Role of Functional MRI in Liver SBRT: Current Use and Future Directions
Simple Summary
Abstract
1. Introduction
2. Liver Function Assessment
2.1. Global Liver Function Tests
2.2. Imaging of Liver Function
3. Functional MRI of the Liver
3.1. Diffusion-Weighted Imaging
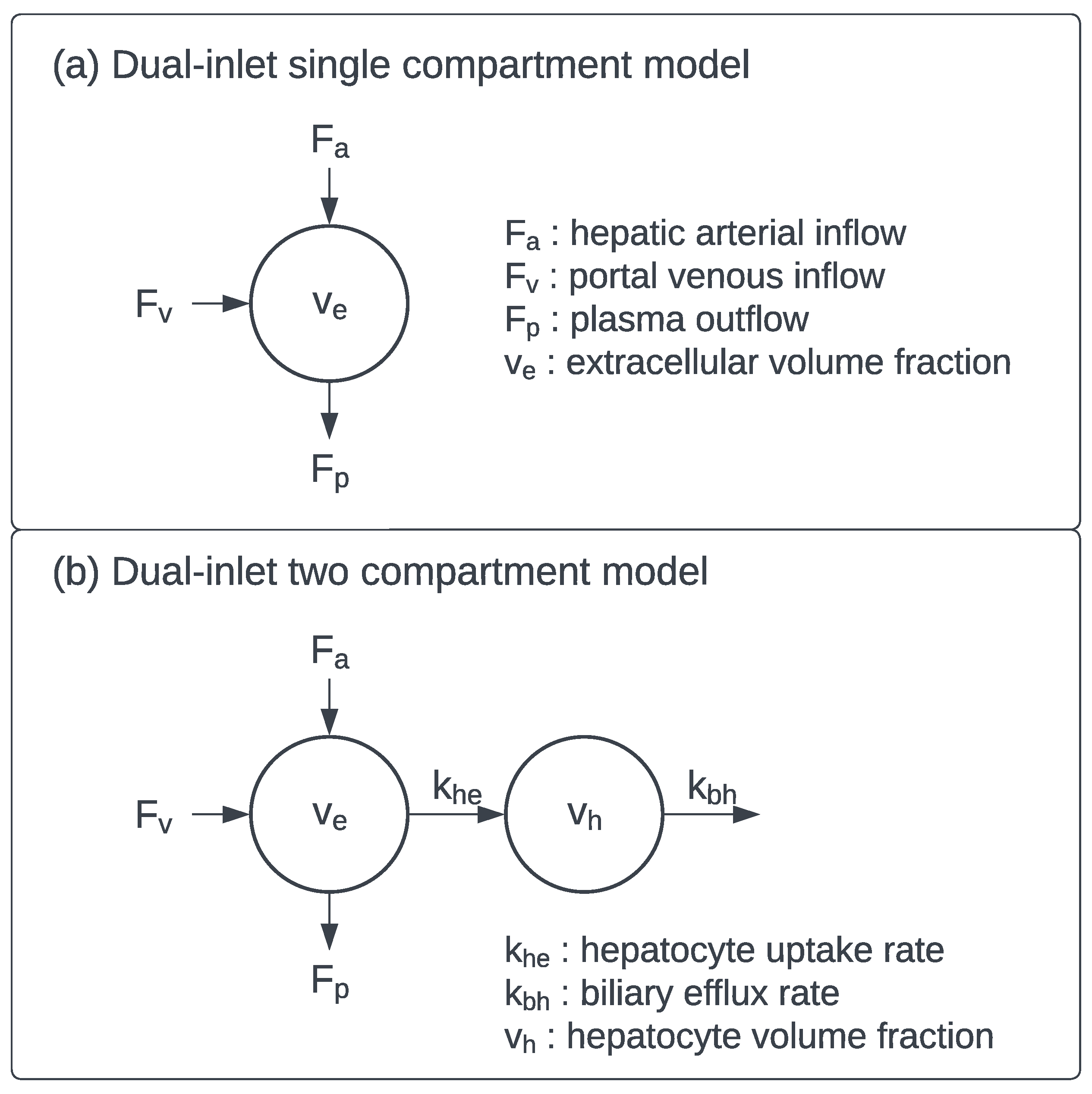
3.2. Dynamic Contrast-Enhanced MRI
3.3. Advanced and Non-Standard Imaging Techniques
4. Current Use of Functional MRI for Liver SBRT
4.1. Use in Treatment Planning
4.2. Use in Response Assessment
4.3. Use for Dose-Response Assessment and Mid-Treatment Adaptation
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SBRT | Stereotactic body radiation therapy |
| HCC | Hepatocellular carcinoma |
| NTCP | Normal tissue complication probability |
| RILD | Radiation induced liver disease |
| MRI | Magnetic resonance imaging |
| CP | Child-Pugh |
| ALBI | Albumin-Bilirubin |
| MELD | Model for end-stage liver disease |
| QUANTEC | Quantitative analysis of normal tissue effects in the clinic |
| ICG | Indocyanine green |
| ICG-R15 | ICG retention at 15 min after injection |
| CT | Computed tomography |
| DCE-CT | Dynamic contrast-enhanced computed tomography |
| DECT | Dual energy CT |
| SPECT | Single-proton emission computed tomography |
| SC | Sulphur colloid |
| HIDA | Hepatobiliary iminodiacetic acid |
| GSA | Galactosyl human serum albumin |
| FLV | Functional liver volume |
| LSR | Liver-to-spleen uptake ratio |
| TLF | Total liver function |
| ASGPR | Asialoglycoprotein receptor |
| LUV | Liver uptake value |
| FLI | Functional liver index |
| PET | Positron emission tomography |
| FDGal | Fluoro-D-galactose |
| T2w | T2-weighted |
| IP | In-phase |
| OP | Opposed phase |
| T1w | T1-weighted |
| DWI | Diffusion-weighted imaging |
| DCE-MRI | Dynamic contrast-enhanced MRI |
| GTV | Gross tumour volume |
| OAR | Organs at risk |
| ADC | Apparent diffusion coefficient |
| IVIM | Intra-voxel incoherent motion |
| DKI | Diffusion kurtosis imaging |
| AUC | Area under the curve |
| MTT | Mean transit time |
| HEF | Hepatic extraction fraction |
| MRE | Magnetic resonance elastography |
| ASL | Arterial spin labeling |
| NID | Normalised iodine density |
| RECIST | Response evaluation criteria in solid tumours |
| mRECIST | modified RECIST |
| EASL | European association of study of the liver |
| LI-RADS | Liver imaging reporting and data system |
| FLR | Focal liver reaction |
| EQD2 | Equivalent dose in 2 Gy fractions |
| BED | Biologically effective dose |
| PRISM | Personalised liver stereotactic body radiation therapy using magnetic resonance imaging |
References
- Gerum, S.; Jensen, A.D.; Roeder, F. Stereotactic body radiation therapy in patients with hepatocellular carcinoma: A mini-review. World J. Gastrointest. Oncol. 2019, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Dawson, L.; Barry, A.; Stanescu, T.; Mohamad, I.; Hosni, A. Stereotactic body radiation therapy for hepatocellular carcinoma: From infancy to ongoing maturity. JHEP Rep. 2022, 4, 100498. [Google Scholar] [CrossRef]
- John, R.G.; Ho, F.; Appalanaido, G.K.; Chen, D.; Tey, J.; Soon, Y.Y.; Vellayappan, B.A. Can radiotherapy finally “go live” in the management of liver metastases? Hepatoma Res. 2020, 6, 56. [Google Scholar] [CrossRef]
- Ohri, N.; Tomé, W.A.; Romero, A.M.; Miften, M.; Ten Haken, R.K.; Dawson, L.A.; Grimm, J.; Yorke, E.; Jackson, A. Local control after stereotactic body radiation therapy for liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-associated liver injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Miften, M.; Vinogradskiy, Y.; Moiseenko, V.; Grimm, J.; Yorke, E.; Jackson, A.; Tomé, W.A.; Ten Haken, R.K.; Ohri, N.; Méndez Romero, A.; et al. Radiation Dose-Volume Effects for Liver SBRT. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.X.; Zhang, Y.; Zhang, Q.B.; Zhang, G.Q.; Yu, H.; Zhang, S.X. Functional Liver Imaging in Radiotherapy for Liver Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 898435. [Google Scholar] [CrossRef]
- Peng, Y.; Qi, X.; Tang, S.; Deng, H.; Li, J.; Ning, Z.; Dai, J.; Hou, F.; Zhao, J.; Wang, R.; et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mo, F.; Hui, E.P.; Chan, S.L.; Koh, J.; Tang, N.L.S.; Yu, S.C.H.; Yeo, W. The association of liver function and quality of life of patients with liver cancer. BMC Gastroenterol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Liu, H.Y.H.; Lee, Y.Y.D.; Sridharan, S.; Choong, E.S.; Le, H.; Wang, W.; Khor, R.; Chu, J.; Oar, A.; Mott, R.; et al. Stereotactic body radiotherapy in the management of hepatocellular carcinoma: An Australian multi-institutional patterns of practice review. J. Med. Imaging Radiat. Oncol. 2021, 65, 365–373. [Google Scholar] [CrossRef]
