Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance
Abstract
Simple Summary
Abstract
1. Introduction
2. Perineural Invasion
2.1. PNI Overview
2.2. PNI in Pancreatic Cancer
3. Attractive Molecular and Cellular Signalling Pathways of PNI in PDAC
4. PNI and Pain Generation in PDAC Patients
5. Role of Extracellular Vesicles in PNI
6. Conventional and Experimental Treatments for PNI
6.1. Surgical Treatment for PNI
6.2. PNI-Targeted Chemotherapy
7. Cannabinoids in Pancreatic Cancer Treatment
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Adamska, A.; Elaskalani, O.; Emmanouilidi, A.; Kim, M.; Razak, N.B.A.; Metharom, P.; Falasca, M. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv. Biol. Regul. 2018, 68, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Reyes, C.M.; Friess, H.; Demir, I.E. Neoadjuvant therapy in pancreatic cancer: What is the true oncological benefit? Langenbecks Arch Surg. 2020, 405, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Nevala-Plagemann, C.; Hidalgo, M.; Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 2019, 17, 108–123. [Google Scholar] [CrossRef]
- Gourgou-Bourgade, S.; Bascoul-Mollevi, C.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Boige, V.; et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J. Clin. Oncol. 2013, 31, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, Y.; Ootsuka, K.; Abue, M. The Appropriate First-Line Chemotherapy Regimen for Incurable Pancreatic Cancer in Clinical Practice: A Consideration of Patients’ Overall Survival and Quality of Life. J. Pancreat. Cancer 2021, 7, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jurcak, N.; Zheng, L. Signaling in the microenvironment of pancreatic cancer: Transmitting along the nerve. Pharmacol. Ther. 2019, 200, 126–134. [Google Scholar] [CrossRef]
- Schorn, S.; Demir, I.E.; Haller, B.; Scheufele, F.; Reyes, C.M.; Tieftrunk, E.; Sargut, M.; Goess, R.; Friess, H.; Ceyhan, G.O. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma – A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 105–115. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 2021, 41, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Gola, M.; Sejda, A.; Godlewski, J.; Cieślak, M.; Starzyńska, A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 5246. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural Invasion in Pancreatic Cancer: The Past, Present and Future. Cancers 2010, 2, 1513–1527. [Google Scholar] [CrossRef]
- Liang, D.; Shi, S.; Xu, J.; Zhang, B.; Qin, Y.; Ji, S.; Xu, W.; Liu, J.; Liu, L.; Liu, C.; et al. New insights into perineural invasion of pancreatic cancer: More than pain. Biochim. et Biophys. Acta 2016, 1865, 111–122. [Google Scholar] [CrossRef]
- Zhang, M.; Xian, H.-C.; Dai, L.; Tang, Y.-L.; Liang, X.-H. MicroRNAs: Emerging driver of cancer perineural invasion. Cell Biosci. 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Scanlon, C.S.; D’Silva, N.J. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750. [Google Scholar] [CrossRef]
- da Silva, S.D.; Kowalski, L.P. Perineural invasion in oral cancer: Challenges, controversies and clinical impact. Chin. Clin. Oncol. 2019, 8, S5. [Google Scholar] [CrossRef]
- Narayan, P.; Flynn, J.; Zhang, Z.; Gillespie, E.F.; Mueller, B.; Xu, A.J.; Cuaron, J.; McCormick, B.; Khan, A.J.; Cahlon, O.; et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci. Rep. 2021, 11, 12781. [Google Scholar] [CrossRef]
- Mirkin, K.A.; Hollenbeak, C.S.; Mohamed, A.; Jia, Y.; El-Deiry, W.; Messaris, E. Impact of perineural invasion on survival in node negative colon cancer. Cancer Biol. Ther. 2017, 18, 740–745. [Google Scholar] [CrossRef]
