Proton Beam Therapy for Pediatric Tumors of the Central Nervous System—Experiences of Clinical Outcome and Feasibility from the KiProReg Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
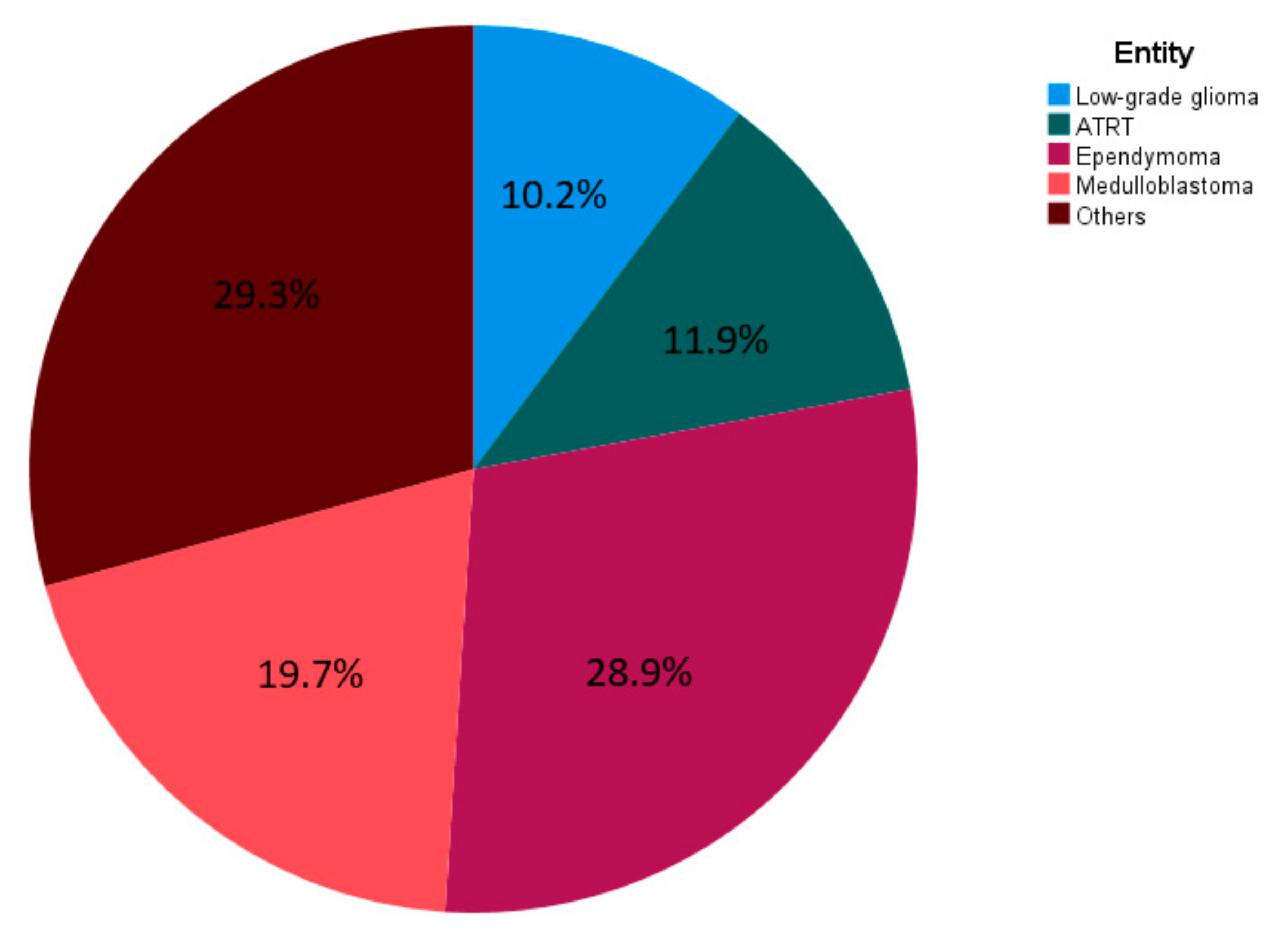
3.1. Patient Characteristics
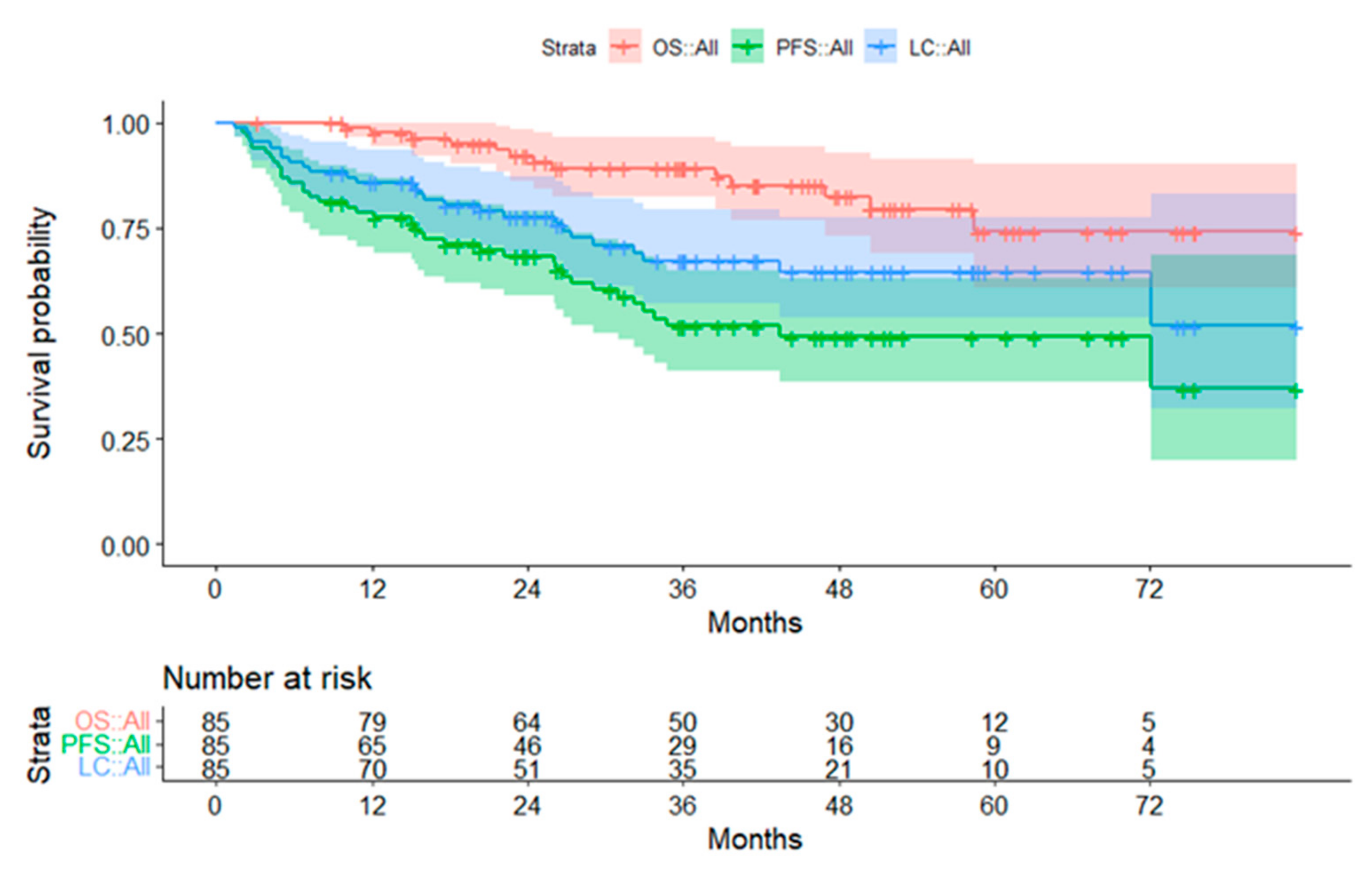
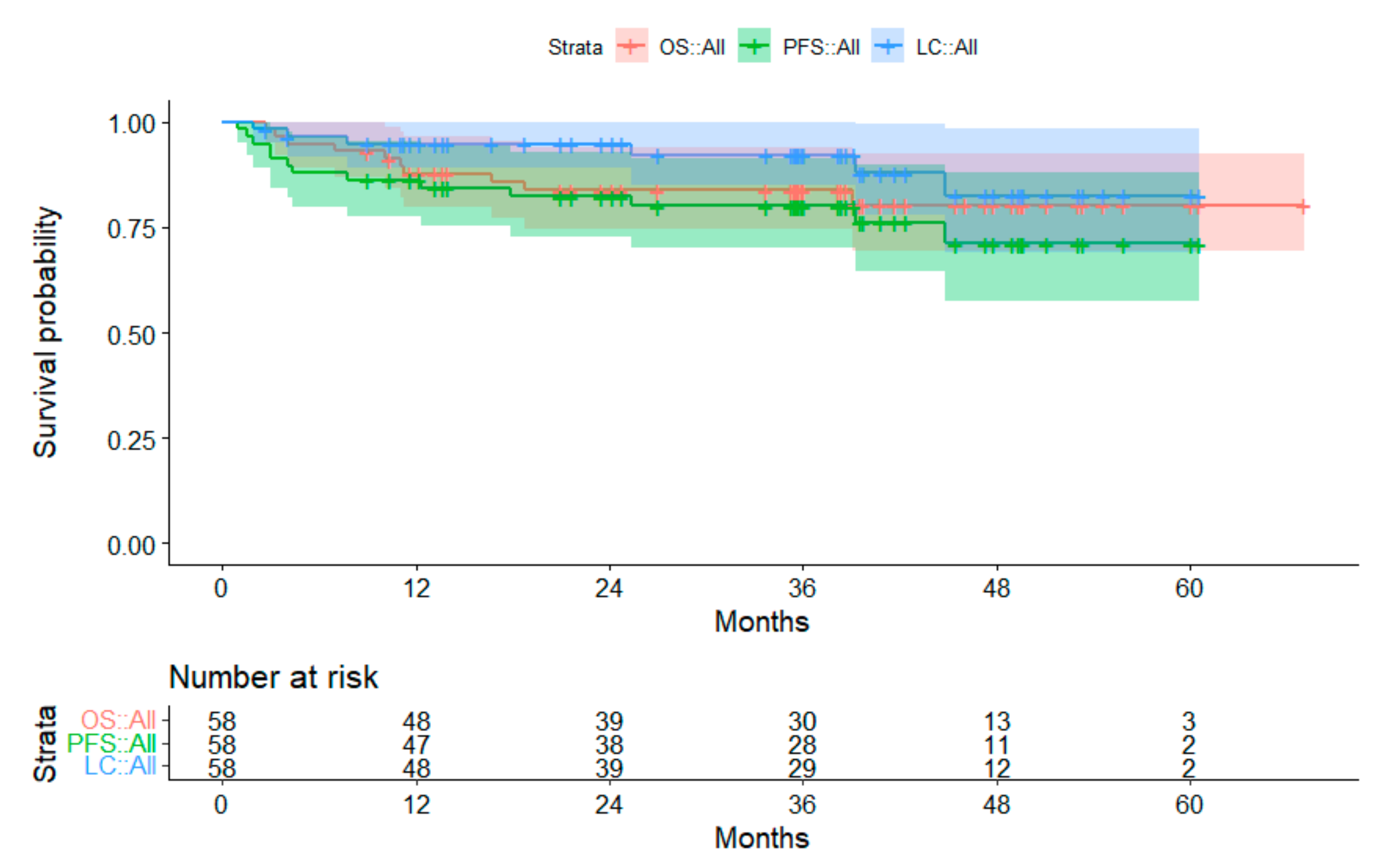
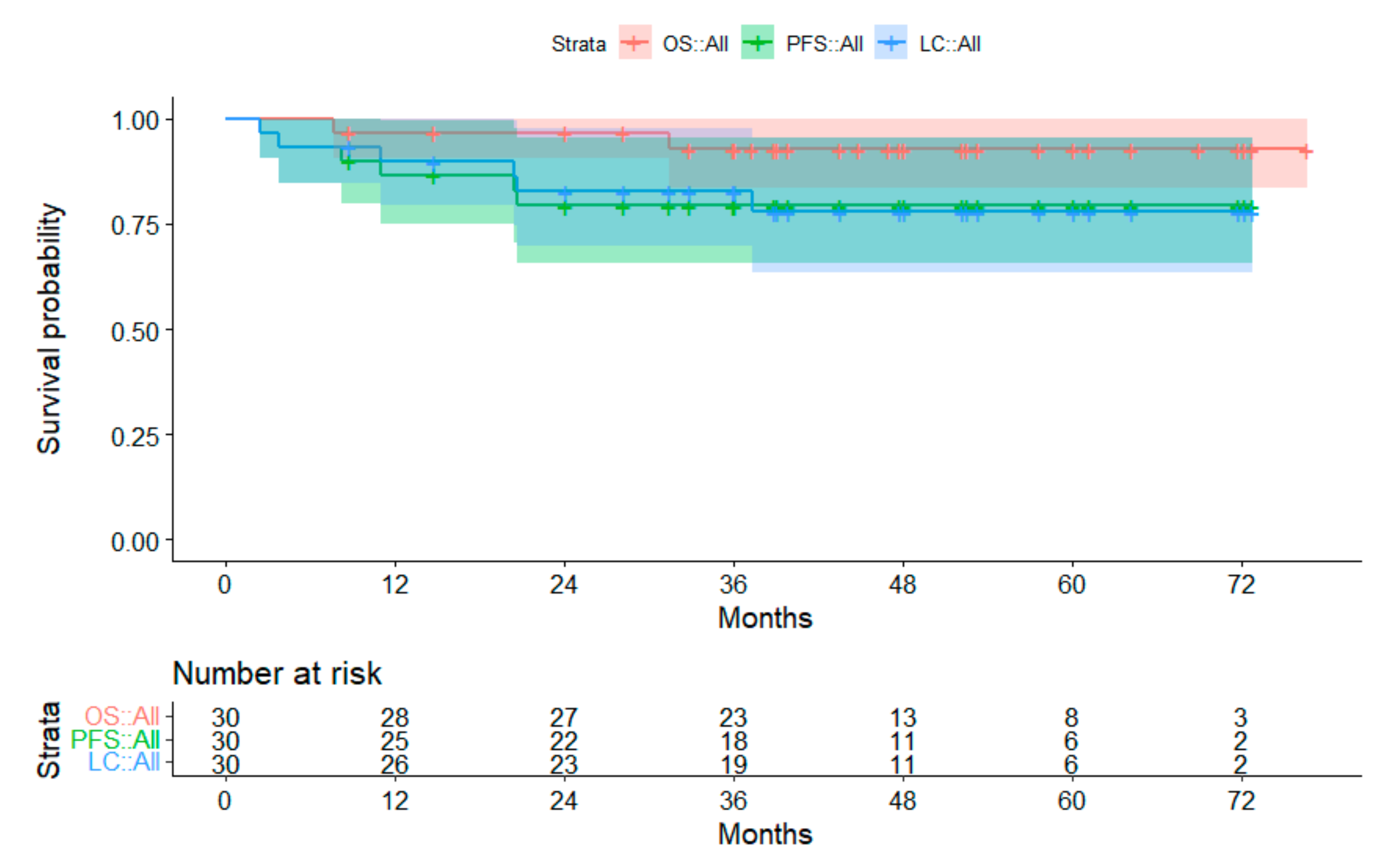
3.2. Survival Data
3.2.1. Subgroup Ependymoma
3.2.2. Subgroup Medulloblastoma
3.2.3. Subgroup Atypical Teratoid Rhabodid Tumors
3.2.4. Subgroup Low-Grade Glioma
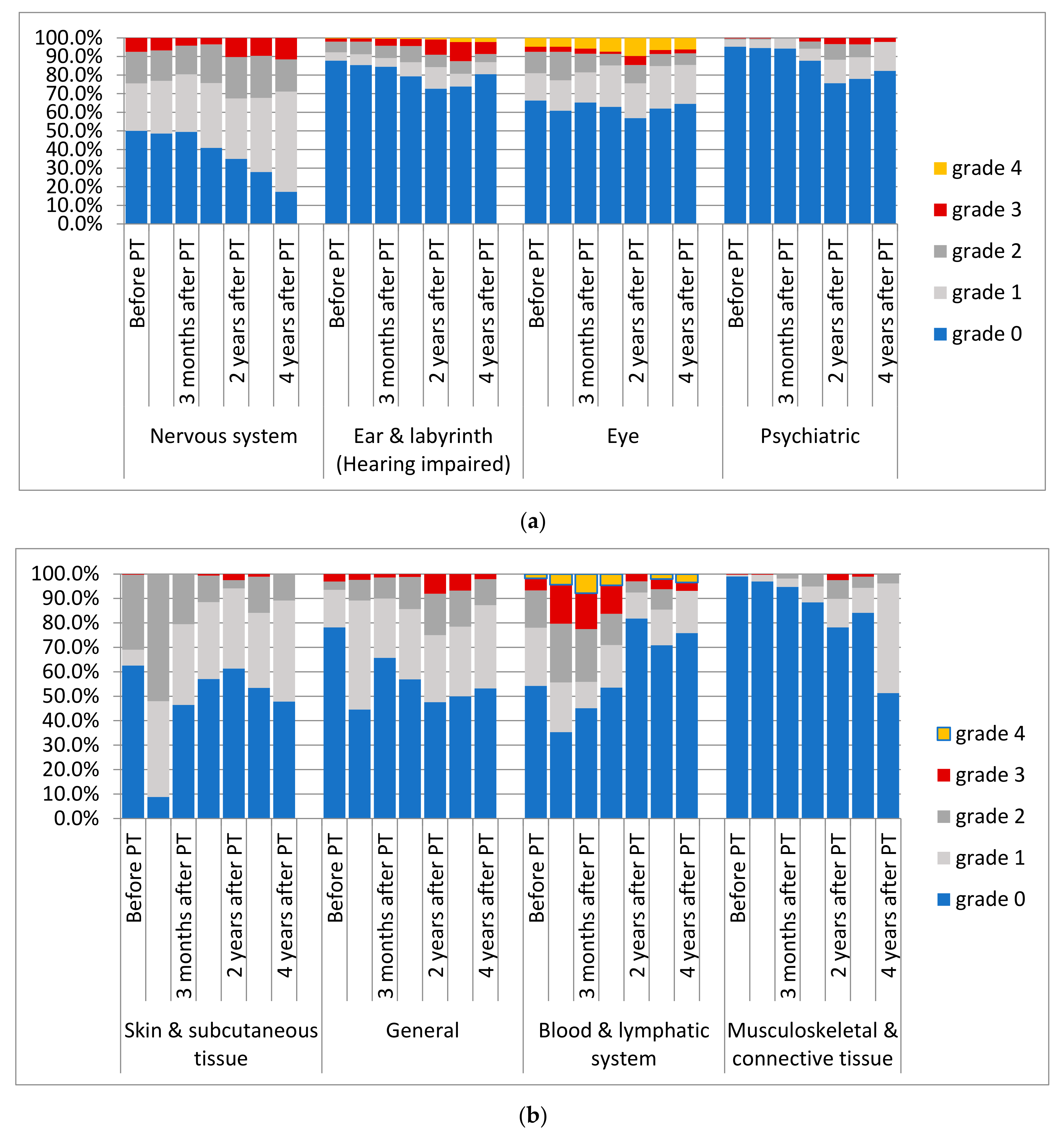
3.3. Adverse Events
3.4. Review of Image Reports
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Histological Entities | n | % |
|---|---|---|
| Ependymoma | 85 | 28.9 |
| Medulloblastoma | 58 | 19.7 |
| Atypical teratoid rhabdoid tumors | 35 | 11.9 |
| Low-grade glioma | 30 | 10.2 |
| Craniopharyngioma | 26 | 8.8 |
| High-grade glioma | 16 | 5.4 |
| Germ cell tumor intracranial | 15 | 5.1 |
| Retinoblastoma | 9 | 3.1 |
| Choroid plexus tumor | 4 | 1.4 |
| Meningioma | 3 | 1 |
| Neuroblastoma | 3 | 1 |
| Pineoblastoma | 3 | 1 |
| Embryonal tumor with multilayered rosettes | 2 | 0.7 |
| Hemangiopericytoma | 1 | 0.3 |
| Germ cell tumor extracranial | 1 | 0.3 |
| Neurocytoma | 1 | 0.3 |
| Paraganglioma | 1 | 0.3 |
| Rosette-forming glioneuronal tumor | 1 | 0.3 |
| Characteristics/Entity | Ependymoma | Low-Grade Glioma | ATRT | Medulloblastoma | Cranio-Pharyngioma | Intracranial Germ Cell Tumor | Retinoblastoma | Others |
|---|---|---|---|---|---|---|---|---|
| gender | male 36 (42.4%)/female 49 (57.6%) | male 15 (50.0%)/female 15 (50.0%) | male 18 (51.4%)/female 17 (48.6%) | male 41 (70.7%)/female 17 (29.3%) | male 11 (42.3%)/female 15 (57.7%) | male 11 (68.7%)/female 5 (31.3%) | male 8 (88.9%)/female 1 (11.1%) | male 14 (50.0%)/female 14 (50.0%) |
| age at diagnosis | median 2.4 years (range 0.1–16.9) | median 7.95 (0.2–17.0) | median 1.5 years (range 0.0–7.5 y.) | median 6.2 years (range 0.9–16.2 y.) | median 9.35 years (range 2.5–15.5 y.) | median 11.1 years (range 2.9–16.3 y.) | median 1.0 years (range 0–3.7 y.) | median 5.95 years (range 1.8–17.7 y.) |
| age at start of PBT | median 3.1 years (range 0.9–17.0) | median 11.4 (4.6–17.9) | median 1.9 years (range 1.0–8.0 y.) | median 6.5 years (range 2.0–16.5 y.) | median 10.65 years (range 5.4–17.1 y.) | median 11.5 years (range 3.3–16.6 y.) | median 2.5 years (range 1.2–8.0 y.) | median 9.0 years (range 2.1–18.0 y.) |
| resection status | ||||||||
| GTR/NTR | 50 (58.8%) | 0 | 10 (28.6%) | 34 (58.6%) | 0 | 6 (37.5%) | 0 | 12 (42.8%) |
| STR | 34 (40%) | 14 (46.7%) | 24 (68.6%) | 20 (34.5%) | 22 (84.6%) | 2 (12.5%) | 0 | 11 (39.3%) |
| none/biopsy only | 1 (1.2%) | 16 (53.3%) | 1 (2.9%) | 4 (6.9%) | 4 (15.4%) | 8 (50%) | 9 (100%) | 5 (17.9) |
| localization | ||||||||
| supratentorial | 15 (17.6%) | 25 (83.3%) | 16 (45.7%) | 0 | 26 (100%) | 16 (100%) | 9 (100%) | 21 (75%) |
| infratentorial | 70 (82.4%) | 5 (16.7%) | 19 (54.3%) | 58 (100%) | 0 | 0 | 0 | 7 (25%) |
