Hearing Loss in Cancer Patients with Skull Base Tumors Undergoing Pencil Beam Scanning Proton Therapy: A Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
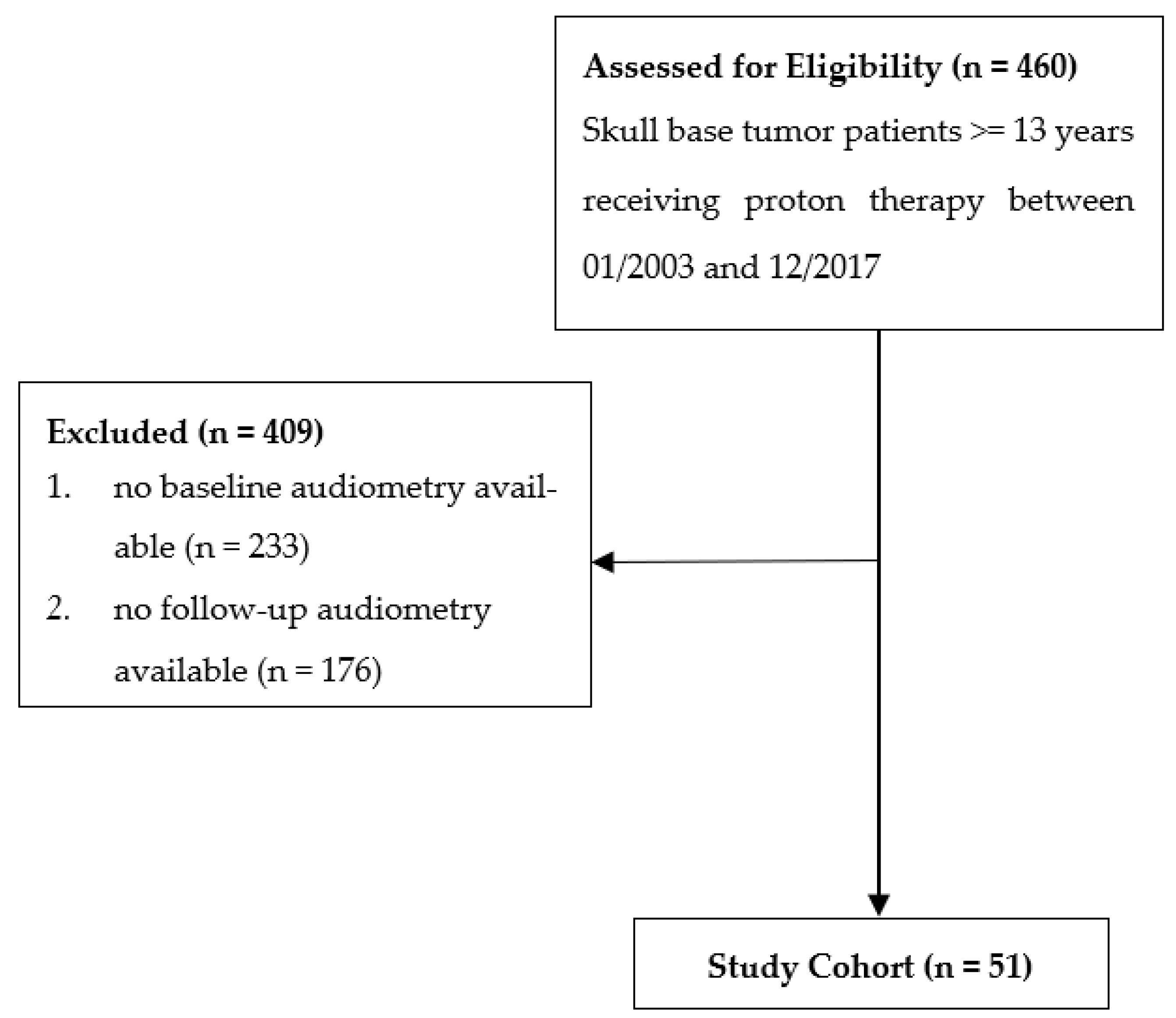
2.1. Study Design and Patient Population
2.2. Treatment
2.3. Follow-Up Evaluation
2.4. Assessment of Hearing
2.5. Statistical Analysis
3. Results
3.1. Patients, Tumors, and Follow-Up
3.2. Analysis of Treated Ears
3.3. CTC Grade Classification of Hearing Loss at First Follow Up
3.4. CTC Grade Classification of Hearing Loss at First Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolai, P.; Bradley, P.J.; Bossi, P.; Ferrari, M. Future Perspectives in the Management of Tumors of the Anterior Skull Base. Adv. Otorhinolaryngol. 2020, 84, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kountouri, M.; Pica, A.; Walser, M.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Bachtiary, B.; Combescure, C.; Lomax, A.J.; Schneider, R.; et al. Radiation-induced optic neuropathy after pencil beam scanning proton therapy for skull-base and head and neck tumours. Br. J. Radiol. 2020, 93, 20190028. [Google Scholar] [CrossRef]
- Pelak, M.J.; Walser, M.; Bachtiary, B.; Hrbacek, J.; Lomax, A.J.; Kliebsch, U.L.; Beer, J.; Pica, A.; Malyapa, R.; Weber, D.C. Clinical outcomes of head and neck adenoid cystic carcinoma patients treated with pencil beam-scanning proton therapy. Oral Oncol. 2020, 107, 104752. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Badiyan, S.; Malyapa, R.; Albertini, F.; Bolsi, A.; Lomax, A.J.; Schneider, R. Long-term outcomes and prognostic factors of skull-base chondrosarcoma patients treated with pencil-beam scanning proton therapy at the Paul Scherrer Institute. Neuro Oncol. 2016, 18, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulino, A.C.; Mahajan, A.; Ye, R.; Grosshans, D.R.; Fatih Okcu, M.; Su, J.; McAleer, M.F.; McGovern, S.; Mangona, V.A.; Chintagumpala, M. Ototoxicity and cochlear sparing in children with medulloblastoma: Proton vs. photon radiotherapy. Radiother. Oncol. 2018, 128, 128–132. [Google Scholar] [CrossRef]