- Olar, A.; He, D.; Florentin, D.; Ding, Y.; Wheeler, T.; Ayala, G. Biological correlates of prostate cancer perineural invasion diameter. Hum. Pathol. 2014, 45, 1365–1369. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Ma, Q.; Xu, Q.; Lei, J.; Li, X.; Wang, Z.; Wu, E. Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2020, 81, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Katz, M.H.; Rashid, A.; Wang, H.; Iuga, A.C.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.; Crane, C.H.; et al. Perineural and Intraneural Invasion in Posttherapy Pancreaticoduodenectomy Specimens Predicts Poor Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 409–417. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Manzoni, A.; Florentin, D.; Fisher, W.; Ding, Y.; Lee, M.; Ayala, G. Biologic effect of neurogenesis in pancreatic cancer. Hum. Pathol. 2016, 52, 182–189. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim. et Biophys. Acta 2019, 1873, 188326. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose Metabolism in Pancreatic Cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Phillips, J.A.; Djamgoz, M.B. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim. et Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188411. [Google Scholar] [CrossRef]
- Gasparini, G.; Pellegatta, M.; Crippa, S.; Lena, M.S.; Belfiori, G.; Doglioni, C.; Taveggia, C.; Falconi, M. Nerves and Pancreatic Cancer: New Insights into A Dangerous Relationship. Cancers 2019, 11, 893. [Google Scholar] [CrossRef]
- Na’Ara, S.; Amit, M.; Gil, Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene 2018, 38, 596–608. [Google Scholar] [CrossRef]
- Coveler, A.L.; Mizrahi, J.; Eastman, B.; Apisarnthanarax, S.J.; Dalal, S.; McNearney, T.; Pant, S. Pancreas Cancer-Associated Pain Management. Oncologist 2021, 26, e971–e982. [Google Scholar] [CrossRef]
- Han, L.; Ma, J.; Duan, W.; Zhang, L.; Yu, S.; Xu, Q.; Lei, J.; Li, X.; Wang, Z.; Wu, Z.; et al. Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 2016, 7, 18146–18158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mascetta, G.; di Mola, F.; Tavano, F.; Selvaggi, F.; Giese, N.; Bassi, C.; Büchler, M.; Friess, H.; di Sebastiano, P. Substance P and Neprilysin in Chronic Pancreatitis. Eur. Surg. Res. 2012, 48, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.W.; Autschbach, F.; Selvaggi, F.; Shi, X.; di Mola, F.F.; Roggo, A.; Müller, M.W.; di Sebastiano, P.; Büchler, M.W.; Giese, T.; et al. Increase in substance P precursor mRNA in noninflamed small-bowel sections in patients with Crohn’s disease. Am. J. Surg. 2007, 193, 476–481. [Google Scholar] [CrossRef]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Khan, A.; Shal, B.; Ali, H.; Kim, Y.S.; Khan, S. Suppression of TRPV1 and P2Y nociceptors by honokiol isolated from Magnolia officinalis in 3rd degree burn mice by inhibiting inflammatory mediators. Biomed. Pharmacother. 2019, 114, 108777. [Google Scholar] [CrossRef] [PubMed]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef] [PubMed]
- Casari, I.; Emmanouilidi, A.; Domenichini, A.; Falasca, M. Extracellular vesicles derived from pancreatic cancer cells are enriched in the growth factor Midkine. Adv. Biol. Regul. 2021, 83, 100857. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Casari, I.; Howard, J.A.; Robless, E.E.; Falasca, M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 2021, 507, 124–134. [Google Scholar] [CrossRef]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; E Barran, P.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Schorey, J.S. Exosomes Released from Infected Macrophages Contain Mycobacterium avium Glycopeptidolipids and Are Proinflammatory. J. Biol. Chem. 2007, 282, 25779–25789. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodríguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; De Maio, A. Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form that Activates Macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Skokos, D.; Botros, H.G.; Demeure, C.; Morin, J.; Peronet, R.; Birkenmeier, G.; Boudaly, S.; Mécheri, S. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses In Vivo. J. Immunol. 2003, 170, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Dong, H.; Cao, H.; Ji, X.; Luan, S.; Liu, J. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer’s Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Yue, S.; Mu, W.; Erb, U.; Zöller, M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 2014, 6, 2366–2384. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wu, Y.; Tan, X. The role of pancreatic cancer-derived exosomes in cancer progress and their potential application as biomarkers. Clin. Transl. Oncol. 2017, 19, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2020, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Hyenne, V.; Labouesse, M.; Goetz, J.G. The Small GTPase Ral orchestrates MVB biogenesis and exosome secretion. Small GTPases 2016, 9, 445–451. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef]
- Guo, Y.; Gil, Z. The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk of the Peripheral Nervous System. Cells 2022, 11, 1294. [Google Scholar] [CrossRef]
- Kovacs, A.; Vermeer, D.W.; Madeo, M.; Reavis, H.D.; Vermeer, S.J.; Williamson, C.S.; Rickel, A.; Stamp, J.; Lucido, C.T.; Cain, J.; et al. Tumor-infiltrating nerves create an electro-physiologically active microenvironment and contribute to treatment resistance. bioRxiv 2020. bioRxiv: 2020.2004.2024.058594. [Google Scholar]
- Hou, W.; Pan, M.; Xiao, Y.; Ge, W. Serum Extracellular Vesicle Stratifin Is a Biomarker of Perineural Invasion in Patients With Colorectal Cancer and Predicts Worse Prognosis. Front. Oncol. 2022, 12, 912584. [Google Scholar] [CrossRef]
- Yao, J.; Li, W.-Y.; Li, S.-G.; Feng, X.-S.; Gao, S.-G. Midkine promotes perineural invasion in human pancreatic cancer. World J. Gastroenterol. 2014, 20, 3018–3024. [Google Scholar] [CrossRef]
- Petrusel, L.; Rusu, I.; Leucuta, D.C.; Seicean, R.; Suharoschi, R.; Zamfir, P.; Seicean, A. Relationship between cachexia and perineural invasion in pancreatic adenocarcinoma. World J. Gastrointest. Oncol. 2019, 11, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Kaduri, M.; Sela, M.; Kagan, S.; Poley, M.; Abumanhal-Masarweh, H.; Mora-Raimundo, P.; Ouro, A.; Dahan, N.; Hershkovitz, D.; Shklover, J.; et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, M.; Lindhammer, F.; Feist, M.; Hillebrandt, K.H.; Timmermann, L.; Benzing, C.; Globke, B.; Zocholl, D.; Hu, M.; Fehrenbach, U.; et al. Perineural Invasion in Pancreatic Ductal Adenocarcinoma (PDAC): A Saboteur of Curative Intended Therapies? J. Clin. Med. 2022, 11, 2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Lau, W.Y.; Sun, J.; Yuan, Z. The concept and controversy of retroperitoneal nerve dissection in pancreatic head carcinoma (Review). Int. J. Oncol. 2015, 47, 2017–2027. [Google Scholar] [CrossRef]
- Selvaggi, F.; Mascetta, G.; Daskalaki, D.; Molin, M.D.; Salvia, R.; Butturini, G.; Cellini, C.; Bassi, C. Outcome of superior mesenteric-portal vein resection during pancreatectomy for borderline ductal adenocarcinoma: Results of a prospective comparative study. Langenbeck’s Arch. Surg. 2014, 399, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Testa, D.C.; Panaccio, P.; Rossi, S.; Raimondi, P.; Ciampaglia, M.; Mazzola, L.; Cotellese, R. Minimally invasive distal pancreatectomy: Mapping surgical maneuvers towards operative standardization. Ann. Ital. Chir. 2022, 92, 122–129. [Google Scholar]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef]
- Hondermarck, H.; Huang, P.S.; Wagner, J.A. The nervous system: Orchestra conductor in cancer, regeneration, inflammation and immunity. FASEB BioAdvances 2021, 3, 944–952. [Google Scholar] [CrossRef]
