Glucose and Inositol Transporters, SLC5A1 and SLC5A3, in Glioblastoma Cell Migration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunostaining
2.3. Cell Volumetry
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. dSTORM (Direct Stochastic Optical Reconstruction Microscopy)
2.6. Gene Expression Profiling Interactive Analysis of SLC5A1 and SLC5A3 in GBM
2.7. Wound Healing Assay
2.8. Statistical Analysis
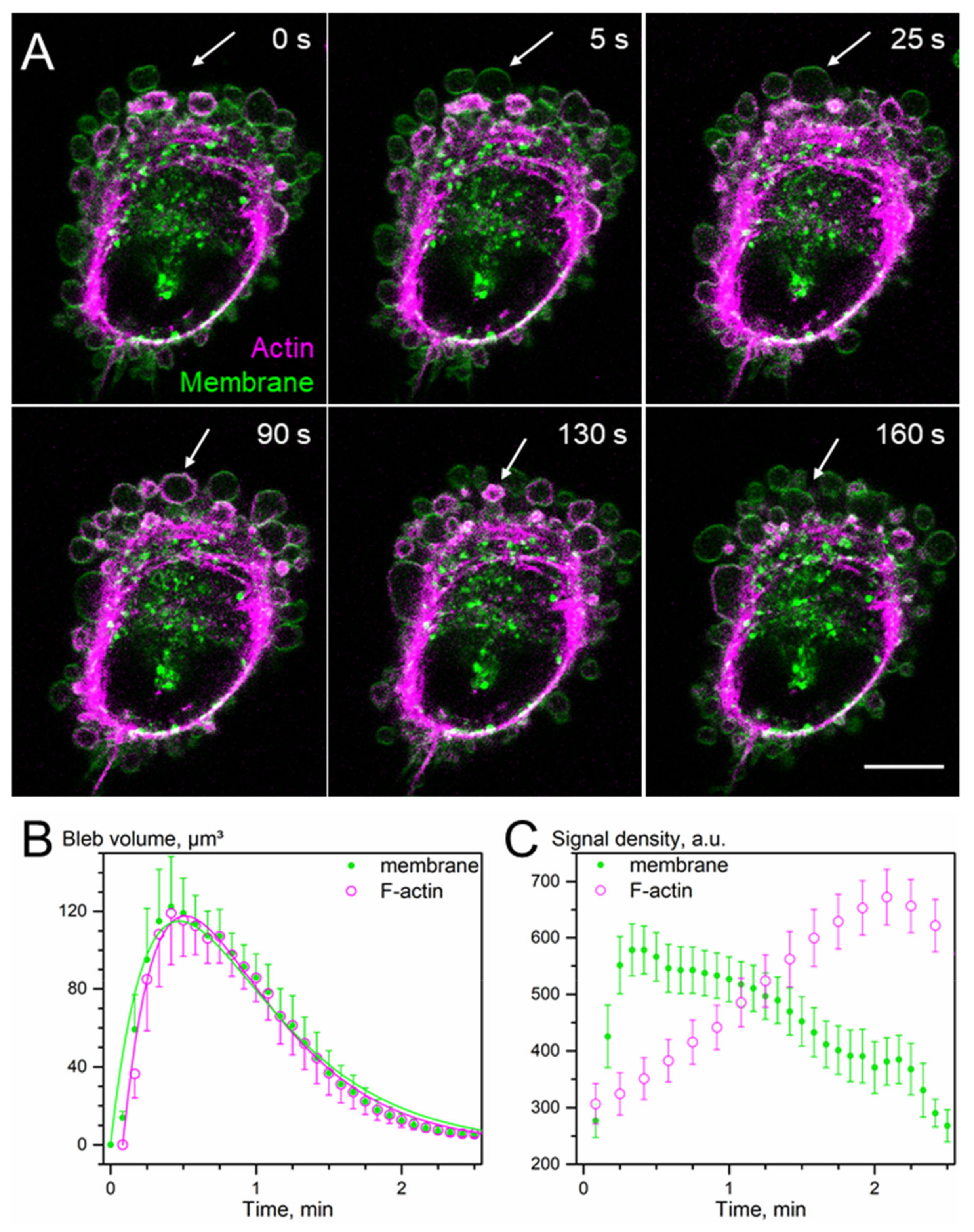
3. Results
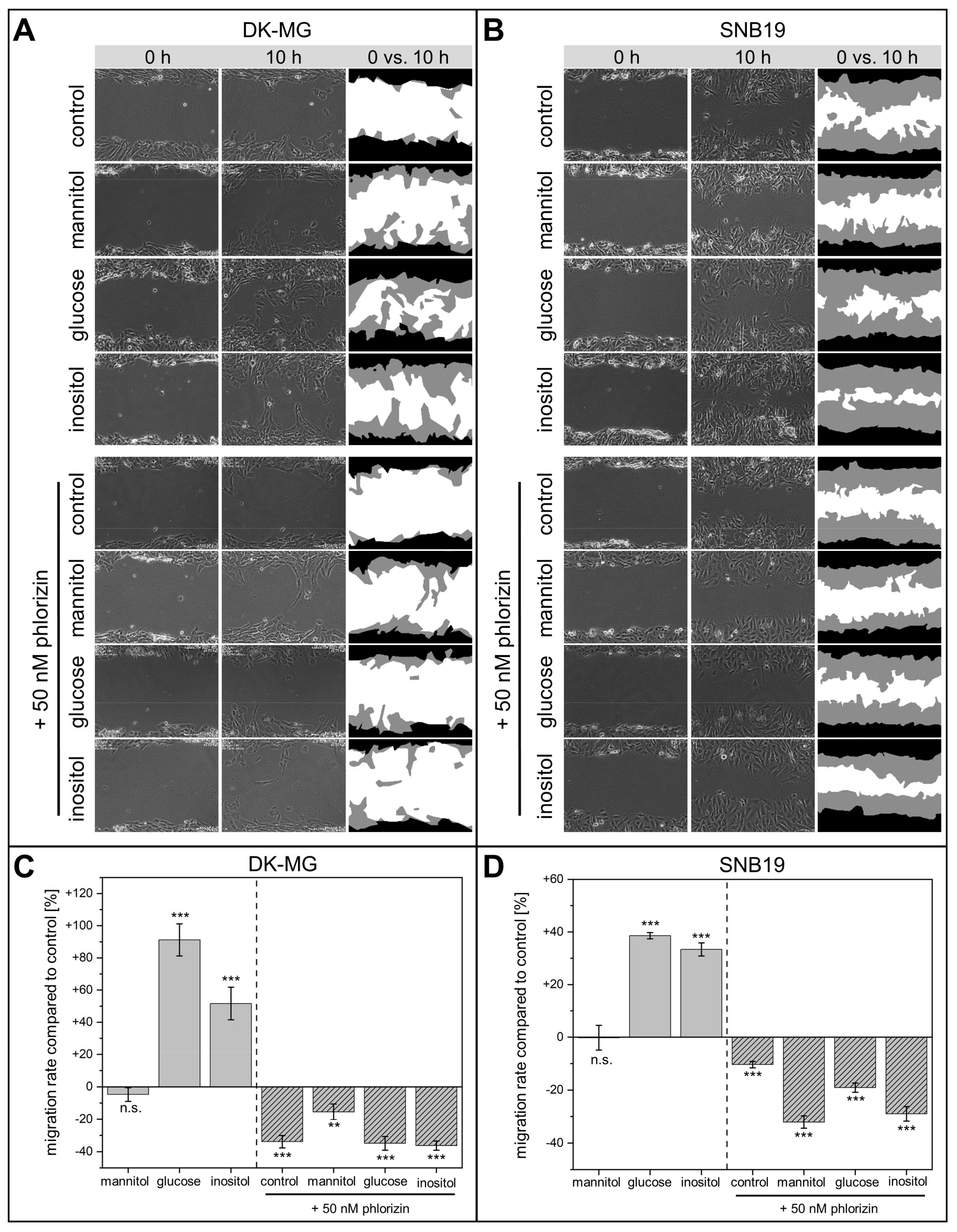
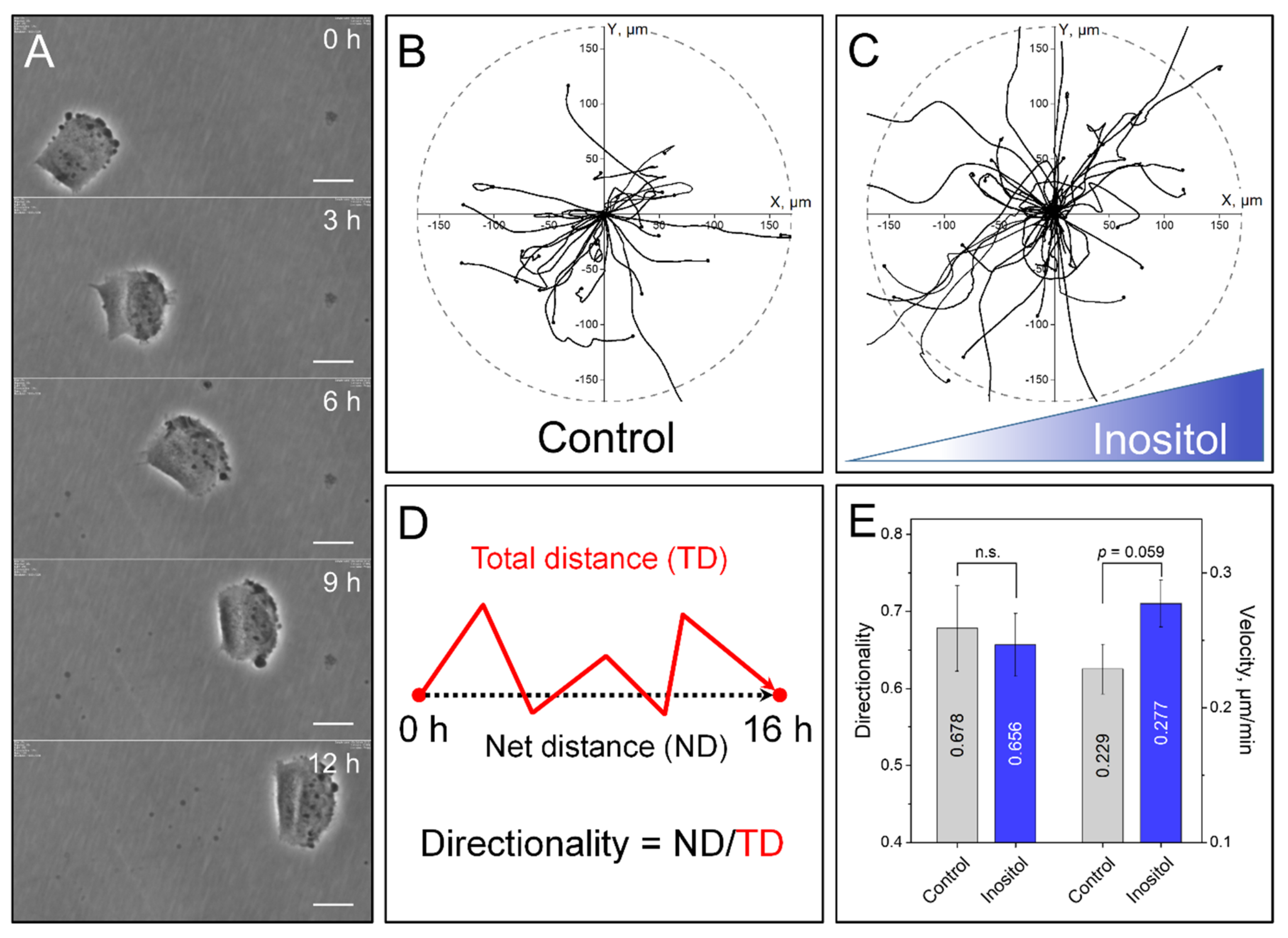
3.1. Wound-Healing and Chemotaxis Assays
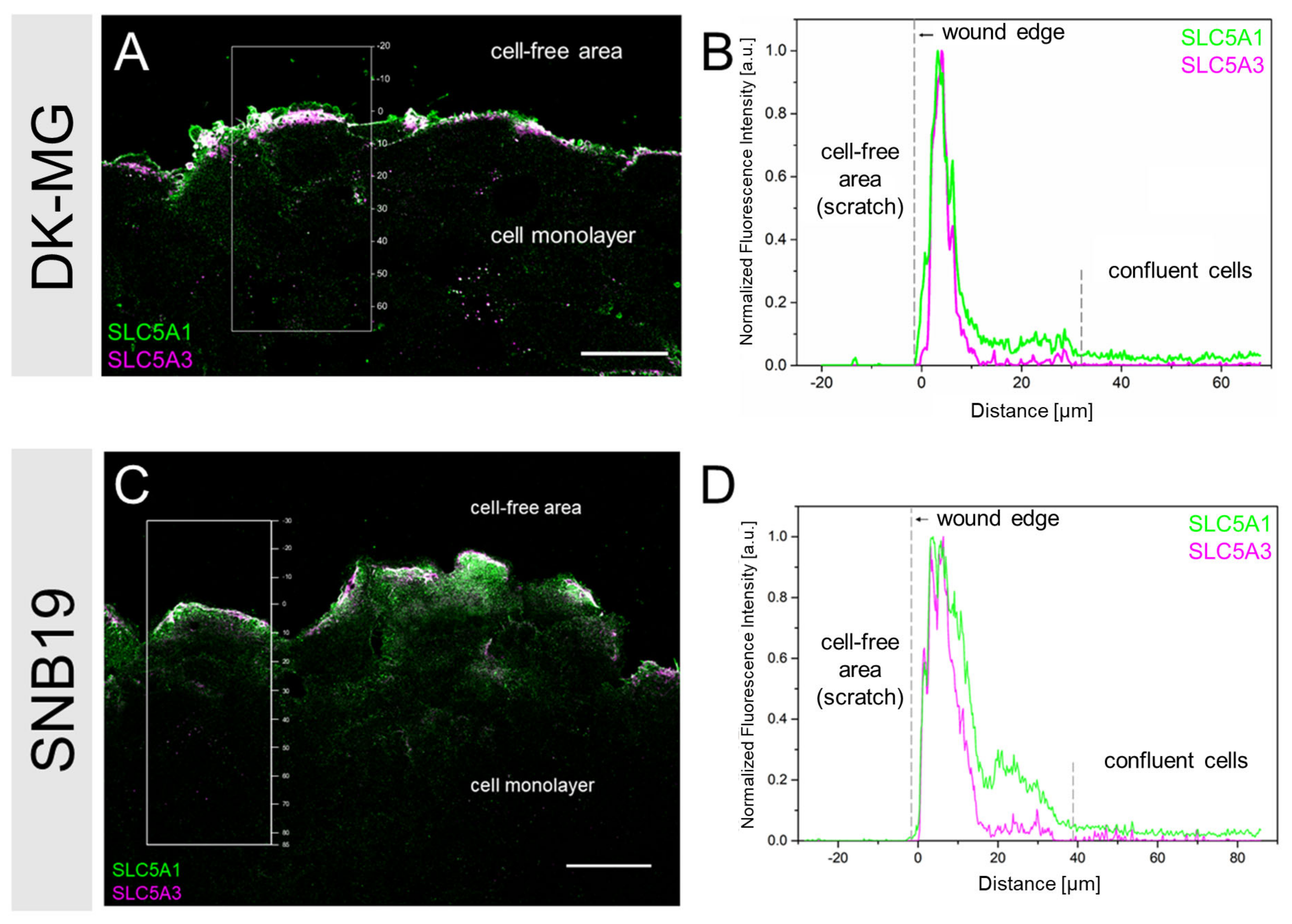
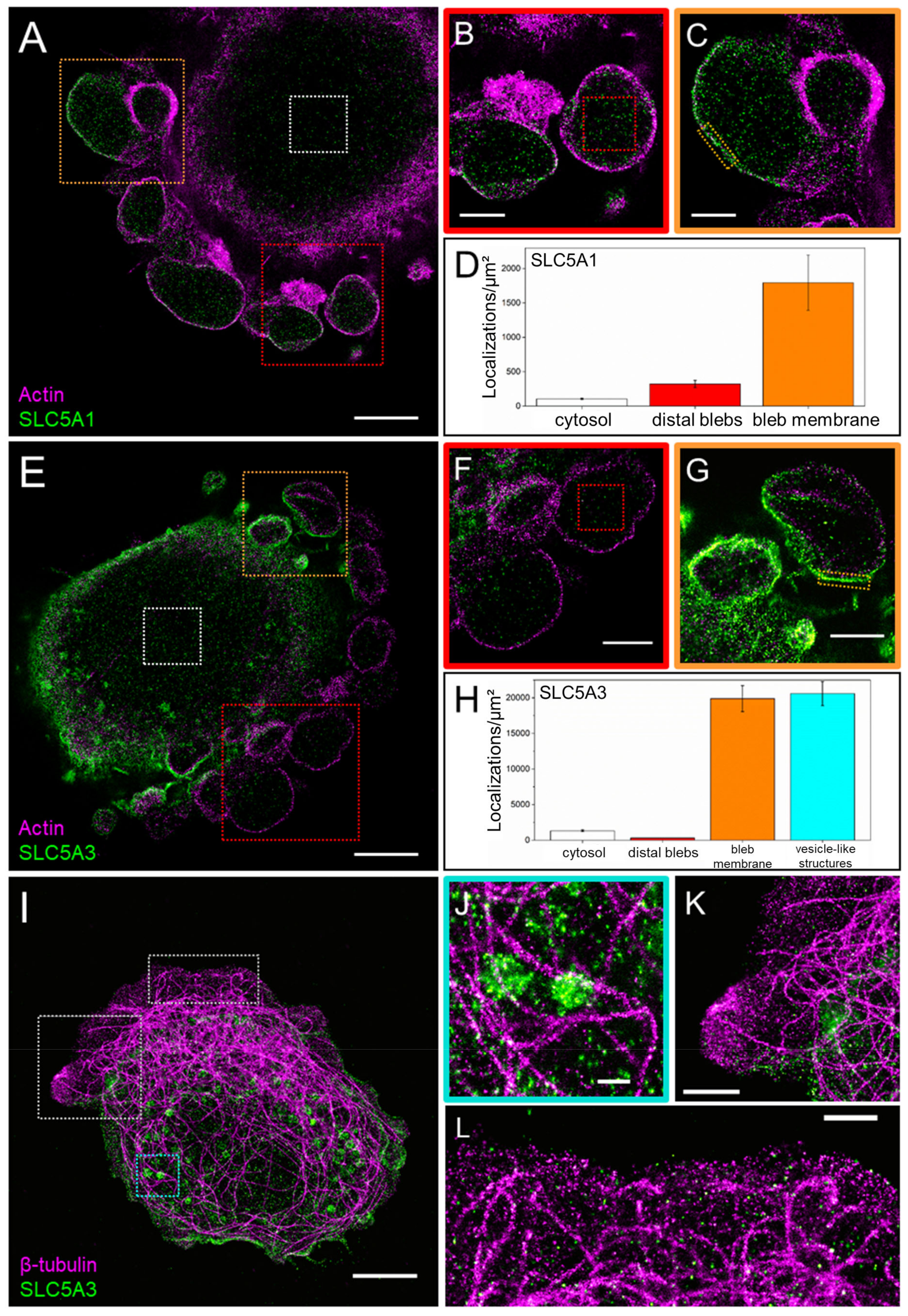
3.2. SLC5A1 and SLC5A3 Preferentially Localize to the Leading Edge of GBM Cells
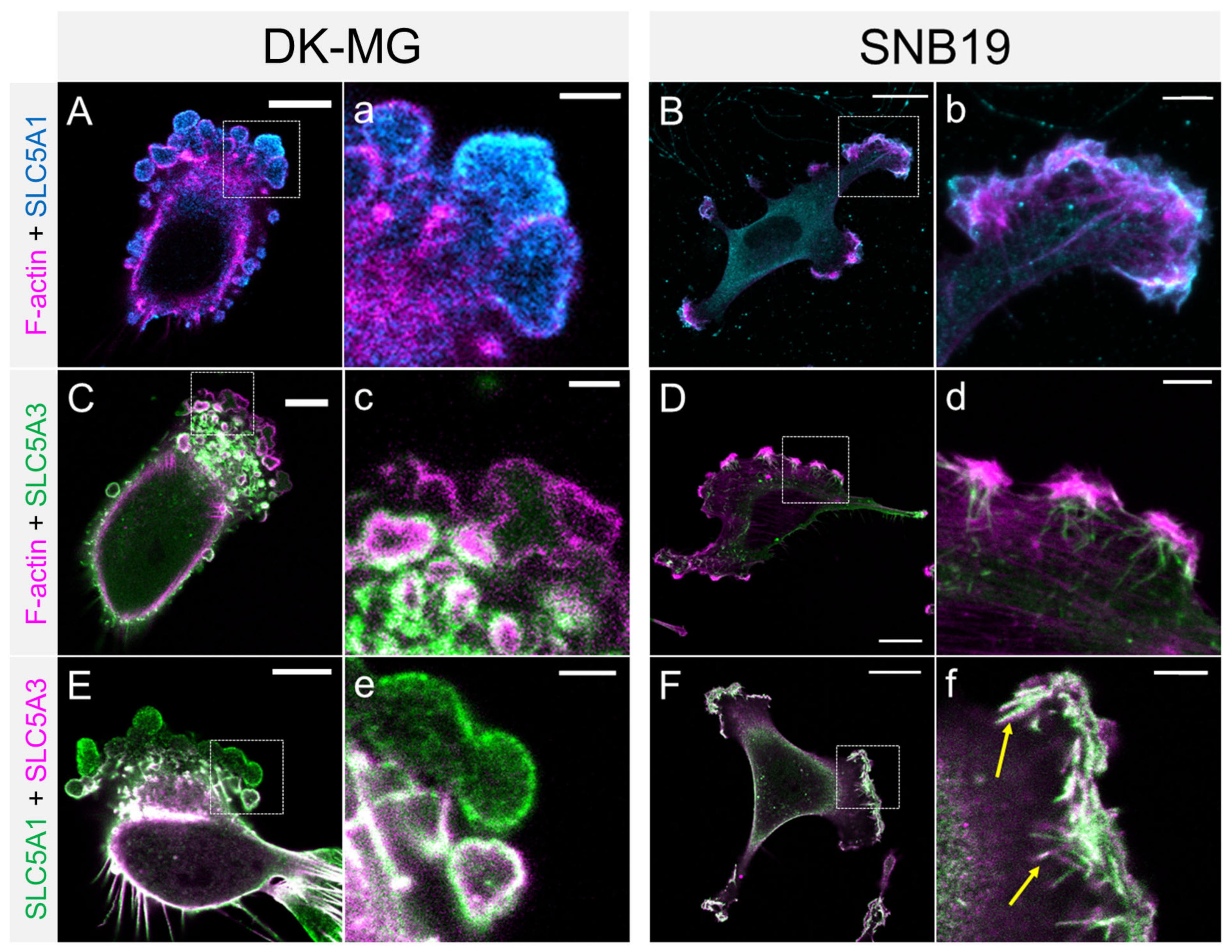
3.3. Super-Resolved (dSTORM) Images of SLC5A1, SLC5A3, F-Actin and Tubulin
3.4. Osmotic Swelling Assay for Glucose and Inositol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Nakada, S.; Demuth, T.; Tran, N.L.; Hoelzinger, D.B.; Berens, M.E. Molecular Targets of Glioma Invasion. Cell. Mol. Life Sci. 2007, 64, 458. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef] [PubMed]
- Memmel, S.; Sisario, D.; Zöller, C.; Fiedler, V.; Katzer, A.; Heiden, R.; Becker, N.; Eing, L.; Ferreira, F.L.R.; Zimmermann, H.; et al. Migration Pattern, Actin Cytoskeleton Organization and Response to PI3K-, MTOR-, and Hsp90-Inhibition of Glioblastoma Cells with Different Invasive Capacities. Oncotarget 2017, 8, 45298–45310. [Google Scholar] [CrossRef]