- Culleton, S.; Jiang, H.; Haddad, C.R.; Kim, J.; Brierley, J.; Brade, A.; Ringash, J.; Dawson, L.A. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother. Oncol. 2014, 111, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dawson, L.A. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2017, 27, 247–255. [Google Scholar] [CrossRef]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.J.; Wietasch, J.K.G.; Absalom, A.R.; Hendriks, H.G.D.; Scheeren, T.W.L. Green light for liver function monitoring using indocyanine green? An overview of current clinical applications. Anaesthesia 2014, 69, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Owen, D.; Bazzi, L.; Jackson, W.; Ten Haken, R.K.; Cuneo, K.; Feng, M.; Lawrence, T.S.; Schipper, M.J. Using Indocyanine Green Extraction to Predict Liver Function After Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 131–137. [Google Scholar] [CrossRef]
- Stenmark, M.H.; Cao, Y.; Wang, H.; Jackson, A.; Ben-Josef, E.; Ten Haken, R.K.; Lawrence, T.S.; Feng, M. Estimating functional liver reserve following hepatic irradiation: Adaptive normal tissue response models. Radiother. Oncol. 2014, 111, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Suresh, K.; Schipper, M.J.; Bazzi, L.; Ben-Josef, E.; Matuszak, M.M.; Parikh, N.D.; Welling, T.H.; Normolle, D.; Ten Haken, R.K.; et al. Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Platt, J.F.; Francis, I.R.; Balter, J.M.; Pan, C.; Normolle, D.; Ben-Josef, E.; Haken, R.K.T.; Lawrence, T.S. The prediction of radiation-induced liver dysfunction using a local dose and regional venous perfusion model. Med. Phys. 2007, 34, 604–612. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, C.; Balter, J.M.; Platt, J.F.; Francis, I.R.; Knol, J.A.; Normolle, D.; Ben-Josef, E.; Ten Haken, R.K.; Lawrence, T.S. Liver function after irradiation based on computed tomographic portal vein perfusion imaging. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 154–160. [Google Scholar] [CrossRef]
- El Naqa, I.; Johansson, A.; Owen, D.; Cuneo, K.; Cao, Y.; Matuszak, M.; Bazzi, L.; Lawrence, T.S.; Ten Haken, R.K. Modeling of normal tissue complications using imaging and biomarkers after radiation therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 335–343. [Google Scholar] [CrossRef]
- Ohira, S.; Kanayama, N.; Toratani, M.; Ueda, Y.; Koike, Y.; Karino, T.; Shunsuke, O.; Miyazaki, M.; Koizumi, M.; Teshima, T. Stereotactic body radiation therapy planning for liver tumors using functional images from dual-energy computed tomography. Radiother. Oncol. 2020, 145, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, Y. Spatially resolved assessment of hepatic function using 99mTc-IDA SPECT. Med. Phys. 2013, 40, 092501. [Google Scholar] [CrossRef]
- Bowen, S.R.; Saini, J.; Chapman, T.R.; Miyaoka, R.S.; Kinahan, P.E.; Sandison, G.A.; Wong, T.; Vesselle, H.J.; Nyflot, M.J.; Apisarnthanarax, S. Differential hepatic avoidance radiation therapy: Proof of concept in hepatocellular carcinoma patients. Radiother. Oncol. 2015, 115, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.R.; Chapman, T.R.; Borgman, J.; Miyaoka, R.S.; Kinahan, P.E.; Liou, I.W.; Sandison, G.A.; Vesselle, H.J.; Nyflot, M.J.; Apisarnthanarax, S. Measuring total liver function on sulfur colloid SPECT/CT for improved risk stratification and outcome prediction of hepatocellular carcinoma patients. EJNMMI Res. 2016, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Schaub, S.K.; Apisarnthanarax, S.; Price, R.G.; Nyflot, M.J.; Chapman, T.R.; Matesan, M.; Vesselle, H.J.; Bowen, S.R. Functional liver imaging and dosimetry to predict hepatotoxicity risk in cirrhotic patients with primary liver cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1339–1348. [Google Scholar] [CrossRef]
- Yoshida, M.; Shiraishi, S.; Sakaguchi, F.; Utsunomiya, D.; Tashiro, K.; Tomiguchi, S.; Okabe, H.; Beppu, T.; Baba, H.; Yamashita, Y. A quantitative index measured on 99mTc GSA SPECT/CT 3D fused images to evaluate severe fibrosis in patients with chronic liver disease. Jpn. J. Radiol. 2012, 30, 435–441. [Google Scholar] [CrossRef]
- Keiding, S.; Sørensen, M.; Frisch, K.; Gormsen, L.C.; Munk, O.L. Quantitative PET of liver functions. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 73–85. [Google Scholar]