| number of surgeries prior to PBT | median 2 (1–6) | median 1 (0–4) | median 1 (0–7) | median 1 (1–3) | median 2 (1–7) | median 1 (0–3) | 0 | median 1 (0–3) |
| metastasis prior to PBT | 5 (5.9%) | 4 (133%) | 6 (17.1%) | 18 (31.0%) | 0 | 2 (12.5%) | 1 (11.1%) | 5 (17.9%) |
| timepoint of PBT | ||||||||
| primary diagnosis | 68 (80%) | 12 (40.0%) | 33 (94.3%) | 47 (81.0%) | 3 (11.5%) | 15 (93.8%) | 4 (44.4%) | 13 (46.4%) |
| recurrence/progression | 17 (20%) | 18 (60.0%) | 2 (5.7%) | 11 (19.0%) | 23 (88.5%) | 1 (6.7%) | 5 (55.6%) | 15 (53.6%) |
| reservoir during PBT | 7 (8.2%) | 3 (10%) | 23 (65.7%) | 12 (20.7%) | 6 (23.1%) | 2 (12.5%) | 0 | 3 (10.7%) |
| VP/VA shunt during PBT | 28 (32.9%) | 6 (20.0%) | 12 (34.3%) | 13 (22.4%) | 5 (19.2%) | 0 | 0 | 2 (7.1%) |
| prior RT in overlapping area | 7 (8.2%) | 0 | 1 (2.9%) | 4 (6.9%) | 1 (3.8%) | 0 | 4 (44.4%) | 1 (3.6%) |
| prior chemotherapy | 41 (48.2%) | 16 (53.3%) | 35 (100%) | 30 (51.7%) | 0 | 16 (100%) | 8 (88.9%) | 14 (50.0%) |
| concomitant CTX | 10 (11.8%) | 1 (3.3%) | 15 (42.9%) | 55 (60.3%) | 0 | 0 | 0 | 9 (32.1%) |
| inpatient treatment besides CTX | 15 (17.6%) | 4 (13.3%) | 13 (37.1%) | 15(19.5%) | 2(7.7%) | 0 | 2 (22.2%) | 3 (10.7%) |
| anesthesia during PBT | 69 (81.2%) | 23 (76.7%) | 34 (97.1%) | 37 (63.8%) | 3 (11.5%) | 1 (6.3%) | 9 (100%) | 14 (50.0%) |
| PBT technique | ||||||||
| PBS | 35 (41.2%) | 13 (5.7%) | 17 (46.6%) | 38 (65.5%) | 18 (59.2%) | 13 (81.3%) | 4 (44.4%) | 20 (71.4%) |
| US | 45 (52.9%) | 16 (45.7%) | 16 (45.7%) | 3 (5.2%) | 8 (30.2%) | 3 (18.7%) | 5 (55.6%) | 4 (14.3%) |
| PBS and US | 5 (5.9%) | 1 (2.7%) | 2 (5.7%) | 17 (29.3%) | 0 | 0 | 0 | 4 (14.3%) |
| median dose | median 54.04 (range 49.6–62.0 Gy) | median 54.0 (range 46.0–554 Gy) | median 54.0 Gy (range 36.6–60.0 Gy) | median 54.0 Gy (range 30.0–72.0 Gy) | 54.0 Gy | 54.0 Gy (24–54 Gy) | 50.0 Gy (49,6–50,0 Gy) | 54.0 Gy (45–72,0 Gy) |
| number of fractions | median 31 (range 30–33) | median 30 (28–33) | median 30 (range 22–35) | median 30 (range 6–72) | 30 | 30 (15–30) | 30 (15–30) | 31 (25–70) |
| CSI | 2 (2.4%) | 1 (3.3%) | 6 (17.1%) | 50 (86.2%) | 0 | 2 (12.5%) | 1 (11.1%) | 8 (28.6%) |
| chemotherapy after PBT | 32 (37.9%) | 9 (24.3%) | 13 (37.1%) | 47 (81.0%) | 0 | 2 (12.5%) | 3 (33.3%) | 13 (46.4%) |
| interruption of PBT treatment > 2 days | 10 (11.8%) | 2 (6.7%) | 4 (11.4%) | 2 (3.4%) | 2 (7.7%) | 1 (6.3%) | 1 (11.1%) | 2 (7.1%) |
| Follow-up since end of PBT | median 39.7 months (3.2–83.3 m) | median 49.95 months (7.7–76.7 m) | median 34.2 months (range 0.7–76.1 m) | median 36.0 months (range 2.7–67.1 m) | median 47.9 months (range 11.7–77.5 m) | median 35.5 months (range 20.3–81.7 m) | median 38.2 months (range 3.0–52.9 m) | median 34.1 months (range 3.9–73.3 m) |
| Characteristic | Patients Not Meeting the Criteria for Imaging Events | Patients Meeting Criteria for Imaging Events |
|---|---|---|
| n (%/Range) | n (%/Range) | |
| gender | male 131 (58.2%)/female 94 (41.8%) | male 41 (59.4%)/female 28 (40.6%) |