- Djuzenova, C.S.; Fiedler, V.; Memmel, S.; Katzer, A.; Hartmann, S.; Krohne, G.; Zimmermann, H.; Scholz, C.-J.; Polat, B.; Flentje, M.; et al. Actin Cytoskeleton Organization, Cell Surface Modification and Invasion Rate of 5 Glioblastoma Cell Lines Differing in PTEN and P53 Status. Exp. Cell Res. 2015, 330, 346–357. [Google Scholar] [CrossRef]
- Djuzenova, C.S.; Fiedler, V.; Memmel, S.; Katzer, A.; Sisario, D.; Brosch, P.K.; Göhrung, A.; Frister, S.; Zimmermann, H.; Flentje, M.; et al. Differential Effects of the Akt Inhibitor MK-2206 on Migration and Radiation Sensitivity of Glioblastoma Cells. BMC Cancer 2019, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell Adhesion: Integrating Cytoskeletal Dynamics and Cellular Tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Cramer, L.P. Forming the Cell Rear First: Breaking Cell Symmetry to Trigger Directed Cell Migration. Nat. Cell Biol. 2010, 12, 628–632. [Google Scholar] [CrossRef]
- Le Clainche, C.; Carlier, M.-F. Regulation of Actin Assembly Associated with Protrusion and Adhesion in Cell Migration. Physiol. Rev. 2008, 88, 489–513. [Google Scholar] [CrossRef]
- Carlier, M.-F.; Clainche, C.L.; Wiesner, S.; Pantaloni, D. Actin-Based Motility: From Molecules to Movement. BioEssays 2003, 25, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Pantaloni, D.; Clainche, C.L.; Carlier, M.-F. Mechanism of Actin-Based Motility. Science 2001, 292, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Borm, B.; Requardt, R.P.; Herzog, V.; Kirfel, G. Membrane Ruffles in Cell Migration: Indicators of Inefficient Lamellipodia Adhesion and Compartments of Actin Filament Reorganization. Exp. Cell Res. 2005, 302, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.; Paluch, E. Blebs Lead the Way: How to Migrate without Lamellipodia. Nat. Rev. Mol. Cell Biol. 2008, 9, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.T.; Coughlin, M.; Mitchison, T.J.; Mahadevan, L. Life and Times of a Cellular Bleb. Biophys. J. 2008, 94, 1836–1853. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Raz, E. The Role and Regulation of Blebs in Cell Migration. Curr. Opin. Cell Biol. 2013, 25, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Jiang, H.; Chen, S.-H.; Tong, Z.; Wirtz, D.; Sun, S.X.; Konstantopoulos, K. Water Permeation Drives Tumor Cell Migration in Confined Microenvironments. Cell 2014, 157, 611–623. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of Ion Channels and Transporters in Cell Migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Andronic, J.; Shirakashi, R.; Pickel, S.U.; Westerling, K.M.; Klein, T.; Holm, T.; Sauer, M.; Sukhorukov, V.L. Hypotonic Activation of the Myo-Inositol Transporter SLC5A3 in HEK293 Cells Probed by Cell Volumetry, Confocal and Super-Resolution Microscopy. PLoS ONE 2015, 10, e0119990. [Google Scholar] [CrossRef]
- Shestov, A.A.; Emir, U.E.; Kumar, A.; Henry, P.-G.; Seaquist, E.R.; Öz, G. Simultaneous Measurement of Glucose Transport and Utilization in the Human Brain. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1040–E1049. [Google Scholar] [CrossRef] [PubMed]