- Brasca, M.G.; Amboldi, N.; Ballinari, D.; Cameron, A.; Casale, E.; Cervi, G.; Colombo, M.; Colotta, F.; Croci, V.; D’Alessio, R.; et al. Identification of N,1,4,4-tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide (PHA-848125), a potent, orally available cyclin dependent kinase inhibitor. J. Med. Chem. 2009, 52, 5152–5163. [Google Scholar] [CrossRef]
- Banh, R.S.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.; Walters, B.; Kuljanin, M.; Gikandi, A.; Wang, H.; Mancias, J.D.; Schneider, R.J.; et al. Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer. Cell 2020, 183, 1202–1218.e25. [Google Scholar] [CrossRef]
- Chan, E.; Mulkerin, D.; Rothenberg, M.; Holen, K.D.; Lockhart, A.C.; Thomas, J.; Berlin, J. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Investig. New Drugs 2008, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pishvaian, M.J.; Petricoin, E. Molecular Profiling of Pancreatic Cancer Patients—Response. Clin. Cancer Res. 2018, 24, 6612. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.; Wang, L.; Fletcher, M.S.; Massarelli, E.; Reckamp, K.L.; Cristea, M.C.; Prajapati, N.; Parikh, A.; Whiting, R.L.; Wang, M.; et al. First-time in-human study of VMD-928, an allosteric and irreversible TrkA selective inhibitor, in patients with solid tumors or lymphoma. J. Clin. Oncol. 2019, 37, TPS3146. [Google Scholar] [CrossRef]
- Lin, C.-C.; Arkenau, H.-T.; Lu, S.; Sachdev, J.; Carpeño, J.D.C.; Mita, M.; Dziadziuszko, R.; Su, W.-C.; Bobilev, D.; Hughes, L.; et al. A phase 1, open-label, dose-escalation trial of oral TSR-011 in patients with advanced solid tumours and lymphomas. Br. J. Cancer 2019, 121, 131–138. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Borazanci, E.; Shaw, A.T.; Katayama, R.; Shimizu, Y.; Zhu, V.W.; Sun, T.Y.; Wakelee, H.A.; Madison, R.; Schrock, A.B.; et al. U.S. Phase I First-in-human Study of Ta-letrectinib (DS-6051b/AB-106), a ROS1/TRK Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2020, 26, 4785–4794. [Google Scholar] [CrossRef]
- Johnson, M.D.; Stone, B.; Thibodeau, B.J.; Baschnagel, A.; Galoforo, S.; E Fortier, L.; Ketelsen, B.; Ahmed, S.; Kelley, Z.; Hana, A.; et al. The significance of Trk receptors in pancreatic cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Yang, A.; Zylberberg, H.M.; Rustgi, S.D.; Amin, S.P.; Bar-Mashiah, A.; Boffetta, P.; Lucas, A.L. Beta-blockers have no impact on survival in pancreatic ductal adenocarcinoma prior to cancer diagnosis. Sci. Rep. 2021, 11, 1038. [Google Scholar] [CrossRef]
- Bressy, C.; Lac, S.; Nigri, J.; Leca, J.; Roques, J.; Lavaut, M.-N.; Secq, V.; Guillaumond, F.; Bui, T.-T.; Pietrasz, D.; et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res. 2018, 78, 909–921. [Google Scholar] [CrossRef]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting nerve growth factor in pain: What is the therapeutic potential? BioDrugs 2008, 22. [Google Scholar] [CrossRef]
- Ugolini, G.; Marinelli, S.; Covaceuszach, S.; Cattaneo, A.; Pavone, F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 2985–2990. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Bouhana, K.S.; Trollinger, D.; Winkler, J.; Lee, P.; Andrews, S.W.; et al. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2011, 48, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; de Vries, E.G.; Walenkamp, A.M. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective Blockade of the Capsaicin Receptor TRPV1 Attenuates Bone Cancer Pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Hartel, M.; di Mola, F.F.; Selvaggi, F.; Mascetta, G.; Wente, M.N.; Felix, K.; Giese, N.A.; Hinz, U.; di Sebastiano, P.; Büchler, M.W.; et al. Vanilloids in pancreatic cancer: Potential for chemotherapy and pain management. Gut 2006, 55, 519–528. [Google Scholar] [CrossRef]
- Koujima, T.; Tazawa, H.; Ieda, T.; Araki, H.; Fushimi, T.; Shoji, R.; Kuroda, S.; Kikuchi, S.; Yoshida, R.; Umeda, Y.; et al. Oncolytic Virus-Mediated Targeting of the ERK Signaling Pathway Inhibits Invasive Propensity in Human Pancreatic Cancer. Mol. Ther. Oncolytics 2020, 17, 107–117. [Google Scholar] [CrossRef]
