 6.7 ± 4.5 | 0.222 | 3.2 ± 1.8 | 3.9 ± 1.9 | 0.403 |
| MTV_N | 22.6 ± 48.9 | 3.3 ± 4.6 | 0.278 | 1.5 ± 1.4 | 37.3 ± 100.9 | 0.451 | 1.4 ± 1.7 | 11.8 ± 14.9 | 0.055 |
| TLG_N | 119.2 ± 268.3 | 23.1 ± 47.1 | 0.327 | 7.9 ± 10.6 | 31.1 ± 31.2 | 0.135 | 4.3 ± 4.7 | 51 ± 70.6 | 0.065 |
| SUVmax_DN | 11.5 ± 8.4 | 5.9 ± 2.5 | 0.289 | 5.7 ± 1.01 | 12.9 ± 11 | 0.317 | 3.4 ± 1.4 | 5.9 ± 2.8 | 0.314 |
| SUVmean_DN | 5.1 ± 2.9 | 3.9 ± 1.9 | 0.518 | 3.8 ± 0.4 | 6.9 ± 6.2 | 0.416 | 2.3 ± 0.4 | 3.5 ± 1.5 | 0.338 |
| MTV_DN | 5.9 ± 5.7 | 0.7 ± 0.4 | 0.150 | 1.1 ± 0.7 | 1.4 ± 1.2 | 0.691 | 0.7 ± 0.2 | 2.2 ± 2.3 | 0.436 |
| TLG_DN | 40 ± 45.6 | 2.13 ± 1.1 | 0.327 | 3.9 ± 2.3 | 9.2 ± 7.1 | 0.281 | 1.6 ± 0.6 | 6.7 ± 5.6 | 0.293 |
| MTV_WB | 83.8 ± 140.9 | 11.3 ± 10.3 | 0.097 | 33.6 ± 101.3 | 8.2 ± 7.1 | 0.310 | 39.1 ± 64.3 | 17.6 ± 20.1 | 0.180 |
| TLG_WB | 382.3 ± 648.5 | 90.4 ± 141.3 | 0.148 | 39.6 ± 26.7 | 50.1 ± 48.6 | 0.449 | 450.9 ± 1057.4 | 145.4 ± 180.7 | 0.224 |
| Luminal B (n = 49) | Luminal B + HER-2 (n = 33) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-off | Sens | Spec | p Value | AUC | Cut-off | Sens | Spec | p Value | |
| MTV_B | 0.72 | ≤3.9 | 63.64% | 84.21% | 0.01 | 0.76 | ≤4.78 | 88.24% | 68.75% | 0.002 |
| TLG_B | 0.68 | ≤32.87 | 72.73% | 65.79% | 0.03 | 0.75 | ≤25.6 | 88.24% | 75% | 0.009 |
| MTV_WB | 0.73 | ≤17.7 | 81.82% | 60.53% | 0.002 | - | - | - | - | - |
| TLG_WB | 0.68 | ≤61.1 | 81.82% | 65.79% | 0.03 | - | - | - | - | - |
| TNBC with pCR after NAC | |||
|---|---|---|---|
| Alive | Dead | P Value | |
| MTV_B | 7.5 ± 9.9 | 35.2 ± 34.1 | 0.009 |
| TLG_B | 85 ± 125 | 317 ± 355 | 0.039 |
| MTV_WB | 12.8 ± 15.2 | 43.2 ± 26.7 | 0.012 |
| TLG_WB | 105.1 ± 131.4 | 360.1 ± 286.4 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers 2022, 14, 5869. https://doi.org/10.3390/cancers14235869
Urso L, Evangelista L, Alongi P, Quartuccio N, Cittanti C, Rambaldi I, Ortolan N, Borgia F, Nieri A, Uccelli L, et al. The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers. 2022; 14(23):5869. https://doi.org/10.3390/cancers14235869
Chicago/Turabian StyleUrso, Luca, Laura Evangelista, Pierpaolo Alongi, Natale Quartuccio, Corrado Cittanti, Ilaria Rambaldi, Naima Ortolan, Francesca Borgia, Alberto Nieri, Licia Uccelli, and et al. 2022. "The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer" Cancers 14, no. 23: 5869. https://doi.org/10.3390/cancers14235869
APA StyleUrso, L., Evangelista, L., Alongi, P., Quartuccio, N., Cittanti, C., Rambaldi, I., Ortolan, N., Borgia, F., Nieri, A., Uccelli, L., Schirone, A., Panareo, S., Arnone, G., & Bartolomei, M. (2022). The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers, 14(23), 5869. https://doi.org/10.3390/cancers14235869