- Duelli, R.; Kuschinsky, W. Brain Glucose Transporters: Relationship to Local Energy Demand. News Physiol. Sci. 2001, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Heilig, C.W.; Stromski, M.E.; Blumenfeld, J.D.; Lee, J.P.; Gullans, S.R. Characterization of the Major Brain Osmolytes That Accumulate in Salt-Loaded Rats. Am. J. Physiol. 1989, 257, F1108–F1116. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, T.; Merboldt, K.D.; Bruhn, H.; Hänicke, W.; Frahm, J. Absolute Concentrations of Metabolites in the Adult Human Brain in Vivo: Quantification of Localized Proton MR Spectra. Radiology 1993, 187, 219–227. [Google Scholar] [CrossRef]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In Vivo Mapping of Brain Myo-Inositol. Neuroimage 2011, 54, 2079–2085. [Google Scholar] [CrossRef]
- Soupart, A.; Silver, S.; Schroöeder, B.; Sterns, R.; Decaux, G. Rapid (24-Hour) Reaccumulation of Brain Organic Osmolytes (Particularly Myo-Inositol) in Azotemic Rats after Correction of Chronic Hyponatremia. J. Am. Soc. Nephrol. 2002, 13, 1433–1441. [Google Scholar] [CrossRef]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR Studies on the Energy Metabolism of Glial and Neuronal Cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef]
- Castillo, M.; Smith, J.K.; Kwock, L. Correlation of Myo-Inositol Levels and Grading of Cerebral Astrocytomas. AJNR Am. J. Neuroradiol. 2000, 21, 1645–1649. [Google Scholar]
- Kim, J.; Dang, C.V. Cancer’s Molecular Sweet Tooth and the Warburg Effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef]
- El-Gebali, S.; Bentz, S.; Hediger, M.A.; Anderle, P. Solute Carriers (SLCs) in Cancer. Mol. Asp. Med. 2013, 34, 719–734. [Google Scholar] [CrossRef]
- Wright, E.M. Glucose Transport Families SLC5 and SLC50. Mol. Asp. Med. 2013, 34, 183–196. [Google Scholar] [CrossRef]