- Bak-Fredslund, K.P.; Lykke Eriksen, P.; Munk, O.L.; Villadsen, G.E.; Keiding, S.; Sørensen, M. Metabolic liver function in humans measured by 2-(18)F-fluoro-2-deoxy-D-galactose PET/CT-reproducibility and clinical potential. EJNMMI Res. 2017, 7, 71. [Google Scholar] [CrossRef]
- Wang, J.; Shao, Y.; Liu, B.; Wang, X.; Geist, B.K.; Li, X.; Li, F.; Zhao, H.; Hacker, M.; Ding, H.; et al. Dynamic (18)F-FDG PET imaging of liver lesions: Evaluation of a two-tissue compartment model with dual blood input function. BMC Med. Imaging 2021, 21, 90. [Google Scholar] [CrossRef]
- Donato, H.; França, M.; Candelária, I.; Caseiro-Alves, F. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Amato, D.M.; Albino, V.; Patrone, R.; Izzo, F.; Petrillo, A. Beyond the vascular profile: Conventional DWI, IVIM and kurtosis in the assessment of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.H.; Park, M.J.; Park, C.K.; Rhim, H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am. J. Roentgenol. 2011, 197, W868–W875. [Google Scholar] [CrossRef]
- Vilgrain, V.; Esvan, M.; Ronot, M.; Caumont-Prim, A.; Aubé, C.; Chatellier, G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur. Radiol. 2016, 26, 4595–4615. [Google Scholar] [CrossRef]
- De Robertis, R.; Tinazzi Martini, P.; Demozzi, E.; Dal Corso, F.; Bassi, C.; Pederzoli, P.; D’Onofrio, M. Diffusion-weighted imaging of pancreatic cancer. World J. Radiol. 2015, 7, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Hayoz, R.; Vietti-Violi, N.; Duran, R.; Knebel, J.F.; Ledoux, J.B.; Dromain, C. The combination of hepatobiliary phase with Gd-EOB-DTPA and DWI is highly accurate for the detection and characterization of liver metastases from neuroendocrine tumor. Eur. Radiol. 2020, 30, 6593–6602. [Google Scholar] [CrossRef] [PubMed]
- Parsai, A.; Zerizer, I.; Roche, O.; Gkoutzios, P.; Miquel, M.E. Assessment of diffusion-weighted imaging for characterizing focal liver lesions. Clin. Imaging 2015, 39, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.; Ghasabeh, M.; Khoshpouri, P.; Kamel, I.; Pawlik, T. Functional hepatic imaging as a biomarker of primary and secondary tumor response to loco-regional therapies. Surg. Oncol. 2017, 26, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Koinuma, M.; Ohashi, I.; Hanafusa, K.; Shibuya, H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J. Magn. Reson. Imaging 2005, 22, 80–85. [Google Scholar] [CrossRef]
- Taouli, B.; Chouli, M.; Martin, A.J.; Qayyum, A.; Coakley, F.V.; Vilgrain, V. Chronic hepatitis: Role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J. Magn. Reson. Imaging 2008, 28, 89–95. [Google Scholar] [CrossRef]
- Bonekamp, S.; Torbenson, M.S.; Kamel, I.R. Diffusion-weighted magnetic resonance imaging for the staging of liver fibrosis. J. Clin. Gastroenterol. 2011, 45, 885. [Google Scholar] [CrossRef]
- Hollingsworth, K.G.; Lomas, D.J. Influence of perfusion on hepatic MR diffusion measurement. NMR Biomed. 2006, 19, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Regini, F.; Colagrande, S.; Mazzoni, L.N.; Busoni, S.; Matteuzzi, B.; Santini, P.; Wyttenbach, R. Assessment of Liver Perfusion by IntraVoxel Incoherent Motion (IVIM) Magnetic Resonance-Diffusion-Weighted Imaging: Correlation With Phase-Contrast Portal Venous Flow Measurements. J. Comput. Assist. Tomogr. 2015, 39, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Y.; Tan, X.; Zheng, Z.; He, M.; Xu, J.; Mei, Y.; Zhang, J.; Zhao, X.; Wang, C.; et al. MRI-based estimation of liver function by intravoxel incoherent motion diffusion-weighted imaging. Magn. Reson. Imaging 2016, 34, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Padhani, A.R.; Chenevert, T.L.; Koh, D.M.; De Keyzer, F.; Taouli, B.; Le Bihan, D. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J. Magn. Reson. Imaging 2015, 42, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Yoshimaru, D.; Takatsu, Y.; Suzuki, Y.; Miyati, T.; Hamada, Y.; Funaki, A.; Tabata, A.; Maruyama, C.; Shimada, M.; Tobari, M.; et al. Diffusion kurtosis imaging in the assessment of liver function: Its potential as an effective predictor of liver function. Br. J. Radiol. 2019, 92, 20170608. [Google Scholar] [CrossRef] [PubMed]
- Goshima, S.; Kanematsu, M.; Noda, Y.; Kondo, H.; Watanabe, H.; Bae, K.T. Diffusion kurtosis imaging to assess response to treatment in hypervascular hepatocellular carcinoma. Am. J. Roentgenol. 2015, 204, W543–W549. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Wang, Z.Y.; Xia, M.Y.; Li, F.Z.; Li, Y.; Shen, Z.; Wang, X.Z. Diffusion Kurtosis Imaging for Assessing the Therapeutic Response of Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma. J. Cancer 2020, 11, 2339–2347. [Google Scholar] [CrossRef]