| age at diagnosis | median 5.0 years (0.0–17.5) | median 3.8 years (0.4–17.7) |
| age at start of PBT | median 6.3 years (1.0–18.0) | median 4.8 years (0.9–17.9) |
| resection status | ||
| GTR/NTR | 77 (24.2.3%) | 34 (49.3%) |
| STR | 103 (45.8%) | 30 (43.5%) |
| none/biopsy only | 45 (20.0%) | 5 (7.2%) |
| localization | ||
| supratentorial | 109 (48.4%) | 26 (37.7%) |
| infratentorial | 116 (51.6%) | 43 (62.3%) |
| timing of PBT | ||
| primary diagnosis | 148 (65.8%) | 54 (78.3%) |
| recurrence/progression | 77 (34.2%) | 15 (21.7%) |
| treatment prior to PBT | ||
| prior radiotherapy in an overlapping area | 14 (6.2%) | 4 (5.8%) |
| prior CTX | 120 (53.3%) | 39 (56.5%) |
| prior intrathecal CTX | 40 (17.8%) | 19 (27.5%) |
| number of tumor surgeries prior to PBT | median 1 (0–6) | median 1 (0–7) |
| treatment during PBT | ||
| concomitant chemotherapy | 51 (22.7%) | 26 (37.7%) |
| reservoir during PBT | 41 (18.2%) | 14 (20.3%) |
| VP/VA shunt during PBT | 49 (21.8%) | 19 (27.5%) |
| PBT | ||
| PBS | 130 (57.8%) | 34 (49.3%) |
| US | 75 (33.3%) | 26 (37.7%) |
| PBS and US | 20 (8.9%) | 9 (13.0%) |
| median dose | median 54 Gy (24.0–72.0) | median 54 Gy (49.6–68.0) |
| number of fractions | median 30 (6.0–72) | median 30 (30–67) |
| CSI | 57 (35.3%) | 13 (18.8%) |
| interruption of treatment > 2 days | 17 (7.6%) | 4 (5.8%) |
| aftercare | ||
| CTX after PBT | 96 (42.7.1%) | 38 (55.1%) |
| FU since end of PBT | median 36.0 months (0.7–81.7) | median 45.3 months (10.1–83.3) |
References
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2015, 16 (Suppl. 10), x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M. Radiotherapy for primary brain tumors in very young children. Cancer 1982, 50, 2785–2789. [Google Scholar] [CrossRef]
- Duffner, P.K.; Armstrong, F.D.; Chen, L.; Helton, K.J.; Brecher, M.L.; Bell, B.; Chauvenet, A.R. Neurocognitive and neuroradiologic central nervous system late effects in children treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) acute lymphoblastic leukemia protocols (ACCL0131): A methotrexate consequence? A report from the Children’s Oncology Group. J. Pediatr. Hematol. Oncol. 2014, 36, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Merchant, T.E.; Conklin, H.M.; Wu, S.; Lustig, R.H.; Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009, 27, 3691–3697. [Google Scholar] [CrossRef]
- Spiegler, B.J.; Bouffet, E.; Greenberg, M.L.; Rutka, J.T.; Mabbott, D.J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J. Clin. Oncol. 2004, 22, 706–713. [Google Scholar] [CrossRef]
- Gondi, V.; Yock, T.I.; Mehta, M.P. Proton therapy for paediatric CNS tumours—Improving treatment-related outcomes. Nat. Rev. Neurol. 2016, 12, 334–345. [Google Scholar] [CrossRef]
- Merchant, T.E.; Hua, C.H.; Shukla, H.; Ying, X.; Nill, S.; Oelfke, U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 2008, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Ulmer, S.; Ahlhelm, F.J.; Fredh, A.S.M.; Kliebsch, U.; Calaminus, G.; Bolsi, A.; Albertini, F.; Leiser, D.; Timmermann, B.; et al. Clinical and Radiologic Outcomes in Adults and Children Treated with Pencil-Beam Scanning Proton Therapy for Low-Grade Glioma. Int. J. Part. Ther. 2017, 3, 450–460. [Google Scholar] [CrossRef]