- Fortunato, S.; Forli, F.; Guglielmi, V.; De Corso, E.; Paludetti, G.; Berrettini, S.; Fetoni, A.R. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol. Ital. 2016, 36, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Punch, J.L.; Hitt, R.; Smith, S.W. Hearing loss and quality of life. J. Commun. Disord. 2019, 78, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hou, X.; Bao, X.; Hou, W.; Jiang, X.; Ma, L.; Jiang, X.; Dong, L. Mechanism and Protection of Radiotherapy Induced Sensorineural Hearing Loss for Head and Neck Cancer. Biomed. Res. Int. 2021, 2021, 3548706. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Bass, J.K.; Khan, R.; Kun, L.E.; Merchant, T.E. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, N.; Jackson, A.; Eisbruch, A.; Pan, C.C.; Flickinger, J.C.; Antonelli, P.; Mendenhall, W.M. Radiation therapy and hearing loss. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S50–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.C.; Eisbruch, A.; Lee, J.S.; Snorrason, R.M.; Ten Haken, R.K.; Kileny, P.R. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Zarowski, A.; Milani, F.; Orecchia, R. Radiotherapy-induced ear toxicity. Cancer Treat. Rev. 2003, 29, 417–430. [Google Scholar] [CrossRef]
- Oh, Y.T.; Kim, C.H.; Choi, J.H.; Kang, S.H.; Chun, M. Sensory neural hearing loss after concurrent cisplatin and radiation therapy for nasopharyngeal carcinoma. Radiother. Oncol. 2004, 72, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.R.; Rezaeyan, A.; Nikoofar, A.; Bakhshandeh, M.; Farahani, S.; Cheraghi, S. Comparison of radiation and chemoradiation-induced sensorineural hearing loss in head and neck cancer patients. J. Cancer Res. Ther. 2020, 16, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Murray, F.; Combescure, C.; Calugaru, V.; Alapetite, C.; Albertini, F.; Bolle, S.; Goudjil, F.; Pica, A.; Walser, M.; et al. Long term outcome of skull-base chondrosarcoma patients treated with high-dose proton therapy with or without conventional radiation therapy. Radiother. Oncol. 2018, 129, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Rutz, H.P.; Pedroni, E.S.; Bolsi, A.; Timmermann, B.; Verwey, J.; Lomax, A.J.; Goitein, G. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: The Paul Scherrer Institut experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.L.; Steenbakkers, R.J.; Bourhis, J.; Budach, W.; Grau, C.; Gregoire, V.; van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olusanya, B.O.; Davis, A.C.; Hoffman, H.J. Hearing loss grades and the International classification of functioning, disability and health. Bull. World Health Organ. 2019, 97, 725–728. [Google Scholar] [CrossRef]
- R Core Team. Available online: https://www.R-project.org/ (accessed on 4 July 2022).