- Nowicki, T.K.; Markiet, K.; Szurowska, E. Diagnostic Imaging of Hepatocellular Carcinoma—A Pictorial Essay. Curr. Med. Imaging Rev. 2017, 13, 140–153. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kimura, T.; Higaki, T.; Honda, Y.; Komoto, D.; Yamagami, T.; Iida, M.; Nagata, Y.; Honda, Y.; Aikata, H.; et al. Clinical utility of gadoxetate disodium-enhanced hepatic MRI for stereotactic body radiotherapy of hepatocellular carcinoma. Jpn. J. Radiol. 2015, 33, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Poetter-Lang, S.; Bastati, N.; Messner, A.; Kristic, A.; Herold, A.; Hodge, J.C.; Ba-Ssalamah, A. Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom. Radiol. 2020, 45, 3532–3544. [Google Scholar] [CrossRef]
- Liao, Y.S.; Lee, L.W.; Yang, P.H.; Kuo, L.M.; Kuan, L.Y.; Tseng, W.Y.; Hwang, D. Assessment of liver cirrhosis for patients with Child’s A classification before hepatectomy using dynamic contrast-enhanced MRI. Clin. Radiol. 2019, 74, 407.e11–407.e17. [Google Scholar] [CrossRef]
- Chen, B.B.; Hsu, C.Y.; Yu, C.W.; Wei, S.Y.; Kao, J.H.; Lee, H.S.; Shih, T.T.F. Dynamic contrast-enhanced magnetic resonance imaging with Gd-EOB-DTPA for the evaluation of liver fibrosis in chronic hepatitis patients. Eur. Radiol. 2012, 22, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Blomqvist, L.; Douglas, L.; Nordell, A.; Janczewska, I.; Näslund, E.; Jonas, E. Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br. J. Radiol. 2013, 86, 20120653. [Google Scholar] [CrossRef] [PubMed]
- Ryeom, H.K.; Kim, S.H.; Kim, J.Y.; Kim, H.J.; Lee, J.M.; Chang, Y.M.; Kim, Y.S.; Kang, D.S. Quantitative evaluation of liver function with MRI Using Gd-EOB-DTPA. Korean J. Radiol. 2004, 5, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Phonlakrai, M.; Ramadan, S.; Simson, J.; Golizda, N.; Arm, J.; Skehan, K.; Goodwin, J.; Trada, Y.; Martin, J.; Sridharan, S.; et al. Determination of hepatic extraction fraction with gadoxetate low-temporal resolution DCE-MRI-based deconvolution analysis: Validation with ALBI score and Child-Pugh class. J. Med. Radiat. Sci. 2022; early view. [Google Scholar] [CrossRef]
- Sourbron, S.P.; Buckley, D.L. On the scope and interpretation of the Tofts models for DCE-MRI. Magn. Reson. Med. 2011, 66, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Materne, R.; Smith, A.; Peeters, F.; Dehoux, J.P.; Keyeux, A.; Horsmans, Y.; Van Beers, B. Assessment of hepatic perfusion parameters with dynamic MRI. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2002, 47, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Yu, M.H.; Hur, B.Y.; Grimm, R.; Sourbron, S.; Chandarana, H.; Son, Y.; Basak, S.; Lee, K.B.; et al. Simultaneous evaluation of perfusion and morphology using GRASP MRI in hepatic fibrosis. Eur. Radiol. 2022, 32, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, S.; Sommer, W.H.; Reiser, M.F.; Zech, C.J. Combined quantification of liver perfusion and function with dynamic gadoxetic acid–enhanced MR imaging. Radiology 2012, 263, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, L.; Penny, J.; Nicholls, G.; Woodhouse, N.; Blé, F.X.; Cristinacce, P.L.H.; Naish, J.H. Quantitative assessment of liver function using gadoxetate-enhanced magnetic resonance imaging: Monitoring transporter-mediated processes in healthy volunteers. Investig. Radiol. 2017, 52, 111. [Google Scholar] [CrossRef] [PubMed]
- Tadimalla, S.; Green, C.; Steinmann, D.; Koehler, S.; Juretschke, H.P.; Laitinen, I.; Waterton, J.C.; Hockings, P.D.; Hines, C.D.; Schütz, G.; et al. Repeatability of hepatocellular uptake and efflux in the rat liver: A comparison of Gadoxetate DCE-MRI models. In Proceedings of the ISMRM 27th Annual Meeting & Exhibition, Montreal, QC, Canada, 10–13 May 2019. [Google Scholar]