- Mizumoto, M.; Oshiro, Y.; Yamamoto, T.; Kohzuki, H.; Sakurai, H. Proton Beam Therapy for Pediatric Brain Tumor. Neurol. Med. Chir. 2017, 57, 343–355. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Bradley, J.A.; Rotondo, R.L.; Nanda, R.H.; Logie, N.; Sandler, E.S.; Aldana, P.R.; Ranalli, N.J.; Beier, A.D.; Morris, C.G.; et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018, 57, 644–648. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Bradley, J.A.; Sandler, E.S.; Aldana, P.R.; Sapp, A.; Gains, J.E.; Crellin, A.; Rotondo, R.L. Clinical outcomes following proton therapy for children with central nervous system tumors referred overseas. Pediatr. Blood Cancer 2017, 64, e26654. [Google Scholar] [CrossRef]
- Jazmati, D.; Steinmeier, T.; Ahamd Khalil, D.; Frisch, S.; Peters, S.; Schulze Schleithoff, S.; Baumer, C.; Rutkowski, S.; Fruhwald, M.C.; Blase, C.; et al. Feasibility of Proton Beam Therapy for Infants with Brain Tumours: Experiences from the Prospective KiProReg Registry Study. Clin. Oncol. 2021, 33, e295–e304. [Google Scholar] [CrossRef]
- Peters, S.; Merta, J.; Schmidt, L.; Jazmati, D.; Kramer, P.H.; Blase, C.; Tippelt, S.; Fleischhack, G.; Stock, A.; Bison, B.; et al. Evaluation of dose, volume, and outcome in children with localized, intracranial ependymoma treated with proton therapy within the prospective KiProReg Study. Neuro-Oncology 2022, 24, 1193–1202. [Google Scholar] [CrossRef]
- Duffner, P.K.; Cohen, M.E.; Myers, M.H.; Heise, H.W. Survival of children with brain tumors: SEER Program, 1973–1980. Neurology 1986, 36, 597–601. [Google Scholar] [CrossRef]
- Fouladi, M.; Chintagumpala, M.; Laningham, F.H.; Ashley, D.; Kellie, S.J.; Langston, J.W.; McCluggage, C.W.; Woo, S.; Kocak, M.; Krull, K.; et al. White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J. Clin. Oncol. 2004, 22, 4551–4560. [Google Scholar] [CrossRef]
- Kralik, S.F.; Ho, C.Y.; Finke, W.; Buchsbaum, J.C.; Haskins, C.P.; Shih, C.S. Radiation Necrosis in Pediatric Patients with Brain Tumors Treated with Proton Radiotherapy. AJNR Am. J. Neuroradiol. 2015, 36, 1572–1578. [Google Scholar] [CrossRef]
- Giantsoudi, D.; Sethi, R.V.; Yeap, B.Y.; Eaton, B.R.; Ebb, D.H.; Caruso, P.A.; Rapalino, O.; Chen, Y.L.; Adams, J.A.; Yock, T.I.; et al. Incidence of CNS Injury for a Cohort of 111 Patients Treated with Proton Therapy for Medulloblastoma: LET and RBE Associations for Areas of Injury. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 287–296. [Google Scholar] [CrossRef]
- Gentile, M.S.; Yeap, B.Y.; Paganetti, H.; Goebel, C.P.; Gaudet, D.E.; Gallotto, S.L.; Weyman, E.A.; Morgan, M.L.; MacDonald, S.M.; Giantsoudi, D.; et al. Brainstem Injury in Pediatric Patients with Posterior Fossa Tumors Treated with Proton Beam Therapy and Associated Dosimetric Factors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 719–729. [Google Scholar] [CrossRef]
