circRNF10 Regulates Tumorigenic Properties and Natural Killer Cell-Mediated Cytotoxicity against Breast Cancer through the miR-934/PTEN/PI3k-Akt Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA-Sequencing and circRNA Microarray Data Collection and Bioinformatics Analysis
2.2. Screening and Prediction of circRNA-Bound miRNAs and miRNA-Targeted mRNAs
2.3. Tissue Specimens and Tissue Microarray
2.4. Xenograft Tumor Model
2.5. Cell Culture and Transfection
2.6. DNA/RNA Extraction, RNase R Treatment and Actinomycin D Assay
2.7. Real-Time PCR Validation and Nucleic Acid Electrophoresis
2.8. Fluorescence In Situ Hybridization (FISH)
2.9. RNA Immunoprecipitation (RIP)
2.10. Luciferase Promoter Assay
2.11. Transwell Migration and Invasion Assays
2.12. Cell Proliferation Assay
2.13. Colony Formation Assay
2.14. Lactate Dehydrogenase (LDH) Assay
2.15. Cytokine Assay
2.16. Western Blot Analysis
2.17. Statistical Analysis
3. Results
3.1. circRNAs Expression Profiles in BC
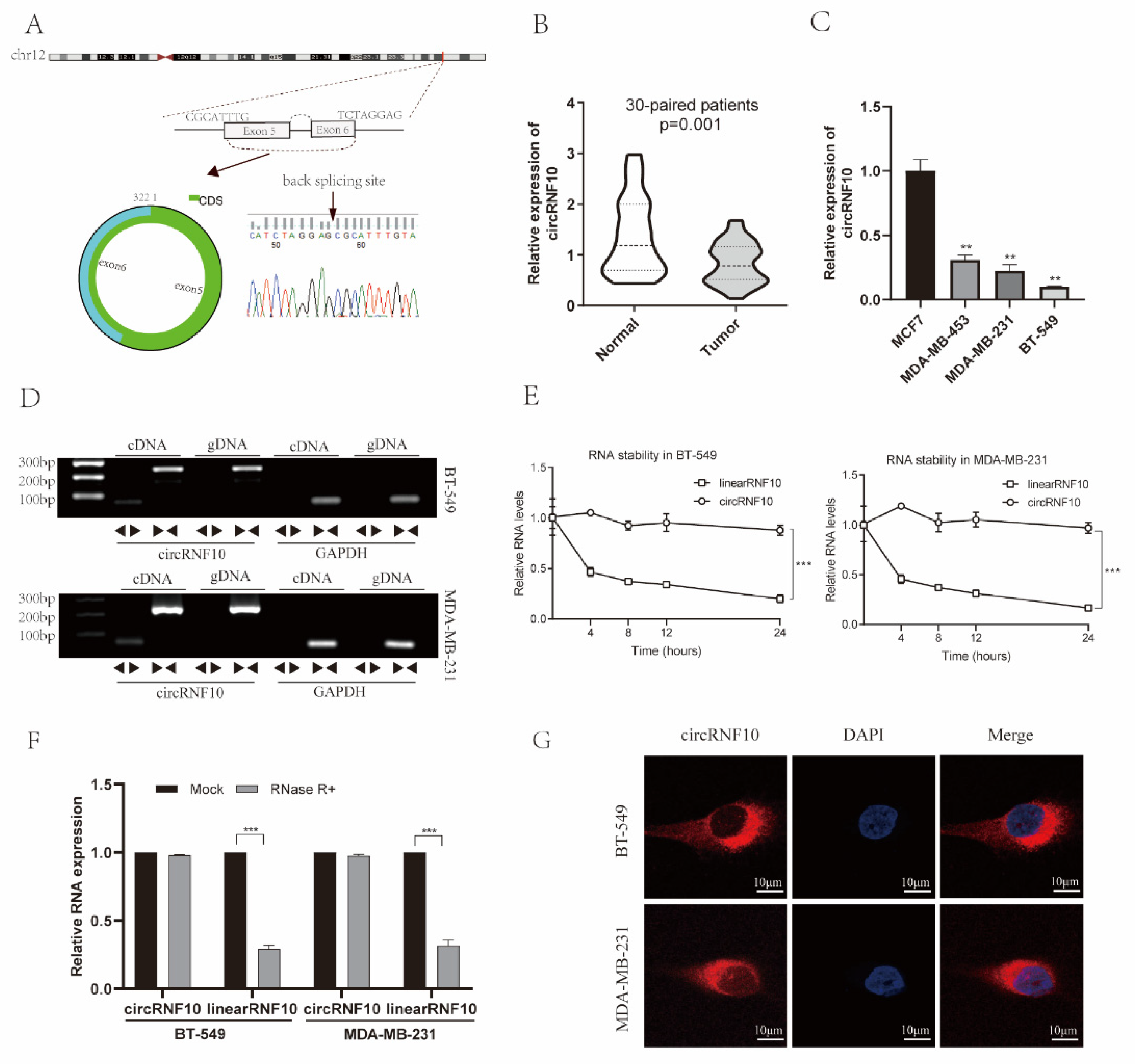
3.2. Characterization of circRNF10 in BC
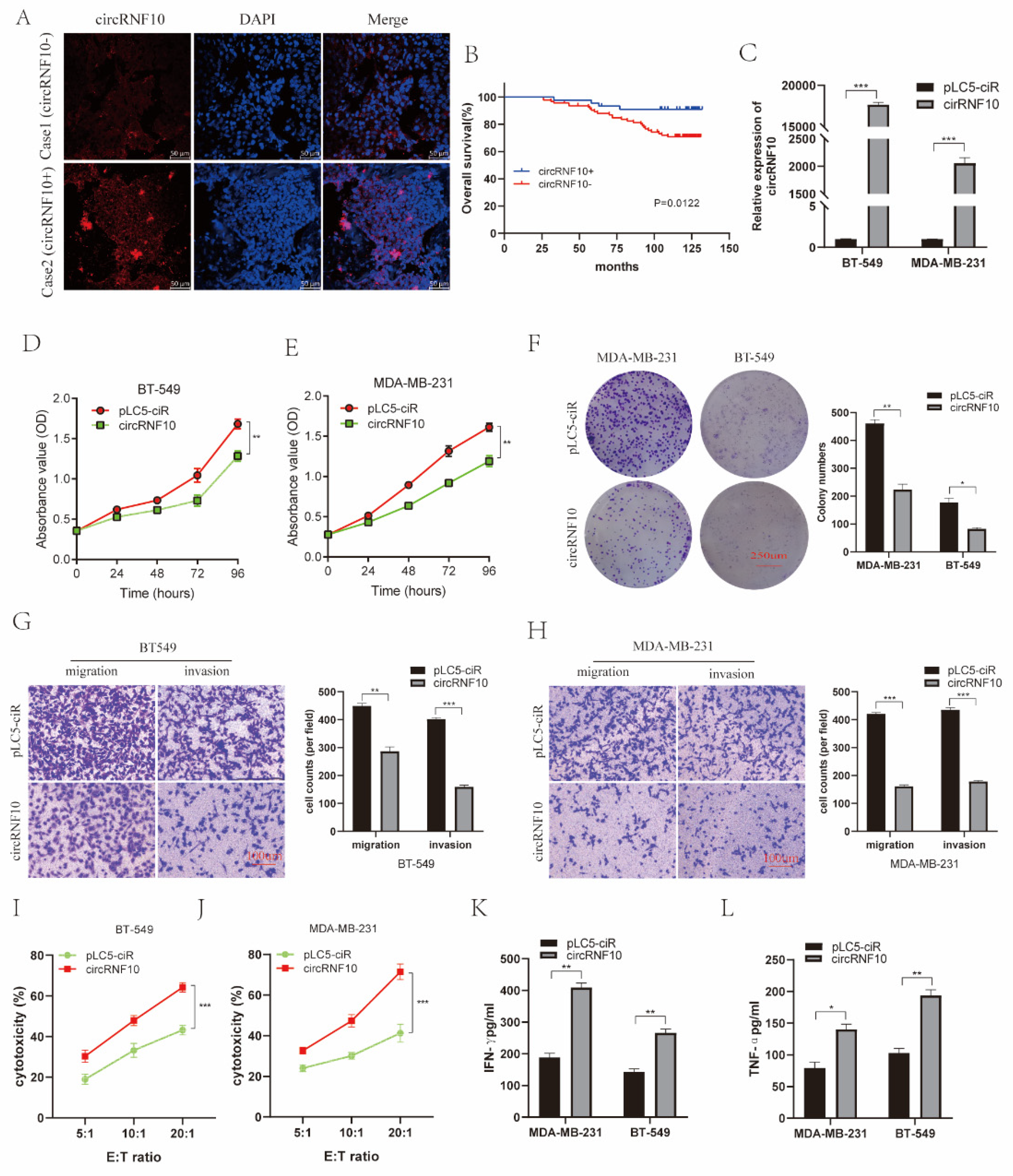
3.3. The Expression of circRNF10 Is Associated with BC Progression