- Nakanishi, T.; Tamai, I. Putative Roles of Organic Anion Transporting Polypeptides (OATPs) in Cell Survival and Progression of Human Cancers. Biopharm. Drug Dispos. 2014, 35, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.F.; Cai, Y.C.; Zhang, B.; Xu, R.H.; Qiu, H.J.; Xia, L.P.; Jiang, W.Q.; Hu, P.L.; Chen, X.X.; Zhou, F.F.; et al. Overexpression of SGLT1 and EGFR in Colorectal Cancer Showing a Correlation with the Prognosis. Med. Oncol. 2011, 28, 197–203. [Google Scholar] [CrossRef]
- Casneuf, V.F.; Fonteyne, P.; Van Damme, N.; Demetter, P.; Pauwels, P.; de Hemptinne, B.; De Vos, M.; Van de Wiele, C.; Peeters, M. Expression of SGLT1, Bcl-2 and P53 in Primary Pancreatic Cancer Related to Survival. Cancer Investig. 2008, 26, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Shorthouse, D.; Riedel, A.; Kerr, E.; Pedro, L.; Bihary, D.; Samarajiwa, S.; Martins, C.P.; Shields, J.; Hall, B.A. Exploring the Role of Stromal Osmoregulation in Cancer and Disease Using Executable Modelling. Nat. Commun. 2018, 9, 3011. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fang, L.; Gibbs, S.; Huang, Y.; Dai, Z.; Wen, P.; Zheng, X.; Sadee, W.; Sun, D. Glucose Uptake Inhibitor Sensitizes Cancer Cells to Daunorubicin and Overcomes Drug Resistance in Hypoxia. Cancer Chemother. Pharmacol. 2007, 59, 495–505. [Google Scholar] [CrossRef]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional Expression of Sodium-Glucose Transporters in Cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119. [Google Scholar] [CrossRef]