- Saito, K.; Ledsam, J.; Sourbron, S.; Otaka, J.; Araki, Y.; Akata, S.; Tokuuye, K. Assessing liver function using dynamic Gd-EOB-DTPA-enhanced MRI with a standard 5-phase imaging protocol. J. Magn. Reson. Imaging 2013, 37, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Simeth, J.; Johansson, A.; Owen, D.; Cuneo, K.; Mierzwa, M.; Feng, M.; Lawrence, T.S.; Cao, Y. Quantification of liver function by linearization of a two-compartment model of gadoxetic acid uptake using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed. 2018, 31, e3913. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ledsam, J.; Sourbron, S.; Hashimoto, T.; Araki, Y.; Akata, S.; Tokuuye, K. Measuring hepatic functional reserve using low temporal resolution Gd-EOB-DTPA dynamic contrast-enhanced MRI: A preliminary study comparing galactosyl human serum albumin scintigraphy with indocyanine green retention. Eur. Radiol. 2014, 24, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Simeth, J.; Aryal, M.; Owen, D.; Cuneo, K.; Lawrence, T.S.; Cao, Y. Gadoxetic Acid Uptake Rate as a Measure of Global and Regional Liver Function as Compared With Indocyanine Green Retention, Albumin-Bilirubin Score, and Portal Venous Perfusion. Adv. Radiat. Oncol. 2022, 7, 100942. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Min, J.; Liang, W.R.; Zhang, G.Q.; Wu, J.J.; Jin, K.; Huang, W.; Ying, C.Y.; Chao, M. Use of magnetic resonance elastography for assessing liver functional reserve: A clinical study. World J. Gastroenterol. 2015, 21, 7522–7528. [Google Scholar] [CrossRef] [PubMed]
- Martirosian, P.; Pohmann, R.; Schraml, C.; Schwartz, M.; Kuestner, T.; Schwenzer, N.F.; Scheffler, K.; Nikolaou, K.; Schick, F. Spatial-temporal perfusion patterns of the human liver assessed by pseudo-continuous arterial spin labeling MRI. Z. Med. Phys. 2019, 29, 173–183. [Google Scholar] [CrossRef]
- Katada, Y.; Shukuya, T.; Kawashima, M.; Nozaki, M.; Imai, H.; Natori, T.; Tamano, M. A comparative study between arterial spin labeling and CT perfusion methods on hepatic portal venous flow. Jpn. J. Radiol. 2012, 30, 863–869. [Google Scholar] [CrossRef]
- Pan, X.; Qian, T.; Fernandez-Seara, M.A.; Smith, R.X.; Li, K.; Ying, K.; Sung, K.; Wang, D.J.J. Quantification of liver perfusion using multidelay pseudocontinuous arterial spin labeling. J. Magn. Reson. Imaging 2016, 43, 1046–1054. [Google Scholar] [CrossRef]
- Potters, L.; Kavanagh, B.; Galvin, J.M.; Hevezi, J.M.; Janjan, N.A.; Larson, D.A.; Mehta, M.P.; Ryu, S.; Steinberg, M.; Timmerman, R.; et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) Practice Guideline for the Performance of Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 326–332. [Google Scholar] [CrossRef]
- Sahgal, A.; Roberge, D.; Schellenberg, D.; Purdie, T.; Swaminath, A.; Pantarotto, J.; Filion, E.; Gabos, Z.; Butler, J.; Letourneau, D.; et al. The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy. Clin. Oncol. 2012, 24, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarax, S.; Barry, A.; Cao, M.; Czito, B.; DeMatteo, R.; Drinane, M.; Hallemeier, C.L.; Koay, E.J.; Lasley, F.; Meyer, J.; et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Bredfeldt, J.S.; Liu, L.; Feng, M.; Cao, Y.; Balter, J.M. Synthetic CT for MRI-based liver stereotactic body radiotherapy treatment planning. Phys. Med. Biol. 2017, 62, 2922–2934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.; Wang, Y.; Wang, T.; Ren, L.; Lin, L.; McDonald, M.; Curran, W.J.; Liu, T.; Zhou, J.; et al. MRI-based treatment planning for proton radiotherapy: Dosimetric validation of a deep learning-based liver synthetic CT generation method. Phys. Med. Biol. 2019, 64, 145015. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yin, F.F.; Cai, J. Evaluation of dosimetric uncertainty caused by MR geometric distortion in MRI-based liver SBRT treatment planning. J. Appl. Clin. Med. Phys. 2019, 20, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.P.; Alshanqity, M.; Moutsatsos, A.; Lababidi, H.; Alsafi, K.; Georgiou, K.; Karaiskos, P.; Georgiou, E. MRI-Related Geometric Distortions in Stereotactic Radiotherapy Treatment Planning: Evaluation and Dosimetric Impact. Technol. Cancer Res. Treat. 2017, 16, 1120–1129. [Google Scholar] [CrossRef]
- Ken, S.; Tournier, A.; Rives, M.; Izar, F.; Aziza, R.; Morel, N.; Sekkal, Y.; Parent, L. 50. Magnetic Resonance Imaging optimization for liver SBRT: Breath-triggered acquisition in treatment position to improve lesion contouring. Phys. Medica 2016, 32, 365–366. [Google Scholar] [CrossRef]
- Oldrini, G.; Taste-George, H.; Renard-Oldrini, S.; Baumann, A.S.; Marchesi, V.; Troufléau, P.; Peiffert, D.; Didot-Moisei, A.; Boyer, B.; Grignon, B.; et al. Implantation of fiducial markers in the liver for stereotactic body radiation therapy: Feasibility and results. Diagn. Interv. Imaging 2015, 96, 589–592. [Google Scholar] [CrossRef]