3.4. circRNF10 Suppresses BC Cell Proliferation, Invasion, and Migration and Enhances the Killing Efficiency of NK-92MI Cells against BC Cells
3.5. circRNF10 Acts as a Sponge for miR-934 in BC Cells
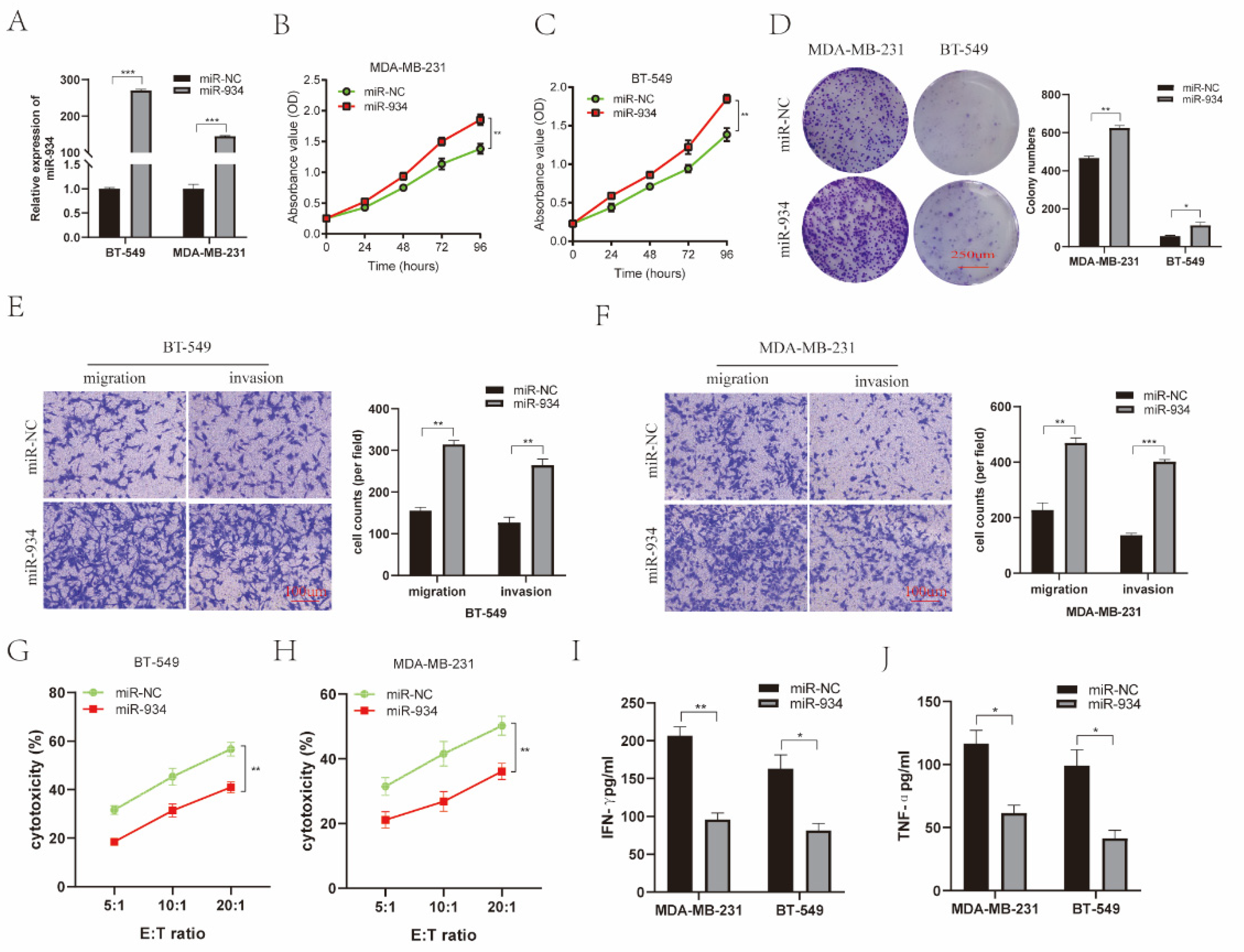
3.6. miR-934 Enhances BC Cell Proliferation, Invasion, and Migration and Reduces the Killing Efficiency of NK-92MI Cells against BC Cells
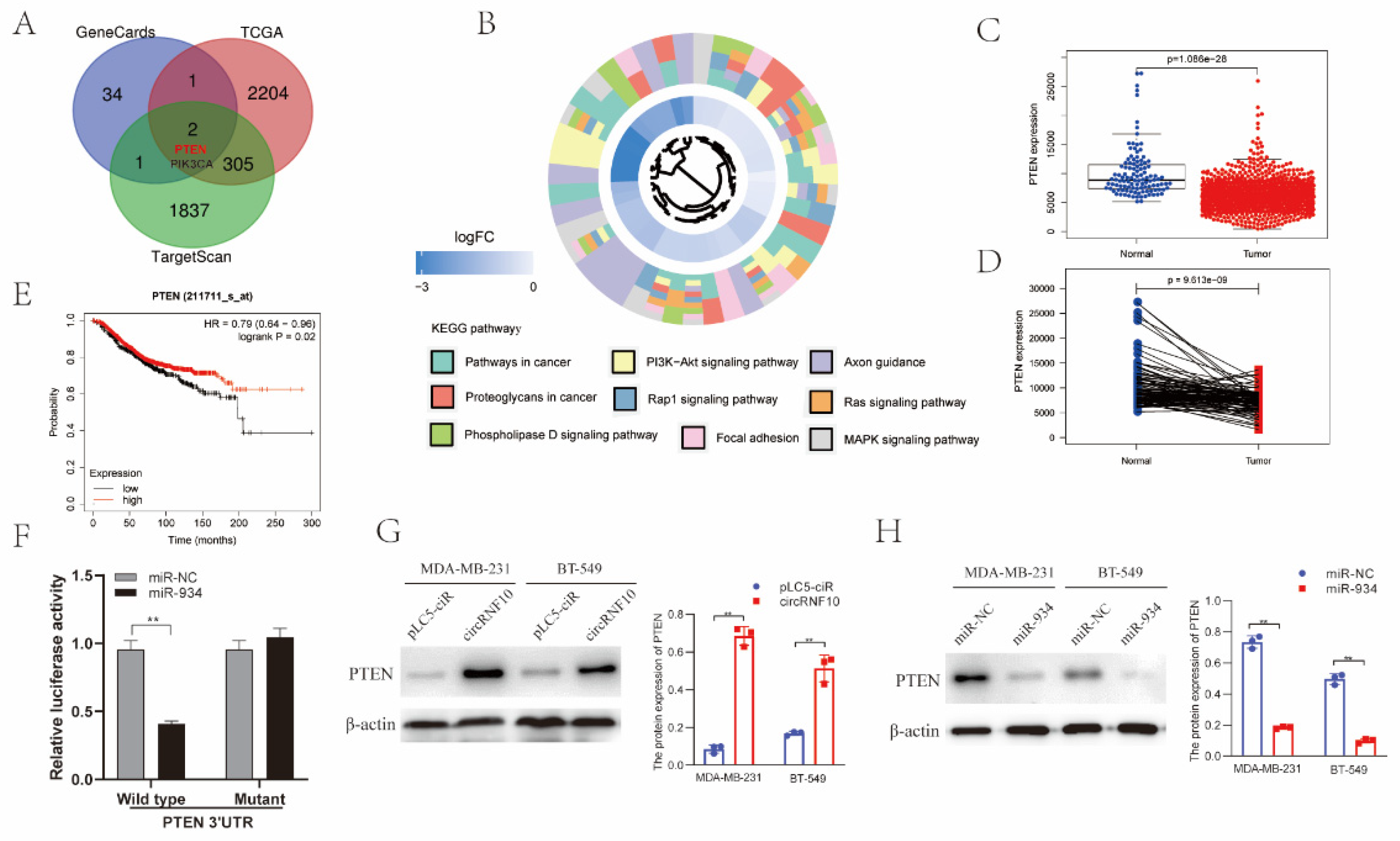
3.7. PTEN Was the Target Gene of miR-934
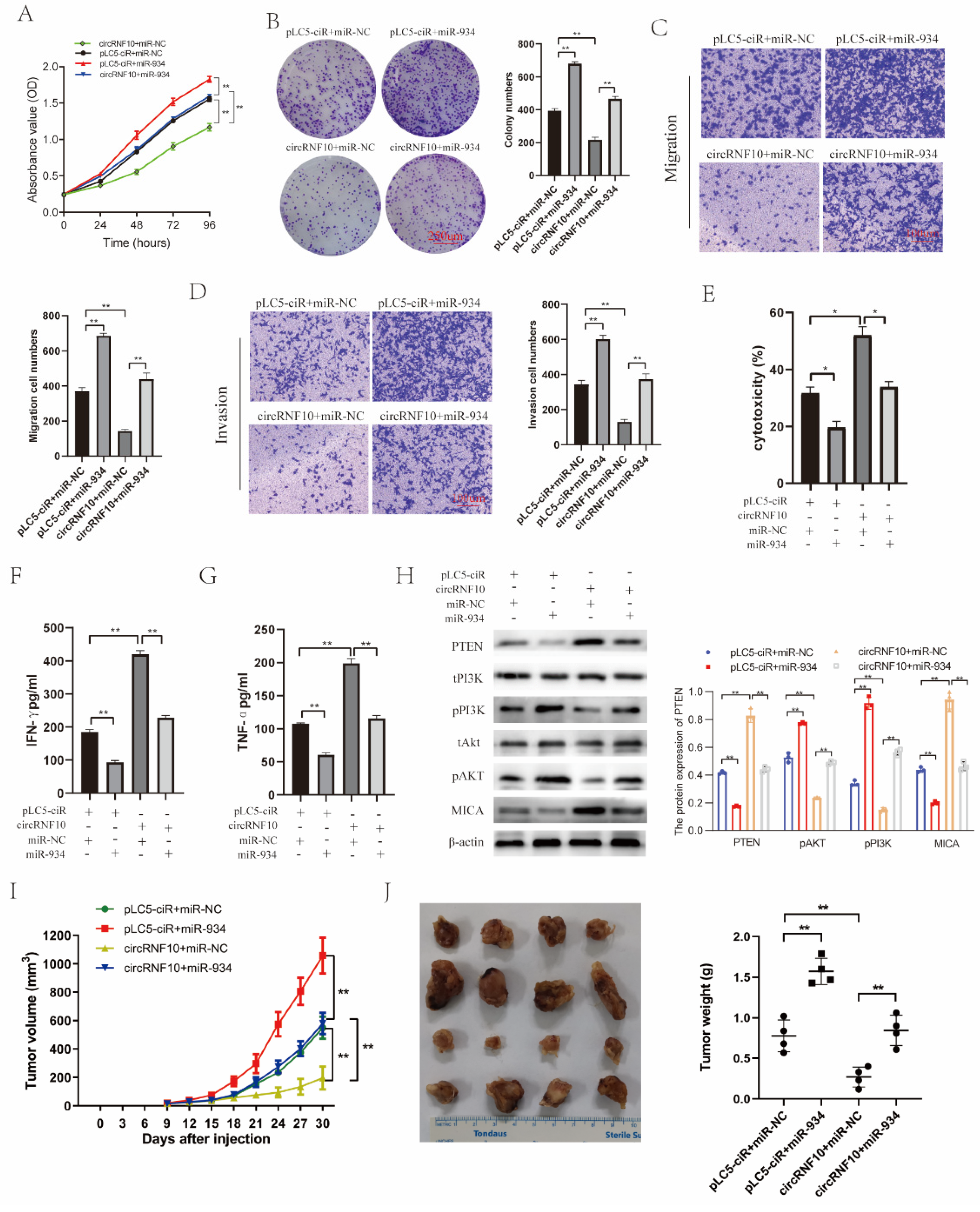
3.8. circRNF10 Suppresses BC Cell Proliferation, Migration, and Invasion and Enhances the Killing Efficiency of NK-92MI Cells on BC Cells via miR-934
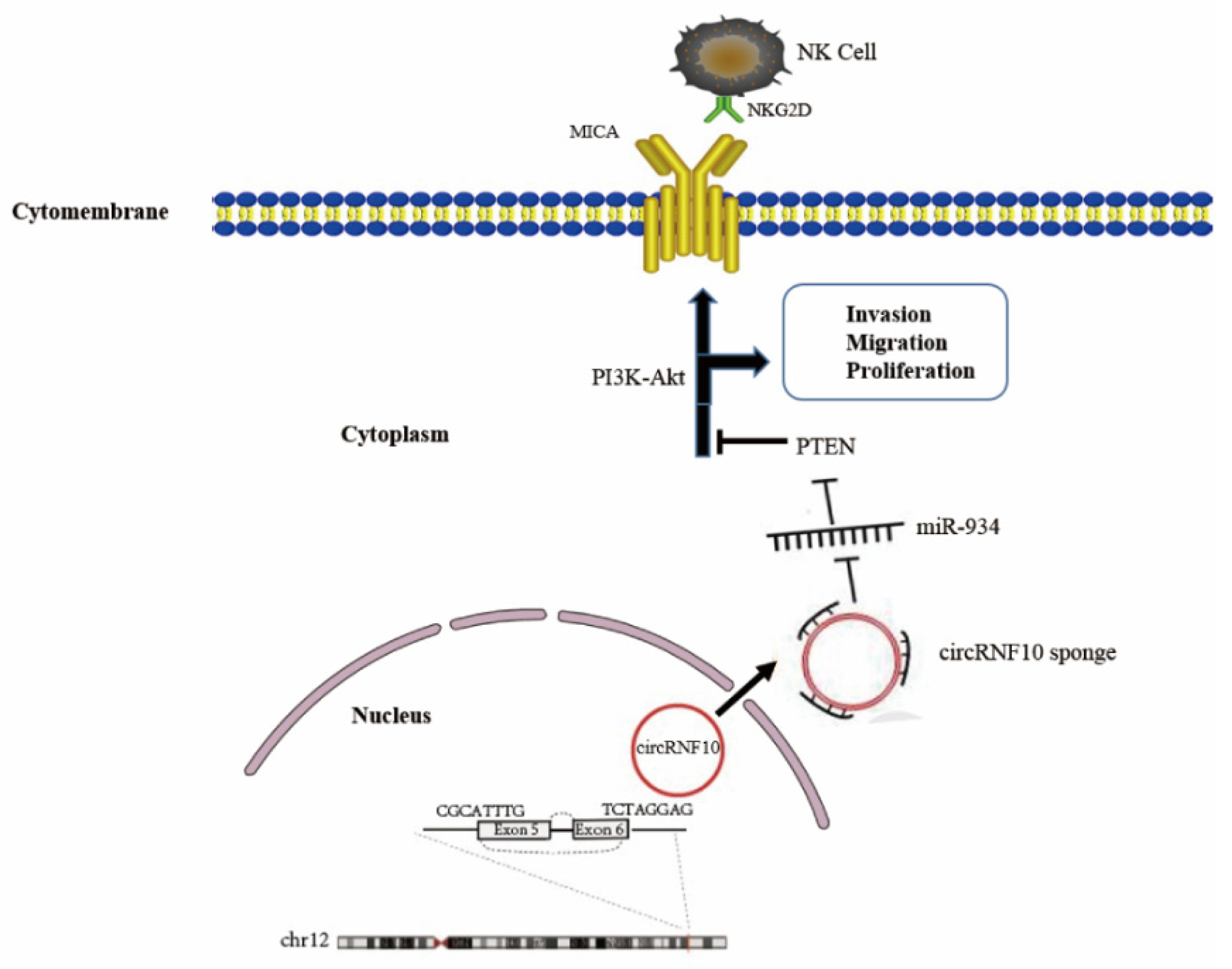
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; Andre, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- Lasda, E.; Parker, R. Circular RNAs: Diversity of form and function. RNA 2014, 20, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Li, Z.; Li, F.; Liang, L.; Zou, Y.; Shen, H.; Li, J.; Xia, Y.; Cheng, Z.; et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J. Hepatol. 2022, 76, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, J.; Zhang, H.; Liao, Z.; Liu, F.; Su, C.; Wang, W.; Han, M.; Zhang, L.; Zhu, H.; et al. EIF4A3-induced circTOLLIP promotes the progression of hepatocellular carcinoma via the miR-516a-5p/PBX3/EMT pathway. J. Exp. Clin. Cancer Res. 2022, 41, 164. [Google Scholar] [CrossRef]