- Chen, W.C.; Jackson, A.; Budnick, A.S.; Pfister, D.G.; Kraus, D.H.; Hunt, M.A.; Stambuk, H.; Levegrun, S.; Wolden, S.L. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer 2006, 106, 820–829. [Google Scholar] [CrossRef] [PubMed]
- De Marzi, L.; Feuvret, L.; Boule, T.; Habrand, J.L.; Martin, F.; Calugaru, V.; Fournier-Bidoz, N.; Ferrand, R.; Mazal, A. Use of gEUD for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br. J. Radiol. 2015, 88, 20140413. [Google Scholar] [CrossRef] [Green Version]
- Merchant, T.E.; Gould, C.J.; Xiong, X.; Robbins, N.; Zhu, J.; Pritchard, D.L.; Khan, R.; Heideman, R.L.; Krasin, M.J.; Kun, L.E. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1194–1207. [Google Scholar] [CrossRef]
- van der Putten, L.; de Bree, R.; Plukker, J.T.; Langendijk, J.A.; Smits, C.; Burlage, F.R.; Leemans, C.R. Permanent unilateral hearing loss after radiotherapy for parotid gland tumors. Head Neck 2006, 28, 902–908. [Google Scholar] [CrossRef]
- Rigters, S.C.; van der Schroeff, M.P.; Papageorgiou, G.; Baatenburg de Jong, R.J.; Goedegebure, A. Progression of Hearing Loss in the Aging Population: Repeated Auditory Measurements in the Rotterdam Study. Audiol. Neurootol. 2018, 23, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Reed, N.S. The Pure-Tone Average as a Universal Metric-Knowing Your Hearing. JAMA Otolaryngol. Head Neck Surg. 2020, 147, 230. [Google Scholar] [CrossRef]


| n = 51 | |
|---|---|
| Age at time of proton therapy (median, IQR) | 49.7 (39.1–61) |
| Sex | |
| - Female, n (%) | 30 (58.8) |
| - Male, n (%) | 21 (41.2) |
| Histology | |
| - Chordoma, n (%) | 24 (47.1) |
| - Chondrosarcoma, n (%) | 15 (29.4) |
| - Head and Neck Tumor, n (%) | 9 (17.6) |
| - Meningioma, n (%) | 3 (5.9) |
| Tumor position | |
| - midline, n (%) | 31 (60.8) |
| - lateralized (ipsi and contralateral), n (%) | 20 (39.2) |
| Mean tumor dose, Gy (RBE), mean (range) | 71.1 (52–77.8) |
| Duration or Proton Therapy (days), median (range) | 51 (27–60) |
| Follow-up (months), median (IQR) | 26 (14–69) |
| Number of audiometric tests during follow-up median (IQR) | 2 (1–3) |
| Time interval between audiometric tests | |
| - Baseline to treatment start (days) median (IQR) | 17 (8.5–34) |
| - Treatment start to first follow-up (months) median (IQR) | 11 (5.5–33.7) |
| Hearing Sensitivity (dB) | Before PBS-PT | First Follow-Up | ||
|---|---|---|---|---|
| n = 101 | (%) | n = 101 | (%) | |
| Excellent (<5) | 5 | 5.0 | 4 | 4.0 |
| Good (5–19.9) | 59 | 58.4 | 34 | 33.7 |
| Mild (20–34.9) | 20 | 19.8 | 23 | 22.8 |
| Moderate (35–49.9) | 9 | 8.9 | 17 | 16.8 |
| Moderately severe (50–64.9) | 2 | 2.0 | 11 | 10.9 |
| Severe (65–79.9) | 2 | 2.0 | 4 | 3.9 |
| Profound (80–94.9) | 3 | 3.0 | 5 | 4.0 |
| Complete (≥95) | 1 | 1.0 | 3 | 3.0 |
| Overall | Contralateral | Ipsilateral | Midline | p | |
|---|---|---|---|---|---|
| n = 101 | n = 20 | n = 19 | n = 62 | ||
| Baseline PTA, dB, median (IQR) | 15.0 (10.0–25.0) | 13.1 (9.7–20.6) | 13.8 (10.0–40.0) | 16.3 (9.1–25. 0) | 0.549 |