- Dai, G.; Yu, H.; Kruse, M.; Traynor-Kaplan, A.; Hille, B. Osmoregulatory Inositol Transporter SMIT1 Modulates Electrical Activity by Adjusting PI(4,5)P2 Levels. Proc. Natl. Acad. Sci. USA 2016, 113, E3290–E3299. [Google Scholar] [CrossRef]
- Leslie, N.R.; Batty, I.H.; Maccario, H.; Davidson, L.; Downes, C.P. Understanding PTEN Regulation: PIP2, Polarity and Protein Stability. Oncogene 2008, 27, 5464–5476. [Google Scholar] [CrossRef]
- Kiesel, M.; Reuss, R.; Endter, J.; Zimmermann, D.; Zimmermann, H.; Shirakashi, R.; Bamberg, E.; Zimmermann, U.; Sukhorukov, V.L. Swelling-Activated Pathways in Human T-Lymphocytes Studied by Cell Volumetry and Electrorotation. Biophys. J. 2006, 90, 4720–4729. [Google Scholar] [CrossRef]
- Bobak, N.; Bittner, S.; Andronic, J.; Hartmann, S.; Mühlpfordt, F.; Schneider-Hohendorf, T.; Wolf, K.; Schmelter, C.; Göbel, K.; Meuth, P.; et al. Volume Regulation of Murine T Lymphocytes Relies on Voltage-Dependent and Two-Pore Domain Potassium Channels. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2036–2044. [Google Scholar] [CrossRef]
- van de Linde, S.; Löschberger, A.; Klein, T.; Heidbreder, M.; Wolter, S.; Heilemann, M.; Sauer, M. Direct Stochastic Optical Reconstruction Microscopy with Standard Fluorescent Probes. Nat. Protoc. 2011, 6, 991–1009. [Google Scholar] [CrossRef]
- Sisario, D.; Memmel, S.; Doose, S.; Neubauer, J.; Zimmermann, H.; Flentje, M.; Djuzenova, C.S.; Sauer, M.; Sukhorukov, V.L. Nanostructure of DNA Repair Foci Revealed by Superresolution Microscopy. FASEB J. 2018, 32, 6469–6477. [Google Scholar] [CrossRef]