- He, J.; Chu, Z.; Lai, W.; Lan, Q.; Zeng, Y.; Lu, D.; Jin, S.; Xu, H.; Su, P.; Yin, D.; et al. Circular RNA circHERC4 as a novel oncogenic driver to promote tumor metastasis via the miR-556-5p/CTBP2/E-cadherin axis in colorectal cancer. J. Hematol. Oncol. 2021, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Yang, Y.; Shen, P.; Ma, J.; Fang, B.; Wang, Q.; Wang, K.; Shi, P.; Fan, S.; Fang, X. circPDE4B prevents articular cartilage degeneration and promotes repair by acting as a scaffold for RIC8A and MID1. Ann. Rheum. Dis. 2021, 80, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Man, C.; Xiang, S.; Yao, L.; Wang, X.; Fan, Y. Circular RNAs’ cap-independent translation protein and its roles in carcinomas. Mol. Cancer 2021, 20, 119. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Weng, W.; Wei, Q.; Toden, S.; Yoshida, K.; Nagasaka, T.; Fujiwara, T.; Cai, S.; Qin, H.; Ma, Y.; Goel, A. Circular RNA ciRS-7-A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin. Cancer Res. 2017, 23, 3918–3928. [Google Scholar] [CrossRef]
- Pan, H.; Li, T.; Jiang, Y.; Pan, C.; Ding, Y.; Huang, Z.; Yu, H.; Kong, D. Overexpression of Circular RNA ciRS-7 Abrogates the Tumor Suppressive Effect of miR-7 on Gastric Cancer via PTEN/PI3K/AKT Signaling Pathway. J. Cell. Biochem. 2018, 119, 440–446. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, G.; Luo, Y.; Li, S.; Xu, Y.; Zhang, Y.; Hu, J.; Li, P.; Pan, H.; Wang, Y. Circular RNA ROCK1, a novel circRNA, suppresses osteosarcoma proliferation and migration via altering the miR-532-5p/PTEN axis. Exp. Mol. Med. 2022, 54, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wu, T.; Zhang, X.; Xu, K.; Yin, X.; Wang, X.; Shi, S.; Wang, P.; Gao, L.; Xu, S.; et al. Immunomodulatory functions of the circ_001678/miRNA-326/ZEB1 axis in non-small cell lung cancer via the regulation of PD-1/PD-L1 pathway. Hum. Mol. Genet. 2022, ddac155. [Google Scholar] [CrossRef]
- Yang, R.; Xing, L.; Zheng, X.; Sun, Y.; Wang, X.; Chen, J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol. Cancer 2019, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Meng, L.; Liu, S.; Ding, P.; Chang, S.; Ju, Y.; Liu, F.; Gu, L.; Lian, Y.; Geng, C. Circular RNA ciRS-7 Maintains Metastatic Phenotypes as a ceRNA of miR-1299 to Target MMPs. Mol. Cancer Res. 2018, 16, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Arnaiz, E.; Sole, C.; Manterola, L.; Iparraguirre, L.; Otaegui, D.; Lawrie, C.H. CircRNAs and cancer: Biomarkers and master regulators. Semin. Cancer Biol. 2019, 58, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.; Coulouarn, C. One stone, two birds: circACTN4, a nexus for a coordinated activation of Hippo and Wnt/beta-catenin pathways in cholangiocarcinoma. J. Hepatol. 2022, 76, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Li, Q.; Li, J.; Chen, K.; He, Q.; Zhao, Y.; Liang, Y.; Zhao, Y.; Qiao, H.; Liu, N.; et al. CircIPO7 Promotes Nasopharyngeal Carcinoma Metastasis and Cisplatin Chemoresistance by Facilitating YBX1 Nuclear Localization. Clin. Cancer Res. 2022, 28, 4521–4535. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, S.; Zhang, Y.; Liu, S.; Meng, L.; Liu, F.; Gu, L.; Ai, N.; Sang, M. Circular RNA circWWC3 augments breast cancer progression through promoting M2 macrophage polarization and tumor immune escape via regulating the expression and secretion of IL-4. Cancer Cell Int. 2022, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, L.; Zhao, L.; Zhang, G.; Wang, Z.; Zhu, P.; Liu, B. circREEP3 Drives Colorectal Cancer Progression via Activation of FKBP10 Transcription and Restriction of Antitumor Immunity. Adv. Sci. 2022, 9, e2105160. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, Y.; Li, J.; Zeng, J.; Wu, L. ALKBH5-mediated m6A modification of circCCDC134 facilitates cervical cancer metastasis by enhancing HIF1A transcription. J. Exp. Clin. Cancer Res. 2022, 41, 261. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef]
- Jin, Y.; Weng, Y.; Wang, Y.; Lin, J.; Deng, X.; Shen, B.; Zhan, Q.; Lu, X. miR-934 as a Prognostic Marker Facilitates Cell Proliferation and Migration of Pancreatic Tumor by Targeting PROX1. OncoTargets Ther. 2020, 13, 3389–3399. [Google Scholar] [CrossRef]
- Yan, H.; Ren, S.; Lin, Q.; Yu, Y.; Chen, C.; Hua, X.; Jin, H.; Lu, Y.; Zhang, H.; Xie, Q.; et al. Inhibition of UBE2N-dependent CDK6 protein degradation by miR-934 promotes human bladder cancer cell growth. FASEB J. 2019, 33, 12112–12123. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Shi, M.; Li, Z.Y.; Zhang, L.M.; Wu, X.Y.; Xiang, S.H.; Wang, Y.G.; Zhang, Y.Q. Hsa_circ_0007456 regulates the natural killer cell-mediated cytotoxicity toward hepatocellular carcinoma via the miR-6852-3p/ICAM-1 axis. Cell Death Dis. 2021, 12, 94. [Google Scholar] [CrossRef]