| Baseline hearing disorder, n (%) | 0.159 | ||||
| conductive | 4 (4.0) | 0 (0.0) | 1 (5.3) | 3 (4.8) | |
| Mixed | 7 (6.9) | 0 (0.0) | 3 (15.8) | 4 (6.5) | |
| normal | 46 (45.5) | 12 (60.0) | 10 (52.6) | 24 (38.7) | |
| sensorineural | 25 (24.8) | 7 (35.0) | 2 (10.5) | 16 (25.8) | |
| unknown | 19 (18.8) | 1 (5.0) | 3 (.15.8) | 15 (24.2) | |
| Follow-up PTA, dB, median (IQR) | 23.8 (11.3–46.3) | 16.9 (10.6–28.8) | 32.5 (11.3–50.0) | 28.8 (14.4–48.1) | 0.120 |
| Follow-up hearing disorder, n (%) | 0.047 | ||||
| conductive | 5 (5.0) | 0 (0.0) | 2 (10.5) | 3 (4.8) | |
| Mixed | 16 (15.8) | 1 (5.0) | 5 (26.3) | 10 (16.1) | |
| normal | 27 (26.7) | 9 (45.0) | 6 (31.6) | 12 (19.4) | |
| sensorineural | 27 (26.7) | 2 (10.0) | 4 (21.1) | 21 (33.9) | |
| unknown | 26 (25.7) | 8 (40.0) | 2 (10.5) | 16 (25.8) | |
| Cochlea Dose Gy (RBE), mean (SD) | 36.7 (22.3) | 13.4 (12.3) | 58.8 (16.7) | 37.51 (18.9) | <0.001 |
| Dose Group, n (%) | <0.001 | ||||
| <32 Gy (RBE) | 45 (44.6) | 17 (85.0) | 2 (10.0) | 26 (41.9) | |
| 32–44.9 Gy (RBE) | 20 (19.8) | 3 (15.0) | 3 (15.0) | 14 (22.6) | |
| ≥45 Gy (RBE) | 36 (35.6) | 0 (0.0) | 14 (73.7) | 22 (35.5) |
| CTCAE Grade | Patients, n (%) |
|---|---|
| 0 | 16 (31) |
| 1 | 11 (22) |
| 2 | 2 (4) |
| 3 | 21 (41) |
| 4 | 1 (2) |
| Estimate | 95% CI | t-Value | p-Value | |
|---|---|---|---|---|
| PTA before proton therapy (dB) | 0.80 | 0.64–0.96 | 9.88 | <0.0001 |
| Age at follow-up (years) | 0.30 | 0.03–0.57 | 2.21 | 0.029 |
| Time since proton therapy (years) | 2.07 | 0.92–3.23 | 3.57 | 0.0005 |
| Mean Dose Cochlea (Gy, RBE) | 0.34 | 0.21–0.46 | 5.43 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachtiary, B.; Veraguth, D.; Roos, N.; Pfiffner, F.; Leiser, D.; Pica, A.; Walser, M.; von Felten, S.; Weber, D.C. Hearing Loss in Cancer Patients with Skull Base Tumors Undergoing Pencil Beam Scanning Proton Therapy: A Retrospective Cohort Study. Cancers 2022, 14, 3853. https://doi.org/10.3390/cancers14163853
Bachtiary B, Veraguth D, Roos N, Pfiffner F, Leiser D, Pica A, Walser M, von Felten S, Weber DC. Hearing Loss in Cancer Patients with Skull Base Tumors Undergoing Pencil Beam Scanning Proton Therapy: A Retrospective Cohort Study. Cancers. 2022; 14(16):3853. https://doi.org/10.3390/cancers14163853
Chicago/Turabian StyleBachtiary, Barbara, Dorothe Veraguth, Nicolaas Roos, Flurin Pfiffner, Dominic Leiser, Alessia Pica, Marc Walser, Stefanie von Felten, and Damien C. Weber. 2022. "Hearing Loss in Cancer Patients with Skull Base Tumors Undergoing Pencil Beam Scanning Proton Therapy: A Retrospective Cohort Study" Cancers 14, no. 16: 3853. https://doi.org/10.3390/cancers14163853
APA StyleBachtiary, B., Veraguth, D., Roos, N., Pfiffner, F., Leiser, D., Pica, A., Walser, M., von Felten, S., & Weber, D. C. (2022). Hearing Loss in Cancer Patients with Skull Base Tumors Undergoing Pencil Beam Scanning Proton Therapy: A Retrospective Cohort Study. Cancers, 14(16), 3853. https://doi.org/10.3390/cancers14163853