- Merchant, T.E.; Li, C.; Xiong, X.; Kun, L.E.; Boop, F.A.; Sanford, R.A. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009, 10, 258–266. [Google Scholar] [CrossRef]
- Tran, S.; Lim, P.S.; Bojaxhiu, B.; Teske, C.; Baust, K.; Zepter, S.; Kliebsch, U.; Timmermann, B.; Calaminus, G.; Weber, D.C. Clinical outcomes and quality of life in children and adolescents with primary brain tumors treated with pencil beam scanning proton therapy. Pediatr. Blood Cancer 2020, 67, e28465. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Mizumoto, M.; Murayama, S.; Akimoto, T.; Demizu, Y.; Fukushima, T.; Ishida, Y.; Oshiro, Y.; Numajiri, H.; Fuji, H.; Okumura, T.; et al. Proton beam therapy for pediatric malignancies: A retrospective observational multicenter study in Japan. Cancer Med. 2016, 5, 1519–1525. [Google Scholar] [CrossRef]
- Sabel, M.; Fleischhack, G.; Tippelt, S.; Gustafsson, G.; Doz, F.; Kortmann, R.; Massimino, M.; Navajas, A.; von Hoff, K.; Rutkowski, S.; et al. Relapse patterns and outcome after relapse in standard risk medulloblastoma: A report from the HIT-SIOP-PNET4 study. J. Neurooncol. 2016, 129, 515–524. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kun, L.E.; Krasin, M.J.; Wallace, D.; Chintagumpala, M.M.; Woo, S.Y.; Ashley, D.M.; Sexton, M.; Kellie, S.J.; Ahern, V.; et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 782–787. [Google Scholar] [CrossRef]
- Lannering, B.; Rutkowski, S.; Doz, F.; Pizer, B.; Gustafsson, G.; Navajas, A.; Massimino, M.; Reddingius, R.; Benesch, M.; Carrie, C.; et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J. Clin. Oncol. 2012, 30, 3187–3193. [Google Scholar] [CrossRef]
- Macdonald, S.M.; Sethi, R.; Lavally, B.; Yeap, B.Y.; Marcus, K.J.; Caruso, P.; Pulsifer, M.; Huang, M.; Ebb, D.; Tarbell, N.J.; et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro-Oncology 2013, 15, 1552–1559. [Google Scholar] [CrossRef]
- Patteson, B.E.; Baliga, S.; Bajaj, B.V.M.; MacDonald, S.M.; Yeap, B.Y.; Gallotto, S.L.; Giblin, M.J.; Weyman, E.A.; Ebb, D.H.; Huang, M.S.; et al. Clinical outcomes in a large pediatric cohort of patients with ependymoma treated with proton radiotherapy. Neuro-Oncology 2021, 23, 156–166. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Ioakeim-Ioannidou, M.; Bradley, J.A.; Mailhot-Vega, R.B.; Morris, C.G.; Tarbell, N.J.; Yock, T.; MacDonald, S.M. Proton Therapy for Pediatric Ependymoma: Mature Results from a Bicentric Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 815–820. [Google Scholar] [CrossRef]
- Buscariollo, D.L.; Park, H.S.; Roberts, K.B.; Yu, J.B. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer 2012, 118, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Valuck, B.W.; Chen, I.; Srivastava, A.J.; Floberg, J.M.; Rao, Y.J.; King, A.A.; Shinohara, E.T.; Perkins, S.M. Assessment of the treatment approach and survival outcomes in a modern cohort of patients with atypical teratoid rhabdoid tumors using the National Cancer Database. Cancer 2017, 123, 682–687. [Google Scholar] [CrossRef]