- Wolter, S.; Löschberger, A.; Holm, T.; Aufmkolk, S.; Dabauvalle, M.-C.; van de Linde, S.; Sauer, M. RapidSTORM: Accurate, Fast Open-Source Software for Localization Microscopy. Nat. Methods 2012, 9, 1040–1041. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Ikeda, T.S.; Hwang, E.S.; Coady, M.J.; Hirayama, B.A.; Hediger, M.A.; Wright, E.M. Characterization of a Na+/Glucose Cotransporter Cloned from Rabbit Small Intestine. J. Membr. Biol. 1989, 110, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Yamauchi, A.; Uchida, S.; Preston, A.S.; Garcia-Perez, A.; Burg, M.B.; Handler, J.S. Cloning of the CDNa for a Na+/Myo-Inositol Cotransporter, a Hypertonicity Stress Protein. J. Biol. Chem. 1992, 267, 6297–6301. [Google Scholar] [CrossRef]
- Steidl, E.; Pilatus, U.; Hattingen, E.; Steinbach, J.P.; Zanella, F.; Ronellenfitsch, M.W.; Bähr, O. Myoinositol as a Biomarker in Recurrent Glioblastoma Treated with Bevacizumab: A 1H-Magnetic Resonance Spectroscopy Study. PLoS ONE 2016, 11, e0168113. [Google Scholar] [CrossRef]
- Rosengren, S.; Henson, P.M.; Worthen, G.S. Migration-Associated Volume Changes in Neutrophils Facilitate the Migratory Process in Vitro. Am. J. Physiol. 1994, 267, C1623–C1632. [Google Scholar] [CrossRef]
- Charras, G.T.; Hu, C.-K.; Coughlin, M.; Mitchison, T.J. Reassembly of Contractile Actin Cortex in Cell Blebs. J. Cell Biol. 2006, 175, 477–490. [Google Scholar] [CrossRef]
- Charras, G.T.; Yarrow, J.C.; Horton, M.A.; Mahadevan, L.; Mitchison, T.J. Non-Equilibration of Hydrostatic Pressure in Blebbing Cells. Nature 2005, 435, 365–369. [Google Scholar] [CrossRef]
- Fletcher, L.M.; Welsh, G.I.; Oatey, P.B.; Tavaré, J.M. Role for the Microtubule Cytoskeleton in GLUT4 Vesicle Trafficking and in the Regulation of Insulin-Stimulated Glucose Uptake. Biochem. J. 2000, 352 Pt 2, 267–276. [Google Scholar] [CrossRef]