- Falasca, V.; Falasca, M. Targeting the Endocannabinoidome in Pancreatic Cancer. Biomolecules 2022, 12, 320. [Google Scholar] [CrossRef]
- Malhotra, P.; Casari, I.; Falasca, M. Therapeutic potential of cannabinoids in combination cancer therapy. Adv. Biol. Regul. 2021, 79, 100774. [Google Scholar] [CrossRef]
- Chung, M.; Kim, H.K.; Abdi, S. Update on cannabis and cannabinoids for cancer pain. Curr. Opin. Anaesthesiol. 2020, 33, 825–831. [Google Scholar] [CrossRef]
- Lian, J.; Casari, I.; Falasca, M. Modulatory role of the endocannabinoidome in the pathophysiology of the gastrointestinal tract. Pharmacol. Res. 2021, 175, 106025. [Google Scholar] [CrossRef]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2007, 122, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Canela, E. Functional Fine-Tuning of Metabolic Pathways by the Endocannabinoid System—Implications for Health and Disease. Int. J. Mol. Sci. 2021, 22, 3661. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, D.; Liu, H.; Abbruzzese, J.L.; Luo, S.; Walsh, K.; Wei, Q. Genetic variants of the peroxisome proliferator-activated receptor (PPAR) signaling pathway genes and risk of pancreatic cancer. Mol. Carcinog. 2020, 59, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Luongo, M.; Marinelli, O.; Zeppa, L.; Aguzzi, C.; Morelli, M.B.; Amantini, C.; Frassineti, A.; Di Costanzo, M.; Fanelli, A.; Santoni, G.; et al. Cannabidiol and Oxygen-Ozone Combination Induce Cytotoxicity in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Cancers 2020, 12, 2774. [Google Scholar] [CrossRef]
- Khan, A.; Wang, F.; Shal, B.; Khan, A.U.; Zahra, S.S.; Haq, I.U.; Khan, S.; Rengasamy, K.R. Anti-neuropathic pain activity of Ajugarin-I via activation of Nrf2 signaling and inhibition of TRPV1/TRPM8 nociceptors in STZ-induced diabetic neuropathy. Pharmacol. Res. 2022, 183, 106392. [Google Scholar] [CrossRef]
- Liang, Q.; Qiao, Z.; Zhou, Q.; Xue, D.; Wang, K.; Shao, L. Discovery of Potent and Selective Transient Receptor Potential Vanilloid 1 (TRPV1) Agonists with Analgesic Effects In Vivo Based on the Functional Conversion Induced by Altering the Orientation of the Indazole Core. J. Med. Chem. 2022, 65, 11658–11678. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Su, T.; Cao, F.; Meng, X.; Pei, L.; Shi, J.; Pan, H.-L.; Li, M. Electroacupuncture Increases CB2 Receptor Expression on Keratinocytes and Infiltrating Inflammatory Cells in Inflamed Skin Tissues of Rats. J. Pain 2010, 11, 1250–1258. [Google Scholar] [CrossRef]
- Auh, Q.S.; Chun, Y.H.; Melemedjian, O.K.; Zhang, Y.; Ro, J.Y. Peripheral interactions between cannabinoid and opioid receptor agonists in a model of inflammatory mechanical hyperalgesia. Brain Res. Bull. 2016, 125, 211–217. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 1748–1758.e1742. [Google Scholar] [CrossRef]
- Ferrè, L.; Nuara, A.; Pavan, G.; Radaelli, M.; Moiola, L.; Rodegher, M.; Colombo, B.; Sarmiento, I.J.K.; Martinelli, V.; Leocani, L.; et al. Efficacy and safety of nabiximols (Sativex®) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol. Sci. 2015, 37, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lohse, I.; Wildermuth, E.; Brothers, S.P. Naturally occurring compounds as pancreatic cancer therapeutics. Oncotarget 2018, 9, 35448–35457. [Google Scholar] [CrossRef][Green Version]
- Zúñiga-Romero, Á.; Rivera-Plata, Q.; Arrieta, J.; Flores-Murrieta, F.J.; Rodríguez-Silverio, J.; Reyes-García, J.G.; Huerta-Cruz, J.C.; Ramírez-Martínez, G.; Rocha-González, H.I. GPR55 and GPR119 Receptors Contribute to the Processing of Neuropathic Pain in Rats. Pharmaceuticals 2022, 15, 67. [Google Scholar] [CrossRef]