- Lee, Y.Y.D.; Nguyen, D.T.; Moodie, T.; O’Brien, R.; McMaster, A.; Hickey, A.; Pritchard, N.; Poulsen, P.; Tabaksblat, E.M.; Weber, B.; et al. Study protocol of the LARK (TROG 17.03) clinical trial: A phase II trial investigating the dosimetric impact of Liver Ablative Radiotherapy using Kilovoltage intrafraction monitoring. BMC Cancer 2021, 21, 494. [Google Scholar] [CrossRef]
- van de Lindt, T.N.; Fast, M.F.; van den Wollenberg, W.; Kaas, J.; Betgen, A.; Nowee, M.E.; Jansen, E.P.; Schneider, C.; van der Heide, U.A.; Sonke, J.J. Validation of a 4D-MRI guided liver stereotactic body radiation therapy strategy for implementation on the MR-linac. Phys. Med. Biol. 2021, 66, 105010. [Google Scholar] [CrossRef]
- Paulson, E.S.; Ahunbay, E.; Chen, X.; Mickevicius, N.J.; Chen, G.P.; Schultz, C.; Erickson, B.; Straza, M.; Hall, W.A.; Li, X.A. 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: Implementation and initial clinical experience. Clin. Transl. Radiat. Oncol. 2020, 23, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Boeke, S.; McNair, H.; Ehlers, J.; Nachbar, M.; Mönnich, D.; Stolte, A.; Boldt, J.; Marks, C.; Winter, J.; et al. Marker-less online MR-guided stereotactic body radiotherapy of liver metastases at a 1.5 T MR-Linac—Feasibility, workflow data and patient acceptance. Clin. Transl. Radiat. Oncol. 2021, 26, 55–61. [Google Scholar] [CrossRef]
- Wojcieszynski, A.P.; Rosenberg, S.A.; Brower, J.V.; Hullett, C.R.; Geurts, M.W.; Labby, Z.E.; Hill, P.M.; Bayliss, R.A.; Paliwal, B.; Bayouth, J.E.; et al. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother. Oncol. 2016, 118, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.R.; Miao, X.; Ferguson, D.; Calvin, C.; Bhaskar Krishnamurthy, U.; Anwar, M.; Feng, M.; Scholey, J. Contrast-enhanced 4D-MRI for internal target volume generation in treatment planning for liver tumors. Radiother. Oncol. 2022, 173, 69–76. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Nemiro, I.; Utehina, O.; Boka, G.; Boka, V.; Riga, L.V. Functional MRI imaging for precise target determination and results evaluation in liver Stereotactic Body Radiotherapy. In Proceedings of the European Congress of Radiology—ECR 2014, Vienna, Austria, 6–10 March 2014. [Google Scholar]
- Long, D.E.; Tann, M.; Huang, K.C.; Bartlett, G.; Galle, J.O.; Furukawa, Y.; Maluccio, M.; Cox, J.A.; Kong, F.M.S.; Ellsworth, S.G. Functional liver image guided hepatic therapy (FLIGHT) with hepatobiliary iminodiacetic acid (HIDA) scans. Pract. Radiat. Oncol. 2018, 8, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Long, D.E.; Ellsworth, S.G. Functional liver-image guided hepatic therapy (FLIGHT): A technique to maximize hepatic functional reserve. Med. Dosim. 2020, 45, 117–120. [Google Scholar] [CrossRef]
- Fode, M.M.; Petersen, J.B.; Sørensen, M.; Holt, M.I.; Keiding, S.; Høyer, M. 2-[18F]fluoro-2-deoxy-d-galactose positron emission tomography guided functional treatment planning of stereotactic body radiotherapy of liver tumours. Phys. Imaging Radiat. Oncol. 2017, 1, 28–33. [Google Scholar] [CrossRef]
- Tsegmed, U.; Kimura, T.; Nakashima, T.; Nakamura, Y.; Higaki, T.; Imano, N.; Doi, Y.; Kenjo, M.; Ozawa, S.; Murakami, Y.; et al. Functional image-guided stereotactic body radiation therapy planning for patients with hepatocellular carcinoma. Med. Dosim. 2017, 42, 97–103. [Google Scholar] [CrossRef]
- Simeth, J.; Baughan, N.; Dow, J.; Ten Haken, R.; Johansson, A.; Aryal, M.; Owen, D.; Cunco, K.; Lawrence, T.; Cao, Y.; et al. Impact of Mis-Match Between Liver Function and Hepatic Perfusion On Functional Avoidance Treatment Planning. In Medical Physics; Wiley: Hoboken, NJ, USA, 2018; Volume 45, p. E582. [Google Scholar]
- Long, D.E.; Huang, C.; Tann, M.; Dawson, B.; Bartlett, G.; Maluccio, M.A.; Rhome, R.; Kong, F.M.S.; Ellsworth, S.G. Prospective trial of functional liver image-guided hepatic therapy (FLIGHT) with hepatobiliary iminodiacetic acid (HIDA) scans and update of institutional experience. J. Clin. Oncol. 2019, 37, 373. [Google Scholar] [CrossRef]
- Groshar, D.; Slobodin, G.; Zuckerman, E. Quantitation of liver and spleen uptake of 99mTc-phytate colloid using SPECT: Detection of liver cirrhosis. J. Nucl. Med. 2002, 43, 312–317. [Google Scholar] [PubMed]
- Matesan, M.M.; Bowen, S.R.; Chapman, T.R.; Miyaoka, R.S.; Velez, J.W.; Wanner, M.F.; Nyflot, M.J.; Apisarnthanarax, S.; Vesselle, H.J. Assessment of functional liver reserve: Old and new in 99mTc-sulfur colloid scintigraphy. Nucl. Med. Commun. 2017, 38, 577–586. [Google Scholar] [CrossRef]