- Zhang, P.F.; Gao, C.; Huang, X.Y.; Lu, J.C.; Guo, X.J.; Shi, G.M.; Cai, J.B.; Ke, A.W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 2020, 19, 110. [Google Scholar] [CrossRef]
- Ke, H.; Zhang, J.; Wang, F.; Xiong, Y. ZNF652-Induced circRHOT1 Promotes SMAD5 Expression to Modulate Tumorigenic Properties and Nature Killer Cell-Mediated Toxicity in Bladder Cancer via Targeting miR-3666. J. Immunol Res. 2021, 2021, 7608178. [Google Scholar] [CrossRef]
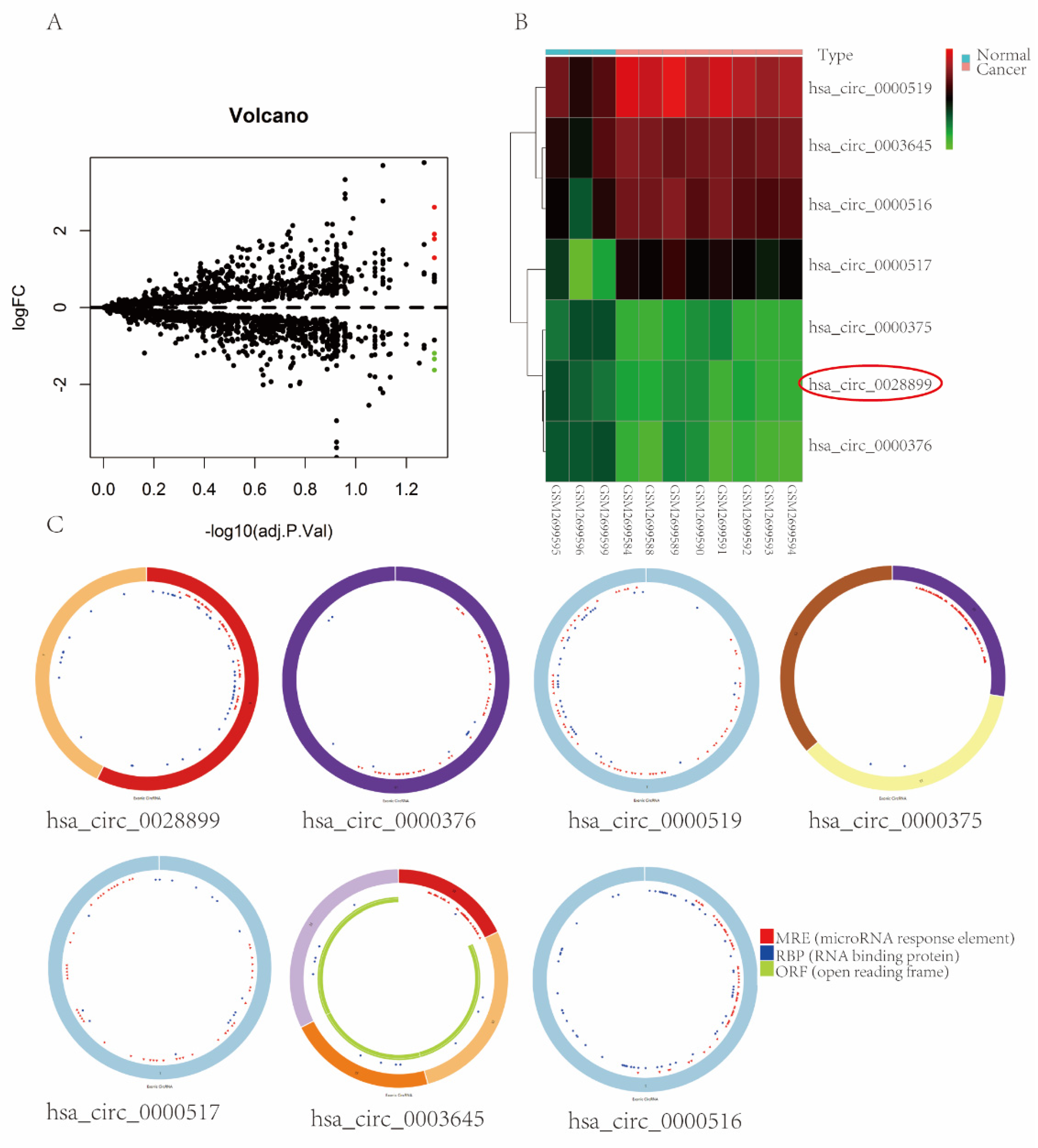

| circRNA | Genomic Length | Position | Strand |
Gene Symbol | Best Transcript | Regulation |
|---|---|---|---|---|---|---|
| hsa_circ_0028899 | 401 | chr12:120995084-120995485 | + | RNF10 | NM_014868 | down |
| hsa_circ_0000376 | 48,782 | chr12:11199618-11248400 | - | PRH1-PRR4 | NR_037918 | down |
| hsa_circ_0000519 | 98 | chr14:20811436-20811534 | - | RPPH1 | NR_002312 | up |
| hsa_circ_0000375 | 401 | chr12:6657590-6657991 | - | IFFO1 | NM_080730 | down |
| hsa_circ_0000517 | 88 | chr14:20811404-20811492 | - | RPPH1 | NR_002312 | up |
| hsa_circ_0003645 | 7205 | chr16:19656207-19663412 | + | C16orf62 | NM_020314 | up |
| hsa_circ_0000516 | 85 | chr14:20811398-20811483 | + | RPPH1 | NR_002312 | up |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Sang, Y.; Zheng, Y.; Gu, L.; Meng, L.; Li, Z.; Dong, Y.; Wei, Z.; Geng, C.; Sang, M. circRNF10 