- Blanton, H.; Armin, S.; Muenster, S.; Abood, M.; Benamar, K. Contribution of G Protein-Coupled Receptor 55 to Periaqueductal Gray-Mediated Antinociception in the Inflammatory Pain. Cannabis Cannabinoid Res. 2022, 7, 274–278. [Google Scholar] [CrossRef]
- Armin, S.; Muenster, S.; Abood, M.; Benamar, K. GPR55 in the brain and chronic neuropathic pain. Behav. Brain Res. 2021, 406, 113248. [Google Scholar] [CrossRef] [PubMed]
- Okine, B.N.; Mc Laughlin, G.; Gaspar, J.C.; Harhen, B.; Roche, M.; Finn, D.P. Antinociceptive Effects of the GPR55 Antagonist CID16020046 Injected into the Rat Anterior Cingulate Cortex. Neuroscience 2020, 443, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Roa-Coria, J.E.; Pineda-Farias, J.B.; Barragán-Iglesias, P.; Quiñonez-Bastidas, G.N.; Zúñiga-Romero, A.; Huerta-Cruz, J.C.; Reyes-García, J.G.; Flores-Murrieta, F.J.; Granados-Soto, V.; Rocha-González, H.I. Possible involvement of peripheral TRP channels in the hydrogen sulfide-induced hyperalgesia in diabetic rats. BMC Neurosci. 2019, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhang, M.Z.; Wei, Y.; Lu, Y.C.; Wang, J.; Yang, S.M.; Zhu, Z.; Chen, Q.; Zhao, M.; Dong, J.; et al. Postsynaptic glutamate response downregulates within presynaptic exaggerated glutamate release by activating TRPV1 in the spinal dorsal horn. Biochem. Biophys. Res. Commun. 2022, 625, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, A.; Yearsley, A.; Brown, N.; Braun, S.; Hayes, C.; Rose, E.; Connolly, B.; Dicks, M.; Beal, C.; Helmonds, B.; et al. Capsaicin 8% Patch for Spinal Cord Injury Focal Neuro-pathic Pain, a Randomized Controlled Trial. Pain Med. 2022. [Google Scholar] [CrossRef]
- Cristiano, C.; Avagliano, C.; Cuozzo, M.; Liguori, F.M.; Calignano, A.; Russo, R. The Beneficial Effects of Ultramicronized Palmitoylethanolamide in the Management of Neuropathic Pain and Associated Mood Disorders Induced by Paclitaxel in Mice. Biomolecules 2022, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. [Google Scholar] [CrossRef] [PubMed]
- Palenca, I.; Seguella, L.; del Re, A.; Franzin, S.B.; Corpetti, C.; Pesce, M.; Rurgo, S.; Steardo, L.; Sarnelli, G.; Esposito, G. N-Palmitoyl-D-Glucosamine Inhibits TLR-4/NLRP3 and Improves DNBS-Induced Colon Inflammation through a PPAR-α-Dependent Mechanism. Biomolecules 2022, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-H.; Zhang, S.; Wang, H.; Xue, J.-L.; Zhang, Z.-G. Emerging experimental models for assessing perineural invasion in human cancers. Cancer Lett. 2022, 535, 215610. [Google Scholar] [CrossRef] [PubMed]
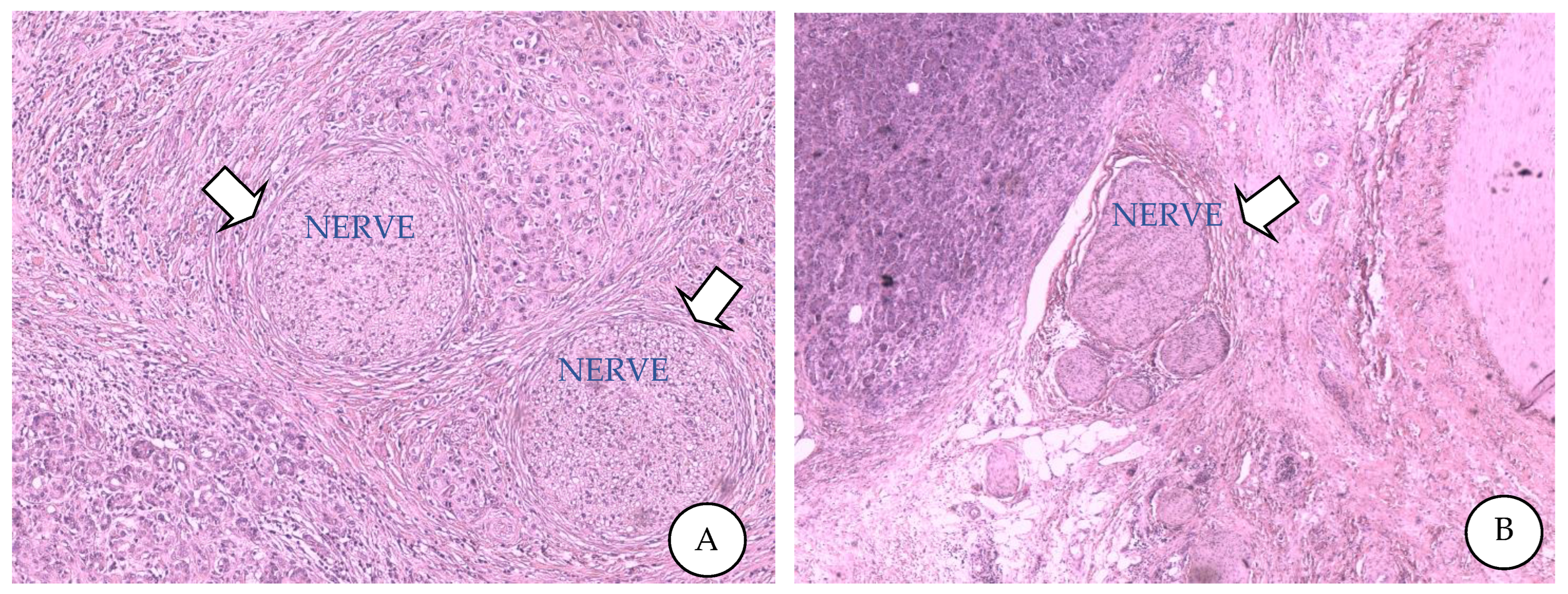


| Molecule | Receptor | Releasing Cell | Function | Inhibitor | Target | Effects |
|---|---|---|---|---|---|---|
| NGF Nerve Growth Factor | NGFR Nerve Growth Factor Receptor | Neuronal cells PDAC cells | cell growth cell survival cell maintenance-neurotrophic pain | Anti-NGF molecules | NGF SERINE | Inhibition of: -tumour growth -neurogenic inflammation -PNI |
| TRPV1 Transient Receptor Potential Cation Channels subfamily V member 1 | ||||||
| BDNF Brain-derived Neurotrophic Factor | --- | Neuronal cells PDAC cells | -cell growth -cell survival -cell maintenance | Anti-NGF molecules | NGF SERINE | Inhibition of: -tumour growth -neurogenic inflammation -PNI |
| NEUROTROPHIN 3 | NTRK1 Neurotrophic Receptor Tyrosine Kinase 1 | Neuronal cells PDAC cells | -cell growth -cell survival -cell maintenance | Anti-NGF molecules | NGF SERINE | Inhibition of: -tumour growth -neurogenic inflammation -PNI |
| NEUROTROPHIN 4 | NTRK2 Neurotrophic receptor Tyrosine Kinase 2 | Neuronal cells PDAC cells | -cell growth -cell survival -cell maintenance | Anti-NGF molecules | NGF SERINE | Inhibition of: -tumour growth -neurogenic inflammation -PNI |