- Fruhwald, M.C.; Biegel, J.A.; Bourdeaut, F.; Roberts, C.W.; Chi, S.N. Atypical teratoid/rhabdoid tumors-current concepts, advances in biology, and potential future therapies. Neuro-Oncology 2016, 18, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, E.; Tippelt, S.; Zeller, J.; Geismar, D.; Jazmati, D.; Peters, S.; Timmermann, B.; Mikasch, R.; Fleischhack, G. RONC-17. Feasibility of proton therapy with and without simultaneous chemotherapy in CNS malignancies of children and adolescents. Neuro-Oncology 2022, 24, i180. [Google Scholar] [CrossRef]
- De Leo, A.N.; Holtzman, A.L.; Ho, M.W.; Morris, C.G.; Rutenberg, M.S.; Rotondo, R.L.; Bates, J.E.; Indelicato, D.J.; Rao, D.; Asa Hasan, M.; et al. Vision loss following high-dose proton-based radiotherapy for skull-base chordoma and chondrosarcoma. Radiother. Oncol. 2021, 158, 125–130. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Rotondo, R.L.; Mailhot Vega, R.B.; Holtzman, A.L.; Looi, W.S.; Morris, C.G.; Sandler, E.S.; Aldana, P.R.; Bradley, J.A. Local Control After Proton Therapy for Pediatric Chordoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Tonning Olsson, I.; Perrin, S.; Lundgren, J.; Hjorth, L.; Johanson, A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr. Neurol. 2014, 51, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; Fong, G.W.; Ingerski, L.M.; Pulsifer, M.B.; Goyal, S.; Zhang, C.; Weyman, E.A.; Esiashvili, N.; Klosky, J.L.; MacDonald, T.J.; et al. Intellectual functioning among case-matched cohorts of children treated with proton or photon radiation for standard-risk medulloblastoma. Cancer 2021, 127, 3840–3846. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, A.M.; Kahalley, L.S.; Minard, C.G.; Stafford, C.; Grosshans, D.R.; Okcu, M.F.; Raghubar, K.P.; Gragert, M.; McCurdy, M.; Warren, E.H.; et al. Treatment age and neurocognitive outcomes following proton beam radiotherapy for pediatric low- and intermediate-grade gliomas. Pediatr. Blood Cancer 2021, 68, e29096. [Google Scholar] [CrossRef]
- Weusthof, K.; Luttich, P.; Regnery, S.; Konig, L.; Bernhardt, D.; Witt, O.; Herfarth, K.; Unterberg, A.; Jungk, C.; Farnia, B.; et al. Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation. Cancers 2021, 13, 3538. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Neurocognitive impairment following proton therapy for paediatric brain tumour: A systematic review of post-therapy assessments. Support. Care Cancer 2021, 29, 3035–3047. [Google Scholar] [CrossRef] [PubMed]
- Gunther, J.R.; Sato, M.; Chintagumpala, M.; Ketonen, L.; Jones, J.Y.; Allen, P.K.; Paulino, A.C.; Okcu, M.F.; Su, J.M.; Weinberg, J.; et al. Imaging Changes in Pediatric Intracranial Ependymoma Patients Treated with Proton Beam Radiation Therapy Compared to Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 54–63. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Chhabda, S.; Lakshmanan, R.; Tan, R.; Warne, R.; Benenati, M.; Michalski, A.; Aquilina, K.; Jacques, T.; Hargrave, D.; et al. Spectrum of neuroimaging findings post-proton beam therapy in a large pediatric cohort. Child’s Nerv. Syst. 2021, 37, 435–446. [Google Scholar] [CrossRef]