- Takahashi, H.; Shigefuku, R.; Yoshida, Y.; Ikeda, H.; Matsunaga, K.; Matsumoto, N.; Okuse, C.; Sase, S.; Itoh, F.; Suzuki, M. Correlation between hepatic blood flow and liver function in alcoholic liver cirrhosis. World J. Gastroenterol. 2014, 20, 17065–17074. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Aryal, M.; Simeth, J.; Cuneo, K.; Matuszak, M.; Lawrence, T.; Ten Haken, R.; Cao, Y.; El Naqa, I. Comparison of NTCP Models Using Liver Function Obtained From Different Contrast Agent-Based DCE-MRI after SBRT in Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e134–e135. [Google Scholar] [CrossRef]
- Wang, H.; Feng, M.; Jackson, A.; Ten Haken, R.K.; Lawrence, T.S.; Cao, Y. Local and Global Function Model of the Liver. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 181–188. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Masch, W.R.; Shampain, K.; Zhang, A.; Jo, A.S.; Moorman, S.; Aslam, A.; Maturen, K.E.; Davenport, M.S. MRI assessment of hepatocellular carcinoma after local-regional therapy: A comprehensive review. Radiol. Imaging Cancer 2020, 2, e190024. [Google Scholar] [CrossRef]
- Price, R.G.; Apisarnthanarax, S.; Schaub, S.K.; Nyflot, M.J.; Chapman, T.R.; Matesan, M.; Vesselle, H.J.; Bowen, S.R. Regional Radiation Dose-Response Modeling of Functional Liver in Hepatocellular Carcinoma Patients With Longitudinal Sulfur Colloid SPECT/CT: A Proof of Concept. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1349–1356. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Gu, E.; Owen, D.; Cuneo, K.C.; Bazzi, L.; Lawrence, T.S.; Hussain, H.K.; Davenport, M.S. Imaging Findings Within the First 12 Months of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta-Lala, M.; Masch, W.; Shankar, P.R.; Hartman, H.E.; Davenport, M.S.; Schipper, M.J.; Maurino, C.; Cuneo, K.C.; Lawrence, T.S.; Owen, D. Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 169–179. [Google Scholar] [CrossRef]
- Tétreau, R.; Llacer, C.; Riou, O.; Deshayes, E. Evaluation of response after SBRT for liver tumors. Rep. Pract. Oncol. Radiother. 2017, 22, 170–175. [Google Scholar] [CrossRef]
- Yip, C.; Cook, G.J.R.; Owczarczyk, K.; Goh, V. Challenges in imaging assessment following liver stereotactic body radiotherapy: Pitfalls to avoid in clinical practice. Chin. Clin. Oncol. 2017, 6, S11. [Google Scholar] [CrossRef] [PubMed]
- Boda-Heggemann, J.; Jahnke, A.; Chan, M.K.H.; Ghaderi Ardekani, L.S.; Hunold, P.; Schäfer, J.P.; Huttenlocher, S.; Wurster, S.; Rades, D.; Hildebrandt, G.; et al. Direct dose correlation of MRI morphologic alterations of healthy liver tissue after robotic liver SBRT. Strahlenther. Onkol. 2018, 194, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Navin, P.J.; Olson, M.C.; Mendiratta-Lala, M.; Hallemeier, C.L.; Torbenson, M.S.; Venkatesh, S.K. Imaging Features in the Liver after Stereotactic Body Radiation Therapy. RadioGraphics 2022, 42, 220084. [Google Scholar] [CrossRef] [PubMed]
- Kellock, T.; Liang, T.; Harris, A.; Schellenberg, D.; Ma, R.; Ho, S.; Yap, W.W. Stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma: Imaging evaluation post treatment. Br. J. Radiol. 2018, 91, 20170118. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Huang, W.Y.; Hsiang, C.W.; Lee, M.S.; Lin, C.S.; Yang, J.F.; Hsu, H.H.; Chang, W.C. Prognostic Significance of Apparent Diffusion Coefficient in Hepatocellular Carcinoma Patients treated with Stereotactic Ablative Radiotherapy. Sci. Rep. 2019, 9, 14157. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.I.; Park, H.C.; Lim, D.H.; Choi, Y.; Jung, S.H.; Paik, S.W.; Kim, S.H.; Jeong, W.K.; Kim, Y.K. The role of diffusion-weighted magnetic resonance imaging in the treatment response evaluation of hepatocellular carcinoma patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 814–821. [Google Scholar] [CrossRef]
- Eccles, C.L.; Haider, E.A.; Haider, M.A.; Fung, S.; Lockwood, G.; Dawson, L.A. Change in diffusion weighted MRI during liver cancer radiotherapy: Preliminary observations. Acta Oncol. 2009, 48, 1034–1043. [Google Scholar] [CrossRef]
- Omiya, Y.; Motosugi, U.; Morisaka, H.; Onishi, H. Liver parenchymal change after stereotactic radiotherapy for hepatocellular carcinoma using DWI and MRE. In Proceedings of the Annual Meeting of the International Society of Magnetic Resonance Imaging 2021, Beijing, China, 10–12 September 2021. [Google Scholar]
- Lewis, B.C. Radiotherapy Response Using Intravoxel Incoherent Motion Magnetic Resonance Imaging in Liver Patients Treated with Stereotactic Body Radiotherapy. Ph.D. Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2019. [Google Scholar]