Regulates Tumorigenic Properties and Natural Killer Cell-Mediated Cytotoxicity against Breast Cancer through the miR-934/PTEN/PI3k-Akt Axis. Cancers 2022, 14, 5862. https://doi.org/10.3390/cancers14235862
Liu F, Sang Y, Zheng Y, Gu L, Meng L, Li Z, Dong Y, Wei Z, Geng C, Sang M. circRNF10 Regulates Tumorigenic Properties and Natural Killer Cell-Mediated Cytotoxicity against Breast Cancer through the miR-934/PTEN/PI3k-Akt Axis. Cancers. 2022; 14(23):5862. https://doi.org/10.3390/cancers14235862
Chicago/Turabian StyleLiu, Fei, Yang Sang, Yang Zheng, Lina Gu, Lingjiao Meng, Ziyi Li, Yuyang Dong, Zishuan Wei, Cuizhi Geng, and Meixiang Sang. 2022. "circRNF10 Regulates Tumorigenic Properties and Natural Killer Cell-Mediated Cytotoxicity against Breast Cancer through the miR-934/PTEN/PI3k-Akt Axis" Cancers 14, no. 23: 5862. https://doi.org/10.3390/cancers14235862
APA StyleLiu, F., Sang, Y., Zheng, Y., Gu, L., Meng, L., Li, Z., Dong, Y., Wei, Z., Geng, C., & Sang, M. (2022). circRNF10 Regulates Tumorigenic Properties and Natural Killer Cell-Mediated Cytotoxicity against Breast Cancer through the miR-934/PTEN/PI3k-Akt Axis. Cancers, 14(23), 5862. https://doi.org/10.3390/cancers14235862