| SLIT 2 SLIT–Guidance ligand 2 | ROBO1 Roundabout Guidance Receptor 1 | PDAC cells CAFs (Cancer-associated Fibroblasts) (from PDAC cells) | -promotion of: cell navigation, Schwann cell migration (by Cadherine 2 pathway), neurite outgrowth -suppression of: cell migration, cell invasion | Anti-SLIT2-ROBO1 | SLIT2/ ROBO1 signalling | -Motility and invasiveness of PDACs increase -Neural remodelling inhibition -PNI inhibition |
| SERINE (stimulated by NGF) | --- | PDAC cells Neurons (Axons and DRG, Dorsal Root Ganglia) | -energy support | Anti-NGF molecules | NGF SERINE | --- |
| -PNI formation | ||||||
| GDNF Glial-cell Derived Neurotrophic Factor | RET 9 Proto-oncogene RET 51 Proto-oncogene (expressed in PDAC cells) | Peripheral and central nervous system: Neural cells (Schwann cells and motor neurons) Macrophage | -KRAS signalling activation -Tumour growth maintenance -Migration of tumour cells to neural cells promotion -upregulation of MMPs - Neural invasion and metastasis promotion | KRAS-inhibitors PI3K-inhibitors | KRAS pathway PI3K | To inhibit tumour cell migration to neuronal cells |
| PERSEPHIN | GFRα1 (RET co-receptor) GDNF family receptor alpha 1 | Peripheral and central nervous system: Neural cells (Schwann cells and motor neurons) Macrophage | -KRAS signalling activation -Tumour growth maintenance -Migration of tumour cells to neural cells promotion -upregulation of MMPs - Neural invasion and metastasis promotion | GFRα1-inhibitors | GDNF– GFRα1– RET axis | To limit cell migration and tumour metastasis |
| ARTN Artemin | GFRα3 GDNF family receptor alpha 3 | Peripheral and central Nervous system: Neurons | -To trigger GFRα3-dependent invasion in PDAC cells -To drive tumour metastasis | GFRα3-inhibitors | ARTN– GFRα3 axis | To limit cell invasion and tumour metastasis |
| Midkine | SDC3 SYNDECAN3 (on pancreatic nerves, neurons and Schwann cells) | PDAC cells | -Nerve proliferation and PNI Neuroplasticity regulation -Nerve damage after PTN accumulation (dual role of PTN–SDC3 in neuroplasticity during PNI) | Anti-Syndecan 3 | PTN–Syndecan3 axis | PNI, nerveoutgrowthand proliferation inhibition |
| PTN Pleiotrophin | SDC3 SYNDECAN3 (on pancreatic nerves, neurons and Schwann cells) | Necrotic PDAC cells | -Nerve proliferation and PNI Neuroplasticity regulation -Nerve damage after PTN accumulation (dual role of PTN–SDC3 in neuroplasticity during PNI) | Anti-Syndecan 3 | PTN–Syndecan3 axis | PNI, nerve outgrowth and proliferation inhibition |
| SEMA 3 D Semaphorine 3D | PLXND1 Plexin D1 | Neurons | -Neuronal networks formation -Nerve density increase -Nerve invasion and PNI promotion | SEMA3D-inhibitors PLXND1-inhibitors | SEMA3D-PLXND1 axis | -Attenuation of the invasion of tumour cells towards the nerves -Nerve density decrease in tumour tissues |
| CX3CL1 C-X3-C motif chemokine ligand 1 | CX3CR-1 C-X3-C motif chemokine receptor 1 | Neurons and nerves | PI3K-AKT activation Chemoattractant for immune cells and neural cells Promoters of PNI process | CX3CR1-inhibitors | CX3CL1-CX3CR1 axis PI3K-AKT pathway | PDAC Inhibition PNI reduction |
| CXCL12 C-X-C motif chemokine ligand 12 | CXCR-4 C-X-C motif chemokine receptor 4 | Dorsal root ganglia (DRG) | Development and progress of PDAC Infiltration of immune cells in the tumour microenvironment | CXCR4-inhibitors | CXCL12-CXCR4 axis | Tumour size, nerve injury degree, PNI reduction |
| CATECHOLAMINES EPINEFRINE NOREPINEFRINE DOPAMINE | ADRB2 Adrenoceptor beta 2 PKA Protein kinase CAMP-activated catalytic STAT 3 Signal transducer and activator of transcription 3 | Neural cells | Tumour invasion PNI promotion Regulator of pancreatic tumorigenesis Tumour stem cells proliferation Maintenance of an inflammatory tumour microenvironment | ADRB2-inhibition CAMP-activated catalytic-inhibitors STAT 3-inhibitors | ADRB2–PKA–STAT 3 signalling pathway | PDAC Reduction PNI inhibition |
| IL-6 ST Interleukin-6 signal transducer | LIF LIF interleukin-6 family cytokine | Schwann cells | --- | --- | --- | --- |