- Niyazi, M.; Niemierko, A.; Paganetti, H.; Sohn, M.; Schapira, E.; Goldberg, S.; Adams, J.; Kim, V.; Oh, K.S.; Hwang, W.L.; et al. Volumetric and actuarial analysis of brain necrosis in proton therapy using a novel mixture cure model. Radiother. Oncol. 2020, 142, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Flampouri, S.; Rotondo, R.L.; Bradley, J.A.; Morris, C.G.; Aldana, P.R.; Sandler, E.; Mendenhall, N.P. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014, 53, 1298–1304. [Google Scholar] [CrossRef] [PubMed]


| Keywords |
|---|
| Radiation necrosis |
| Imaging changes |
| White matter lesions |
| Radiogenic change |
| Conspicuous, increased T2 intensity/signal |
| Contrast image (outside tumor remnant) |
| Gadolinium uptake (outside tumor remnant) |
| Hemorrhage |
| Signs of bleeding |
| Edema |
| Diffusion disturbance |
| Barrier disturbance |
| Characteristics | n (%/Range) |
|---|---|
| gender | male 172 (58.5%)/female 122 (41.5%) |
| age at diagnosis | median 4.3 years (0.0–17.7) |
| age at start of proton therapy | median 5.8 years (0.9–18.0) |
| WHO grade | |
| I | 44 (15.0%) |
| II | 30 (10.2%) |
| III | 84 (28.6%) |
| IV | 103 (35.0%) |
| not available/not applicable | 33 (11.2%) |
| resection status | |
| GTR/NTR | 111 (37.8%) |
| STR | 133 (45.2%) |
| none/biopsy only | 50 (17.0%) |
| localization | |
| supratentorial | 125 (45.8%) |
| infratentorial | 159 (54.2%) |
| timing of PBT | |
| primary diagnosis | 202 (68.7%) |
| recurrence/progression | 92 (31.3%) |
| treatment prior to PBT | |
| prior radiotherapy in an overlapping area | 18 (6.1%) |
| prior chemotherapy | 159 (54.1%) |
| number of tumor surgeries prior to PBT | median 1 (0–7) |
| treatment during PBT | |
| concomitant CTX | 76 (25.9%) |
| inpatient treatment besides CTX | 51 (17.3%) |
| time from diagnosis to PBT start | 5.4 months (0.8–136.0) |
| anesthesia during PBT | 177 (60.2%) |
| intraventricular catheter system during PBT | 54 (18.4%) |
| VP/VA shunt during PBT | 68 (23.1%) |
| PBT | |
| PBS | 163 (55.4%) |
| US | 102 (34.7%) |
| PBS and US | 29 (9.9%) |
| median dose | median 54 Gy (24.0–74.0) |
| number of fractions | median 30 (6–72) |
| CSI | 70 (23.8%) |
| interruption of treatment >2 days | 22 (7.5%) |
| time from PBT start to PBT end | median 43 days (11–78) |
| aftercare | |
| CTX after PBT | 134 (45.9%) |
| follow-up since first diagnosis | 49.2 months (7.2–185.3) |
| follow-up since end of PBT | median 38.1 months (0.7–83.3) |
| Variable | 3-Year OS | p-Value | 3-Year PFS | p-Value | 3-Year LC | p-Value |
|---|---|---|---|---|---|---|
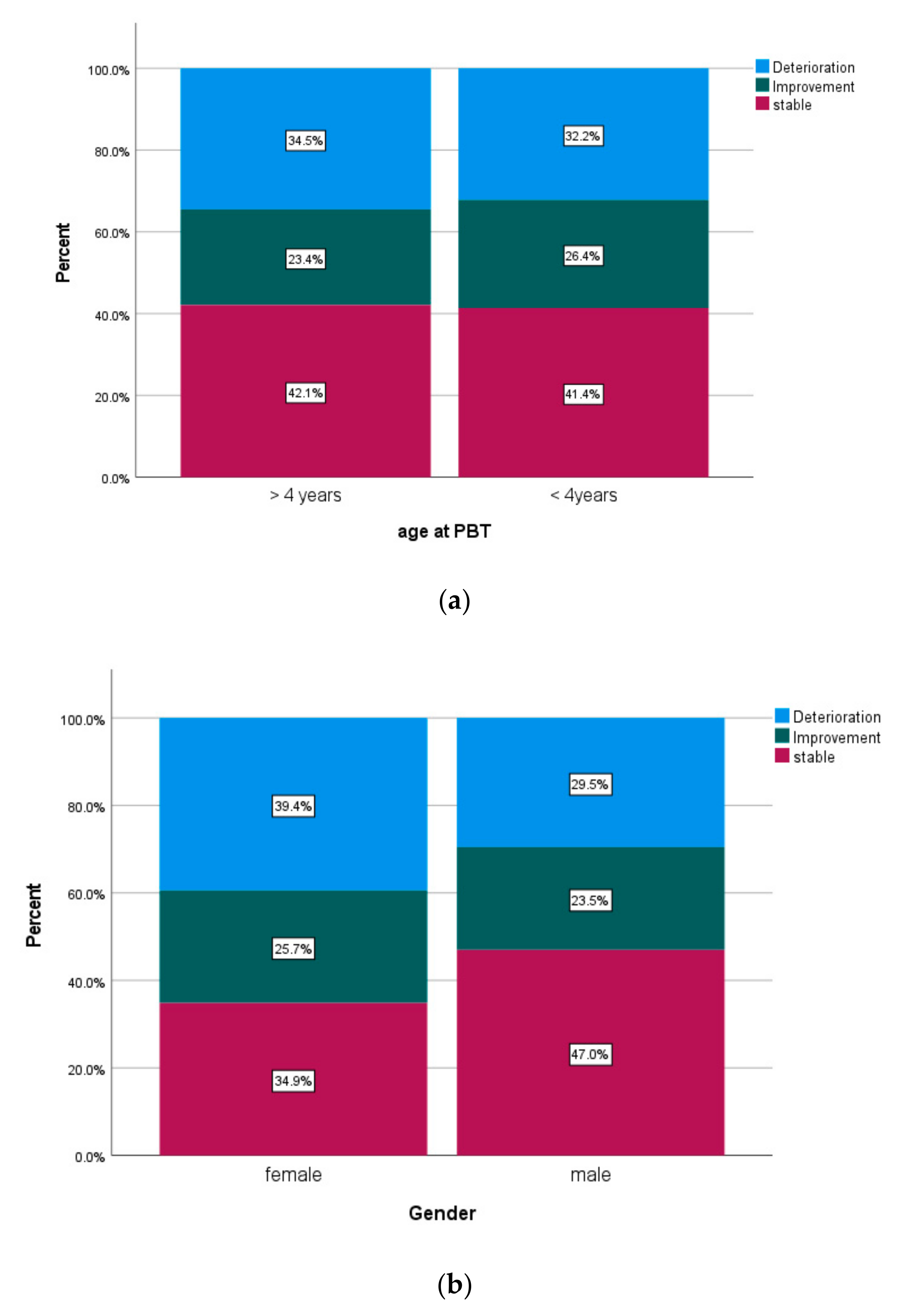
| gender | ||||||
| female | 85.5% | 68.7% | 80.1% | |||
| male | 80.8% | 0.233 | 66.4% | 0.549 | 79.0% | 0.939 |
| age ≥ 4 years | ||||||
| yes | 85.7% | 75.1% | 84.2% | |||
| no | 77.4% | 0.067 | 53.8% | 0.001 | 70.8% | 0.009 |
| metastatic disease | ||||||
| yes | 70.6% | 57.7% | 74.1% | |||
| no | 84.7% | 0.005 | 68.9% | 0.055 | 80.3% | 0.392 |