- Liang, P.C.; Ch’ang, H.J.; Hsu, C.; Tseng, S.S.; Shih, T.T.F.; Wu Liu, T. Dynamic MRI signals in the second week of radiotherapy relate to treatment outcomes of hepatocellular carcinoma: A preliminary result. Liver Int. 2007, 27, 516–528. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Johnson, T.D.; Pan, C.; Hussain, H.; Balter, J.M.; Normolle, D.; Ben-Josef, E.; Ten Haken, R.K.; Lawrence, T.S.; et al. Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 258–263. [Google Scholar] [CrossRef]
- Wei, L.; Simeth, J.; Aryal, M.P.; Matuszak, M.; Ten Haken, R.K.; Cuneo, K.; Lawrence, T.S.; Cao, Y. The Effect of Stereotactic Body Radiation Therapy for Hepatocellular Cancer on Regional Hepatic Liver Function. Int. J. Radiat. Oncol. Biol. Phys. 2022. [Google Scholar] [CrossRef]
- Wu, V.W.; Epelman, M.A.; Wang, H.; Edwin Romeijn, H.; Feng, M.; Cao, Y.; Ten Haken, R.K.; Matuszak, M.M. Optimizing global liver function in radiation therapy treatment planning. Phys. Med. Biol. 2016, 61, 6465–6484. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Kuczmarska-Haas, A.; Musunuru, H.B.; Witt, J.; Blitzer, G.; Mahler, P.; Bassetti, M.F. Evaluating dose constraints for radiation induced liver damage following magnetic resonance image guided Stereotactic Body radiotherapy. Phys. Imaging Radiat. Oncol. 2021, 17, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Shiomi, H.; Masai, N.; Tatsumi, D.; Igura, T.; Imai, Y.; Oh, R.J. Threshold doses and prediction of visually apparent liver dysfunction after stereotactic body radiation therapy in cirrhotic and normal livers using magnetic resonance imaging. J. Radiat. Res. 2016, 57, 294–300. [Google Scholar] [CrossRef]
- Ghita, M.; Drexler, D.A.; Kovács, L.; Copot, D.; Muresan, C.I.; Ionescu, C.M. Model-Based Management of Lung Cancer Radiation Therapy. IFAC-PapersOnLine 2020, 53, 15928–15933. [Google Scholar] [CrossRef]
- Ghita, M.; Billiet, C.; Copot, D.; Verellen, D.; Ionescu, C.M. Model Calibration of Pharmacokinetic-Pharmacodynamic Lung Tumour Dynamics for Anticancer Therapies. J. Clin. Med. 2022, 11, 1006. [Google Scholar] [CrossRef]
- Jackson, W.C.; Tang, M.; Maurino, C.; Mendiratta-Lala, M.; Parikh, N.D.; Matuszak, M.M.; Dow, J.S.; Cao, Y.; Mayo, C.S.; Ten Haken, R.K.; et al. Individualized Adaptive Radiation Therapy Allows for Safe Treatment of Hepatocellular Carcinoma in Patients With Child-Turcotte-Pugh B Liver Disease. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 212–219. [Google Scholar] [CrossRef]
- Boraschi, P.; Donati, F.; Cervelli, R.; Pacciardi, F.; Tarantini, G.; Castagna, M.; Urbani, L.; Lencioni, R. Colorectal liver metastases: ADC as an imaging biomarker of tumor behavior and therapeutic response. Eur. J. Radiol. 2021, 137, 109609. [Google Scholar] [CrossRef]
- Sahin, H.; Harman, M.; Cinar, C.; Bozkaya, H.; Parildar, M.; Elmas, N. Evaluation of Treatment Response of Chemoembolization in Hepatocellular Carcinoma with Diffusion-Weighted Imaging on 3.0-T MR Imaging. J. Vasc. Interv. Radiol. 2012, 23, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sobhani, F.; Xu, C.; Pan, L.; Ghasebeh, M.A.; Kamel, I.R. Quantitative volumetric functional MR imaging: An imaging biomarker of early treatment response in hypo-vascular liver metastasis patients after yttrium-90 transarterial radioembolization. Abdom. Radiol. 2016, 41, 1495–1504. [Google Scholar] [CrossRef]
| Model | Model Equation | Parameters |
|---|---|---|
| Mono-exponential | ADC: apparent diffusion coefficient | |
| IVIM | D*: Pseudo-diffusion coefficient; f: perfusion fraction; D: diffusion coefficient | |
| Kurtosis | DK: diffusion coefficient; Kapp: diffusion kurtosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadimalla, S.; Wang, W.; Haworth, A. Role of Functional MRI in Liver SBRT: Current Use and Future Directions. Cancers 2022, 14, 5860. https://doi.org/10.3390/cancers14235860
Tadimalla S, Wang W, Haworth A. Role of Functional MRI in Liver SBRT: Current Use and Future Directions. Cancers. 2022; 14(23):5860. https://doi.org/10.3390/cancers14235860
Chicago/Turabian StyleTadimalla, Sirisha, Wei Wang, and Annette Haworth. 2022. "Role of Functional MRI in Liver SBRT: Current Use and Future Directions" Cancers 14, no. 23: 5860. https://doi.org/10.3390/cancers14235860
APA StyleTadimalla, S., Wang, W., & Haworth, A. (2022). Role of Functional MRI in Liver SBRT: Current Use and Future Directions. Cancers, 14(23), 5860. https://doi.org/10.3390/cancers14235860