| S P Substance P | KLRB1 Killer cell lectin-like receptor B1 | CD8+ T-cells | PNI induction activating MAPK pathway | --- | --- | --- |
| LIF LIF Interleukin-6 Family cytokine | --- | Macrophage | --- | --- | --- | --- |
| SNCG Synuclein gamma | --- | PDAC cells | To promote PNI and metastasis | --- | --- | --- |
| MUC 1 Mucin 1 (Cell surface-associated) | --- | Pancreatic cancer cells | --- | --- | --- | --- |
| MAG Myelin-associated glycoprotein | --- | Schwann cells | --- | --- | --- | --- |
| NCAM 1 Neural cell adhesion molecule 1 | --- | PDAC cells | To elicit structural changes in PNI cells, promoting PNI | Anti-NCAM antibodies | PDAC cells | To alleviate PNI |
| L1CAM L1 cell adhesion molecule | STAT 3 Signal transducer and activator of transcription 3 | Schwann cells | To enhance PNI-activating STAT3 pathway, promoting chemotaxis and upregulating the expression of MMP2 and MMP9 | Anti-L1CAM antibodies | PDAC cells | To alleviate PNI |
| CCL 2 C-C motif chemokine Ligand 2 | CCR 2 C-C motif chemokine receptor 2 | Schwann cells Macrophages | Inflammatory macrophages recruitment from the circulation to the site of PNI | CCL 2-inhibitors | PDAC cells | To alleviate PNI |
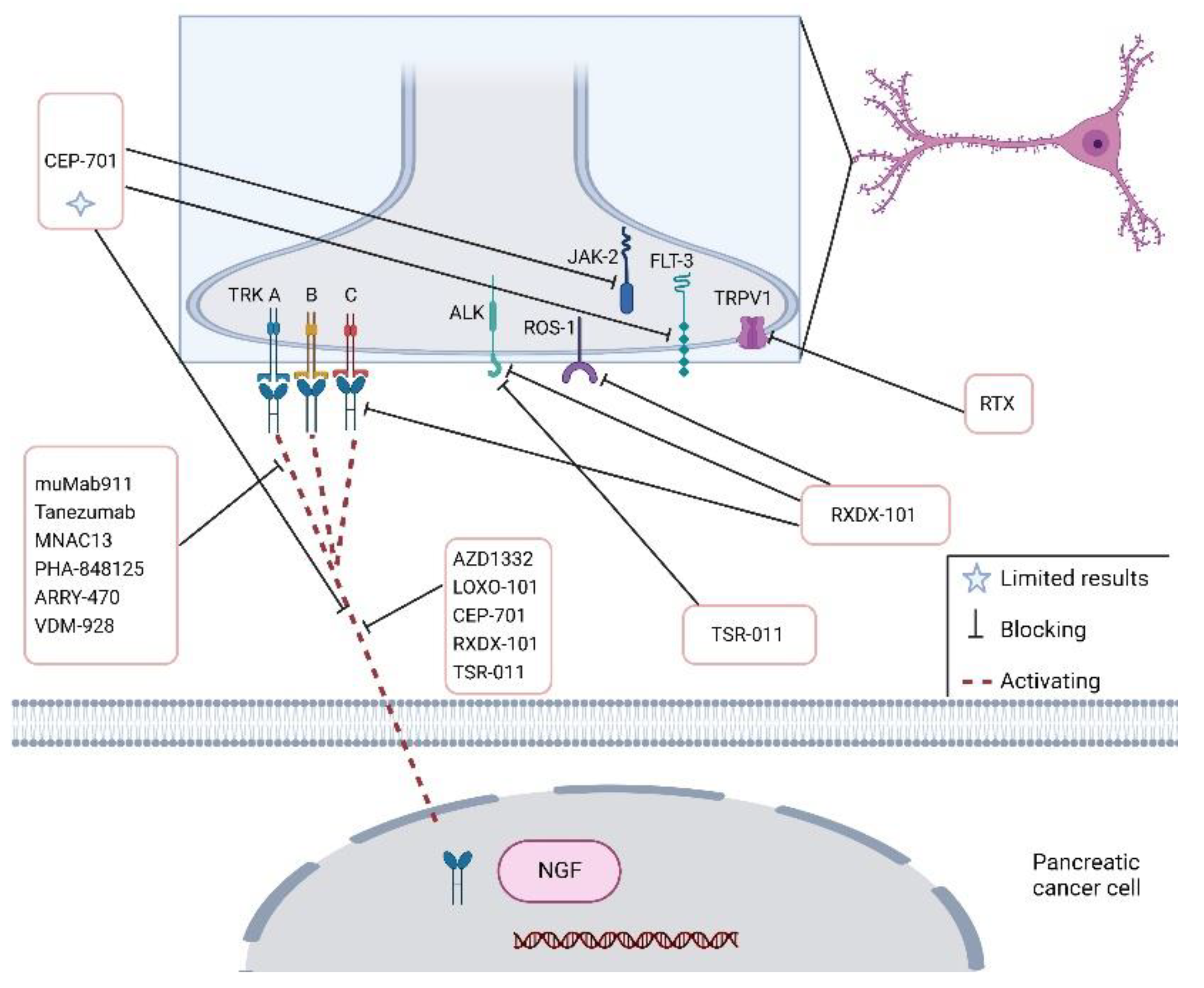
| Target | Drug | Effects | Study | References |
|---|---|---|---|---|
| Trk-A/B | CEP-701 | - | Phase I | [71] |
| TrkA, TrkB, TrkC | Entrectnib/RXDX-101 | Stable disease/reduction | Phase I- II (NCT02097810); (NCT02568267); (NCT02650401); | [72] |
| TrkA, TrkB, TrkC | NOV1601(CHC2014) | - | Phase I NCT04014257 | - |
| TrkA | VMD-928 | - | Phase I (NCT03556228) | [73] |
| TrkA, TrkB, TrkC | TSR-011 | - | Phase I-II NCT02048488. | [74] |
| Trk | DS-6051b | - | Phase I NCT02279433 | [75] |
| TrkA, TrkB, TrkC | AZD1332 | Increases radiosensitivity | Preclinical | [76] |
| βAR | β-blockers | No benefits on survival | Clinical | [77] |
| NGF | Tanezumab | - | Phase III NCT02609828 | - |
| CXCR4 | MSX-122 Inhibitor (partial antagonist of CXCR4) | - | Phase I NCT00591682 | - |
| LIF | Ab-LIFR | Reduces PDAC-associated neural remodelling | In vitro (cocultures); In vivo (PDAC-bearing mice) | [78] |
| NGF | muMab911 | Prevents hyperalgesia | In vivo | [79] |
| TrkA | MNAC13 | analgesic effects | In vivo (CD1 mice) | [80] |
| TrkA | PHA-848125 | Synergistic effects with Gemcitabine | Phase II | [69] |
| TrkA | ARRY-470 | Reduces pain | In vivo (C3H/HeJ mice) | [81] |
| CXC4R/CXCL12 | CTCE-9908 | - | Phase I | [82] |
| TRPV1 | Resiniferatoxin | Reduces pain | In vivo | [83] |
| Neuron ablation | Neonatal Capsaicin | Delays PanIN formation; prolongs survival | In vivo PKC mice | [67] |
| NGF | GNC–siRNA | Inhibits tumour progression | In vivo (subcutaneous model, orthotopic model and patient-derived xenograft model) | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaggi, F.; Melchiorre, E.; Casari, I.; Cinalli, S.; Cinalli, M.; Aceto, G.M.; Cotellese, R.; Garajova, I.; Falasca, M. Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers 2022, 14, 5793. https://doi.org/10.3390/cancers14235793
Selvaggi F, Melchiorre E, Casari I, Cinalli S, Cinalli M, Aceto GM, Cotellese R, Garajova I, Falasca M. Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers. 2022; 14(23):5793. https://doi.org/10.3390/cancers14235793
Chicago/Turabian StyleSelvaggi, Federico, Eugenia Melchiorre, Ilaria Casari, Sebastiano Cinalli, Massimiliano Cinalli, Gitana Maria Aceto, Roberto Cotellese, Ingrid Garajova, and Marco Falasca. 2022. "Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance" Cancers 14, no. 23: 5793. https://doi.org/10.3390/cancers14235793
APA StyleSelvaggi, F., Melchiorre, E., Casari, I., Cinalli, S., Cinalli, M., Aceto, G. M., Cotellese, R., Garajova, I., & Falasca, M. (2022). Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers, 14(23), 5793. https://doi.org/10.3390/cancers14235793