| time point of PBT | ||||||
| primary diagnosis | 85.6% | 68.8% | 72.2% | |||
| recurrence/progression | 76.2% | 0.238 | 65.6% | 0.530 | 82.2% | 0.081 |
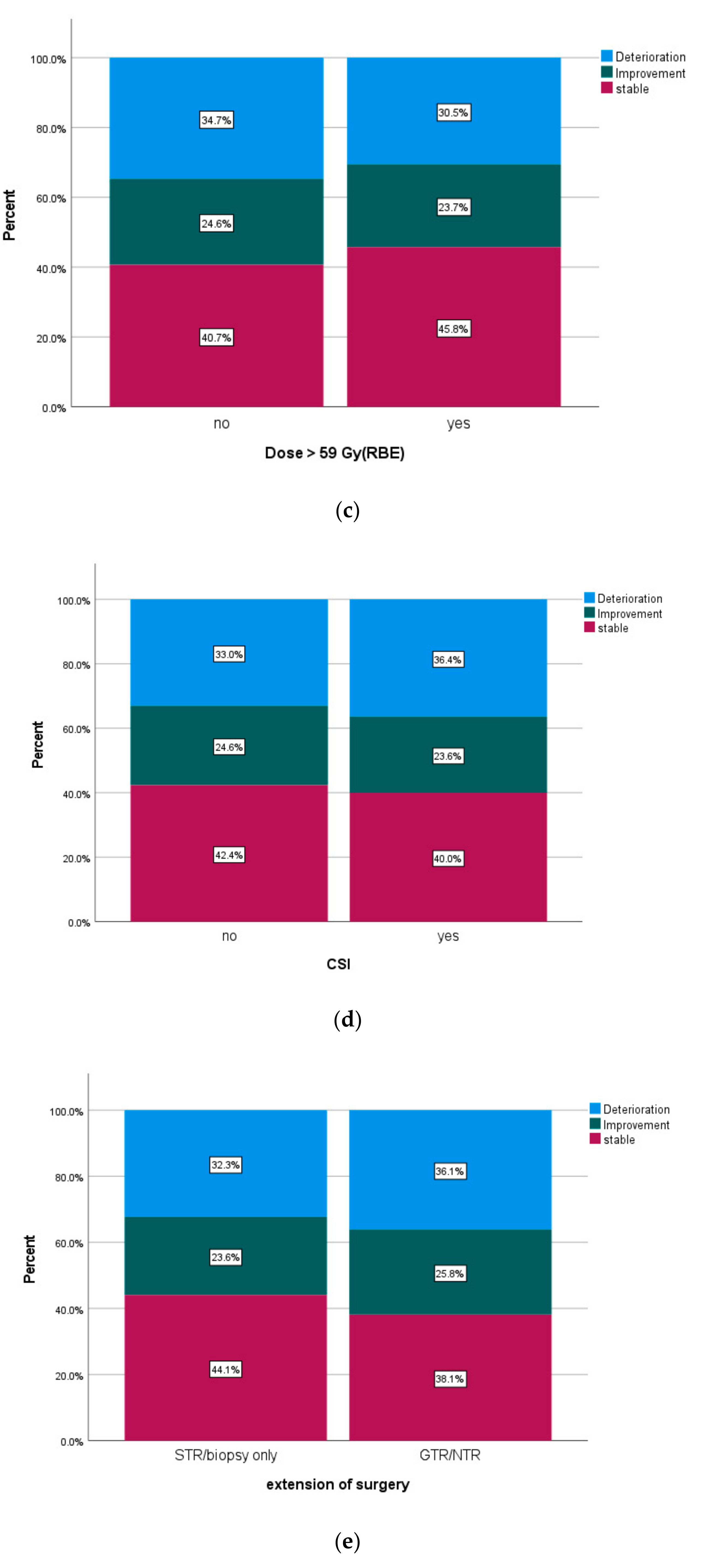
| status of resection | ||||||
| GTR/NTR | 87.8% | 68.5% | 81.3% | |||
| STR/biopsy only | 79.5% | 0.126 | 66.9% | 0.610 | 78.4% | 0.432 |
| PBT dose | ||||||
| ≥59 Gy (RBE) | 76.6% | 57.3% | 67.4% | |||
| <59 Gy (RBE) | 82.8% | 0.344 | 70.1% | 0.070 | 83.2% | 0.002 |
| CSI | ||||||
| yes | 79.7% | 73.4% | 85.6% | |||
| no | 83.8% | 0.186 | 65.6% | 0.599 | 77.8% | 0.335 |
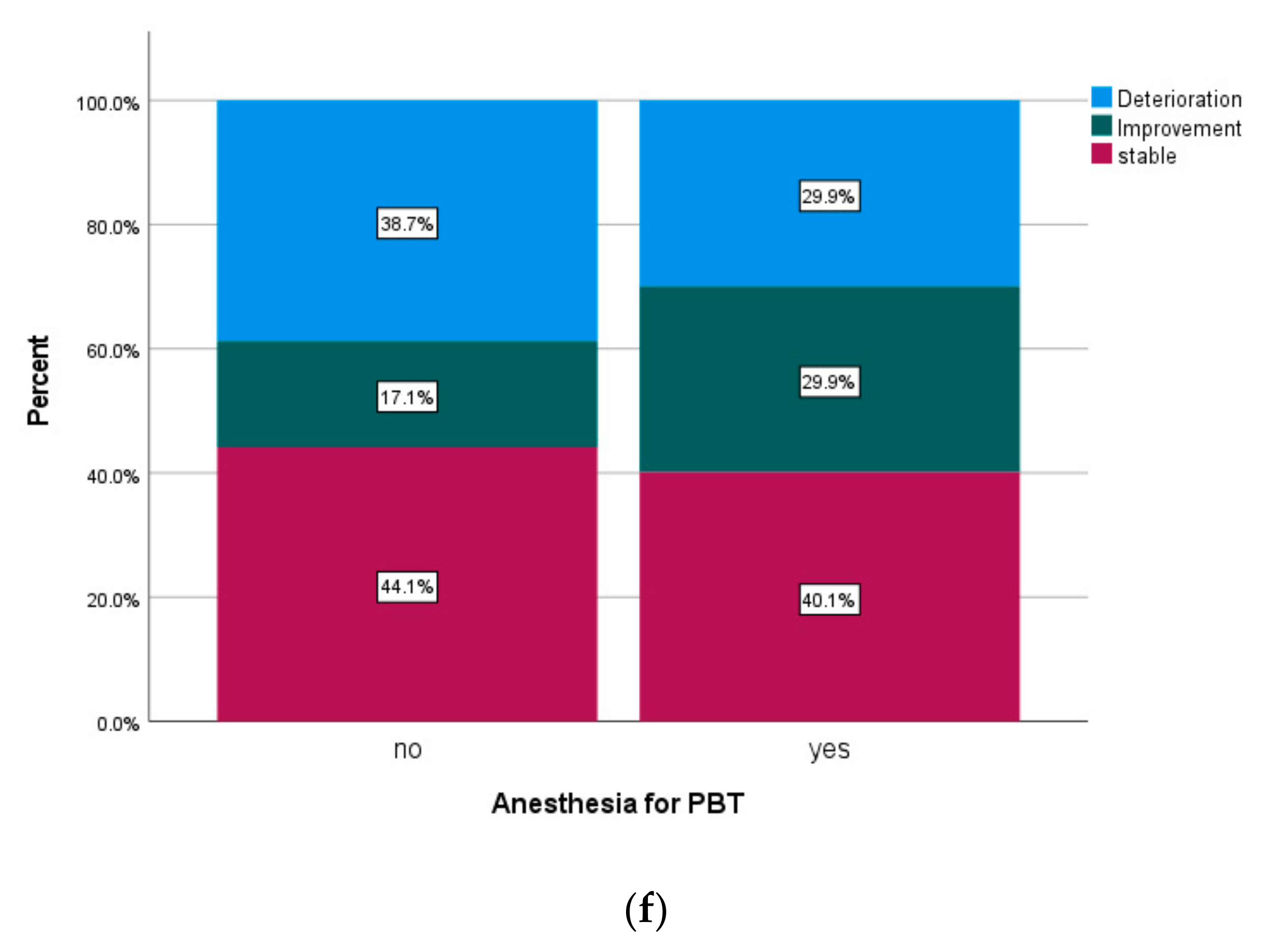
| anesthesia | ||||||
| yes | 76.9% | 57.6% | 72.6% | |||
| no | 91.7% | 0.001 | 82.5% | <0.001 | 89.7% | <0.001 |
| concomitant CTX | ||||||
| yes | 80.3% | 76.8% | 88.4% | |||
| no | 83.5% | 0.750 | 63.9% | 0.148 | 76.3% | 0.115 |
| Risk factors for OS | p-value | HR | 95% confidence interval of HR |
| metastatic disease | 0.013 | 2.214 | 1.181–4.153 |
| status of resection | 0.027 | 1.961 | 1.079–3.564 |
| anesthesia during PBT | <0.001 | 0.271 | 0.135–0.545 |
| Risk factors for PFS | p-value | HR | 95% confidence interval of HR |
| PBT dose ≥ 59 Gy (RBE) | 0.018 | 0.578 | 0.367–0.912 |
| anesthesia during PBT | <0.001 | 0.530 | 0.177–0.487 |
| Risk factors for LC | p-value | HR | 95% confidence interval of HR |
| PBT dose ≥ 59 Gy (RBE) | 0.001 | 0.414 | 0.245–0.702 |
| anesthesia during PBT | <0.001 | 0.334 | 0.180–0.617 |
| concomitant CTX | 0.028 | 2.063 | 1.082–3.931 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, S.; Frisch, S.; Stock, A.; Merta, J.; Bäumer, C.; Blase, C.; Schuermann, E.; Tippelt, S.; Bison, B.; Frühwald, M.; et al. Proton Beam Therapy for Pediatric Tumors of the Central Nervous System—Experiences of Clinical Outcome and Feasibility from the KiProReg Study. Cancers 2022, 14, 5863. https://doi.org/10.3390/cancers14235863
Peters S, Frisch S, Stock A, Merta J, Bäumer C, Blase C, Schuermann E, Tippelt S, Bison B, Frühwald M, et al. Proton Beam Therapy for Pediatric Tumors of the Central Nervous System—Experiences of Clinical Outcome and Feasibility from the KiProReg Study. Cancers. 2022; 14(23):5863. https://doi.org/10.3390/cancers14235863
Chicago/Turabian StylePeters, Sarah, Sabine Frisch, Annika Stock, Julien Merta, Christian Bäumer, Christoph Blase, Eicke Schuermann, Stephan Tippelt, Brigitte Bison, Michael Frühwald, and et al. 2022. "Proton Beam Therapy for Pediatric Tumors of the Central Nervous System—Experiences of Clinical Outcome and Feasibility from the KiProReg Study" Cancers 14, no. 23: 5863. https://doi.org/10.3390/cancers14235863
APA StylePeters, S., Frisch, S., Stock, A., Merta, J., Bäumer, C., Blase, C., Schuermann, E., Tippelt, S., Bison, B., Frühwald, M., Rutkowski, S., Fleischhack, G., & Timmermann, B. (2022). Proton Beam Therapy for Pediatric Tumors of the Central Nervous System—Experiences of Clinical Outcome and Feasibility from the KiProReg Study. Cancers, 14(23), 5863. https://doi.org/10.3390/cancers14235863