- Caviston, J.P.; Holzbaur, E.L.F. Microtubule Motors at the Intersection of Trafficking and Transport. Trends Cell Biol. 2006, 16, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Andronic, J.; Bobak, N.; Bittner, S.; Ehling, P.; Kleinschnitz, C.; Herrmann, A.M.; Zimmermann, H.; Sauer, M.; Wiendl, H.; Budde, T.; et al. Identification of Two-Pore Domain Potassium Channels as Potent Modulators of Osmotic Volume Regulation in Human T Lymphocytes. Biochim. Biophys. Acta 2013, 1828, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Memmel, S.; Sukhorukov, V.L.; Höring, M.; Westerling, K.; Fiedler, V.; Katzer, A.; Krohne, G.; Flentje, M.; Djuzenova, C.S. Cell Surface Area and Membrane Folding in Glioblastoma Cell Lines Differing in PTEN and P53 Status. PLoS ONE 2014, 9, e87052. [Google Scholar] [CrossRef] [PubMed]
- Djuzenova, C.S.; Krasnyanska, J.; Kiesel, M.; Stingl, L.; Zimmermann, U.; Flentje, M.; Sukhorukov, V.L. Intracellular Delivery of 2-Deoxy-D-Glucose into Tumor Cells by Long-Term Cultivation and through Swelling-Activated Pathways: Implications for Radiation Treatment. Mol. Med. Rep. 2009, 2, 633–640. [Google Scholar] [CrossRef]
- McCoy, E.; Sontheimer, H. Expression and Function of Water Channels (Aquaporins) in Migrating Malignant Astrocytes. Glia 2007, 55, 1034–1043. [Google Scholar] [CrossRef]
- Ernest, N.J.; Weaver, A.K.; Van Duyn, L.B.; Sontheimer, H.W. Relative Contribution of Chloride Channels and Transporters to Regulatory Volume Decrease in Human Glioma Cells. Am. J. Physiol. Cell Physiol. 2005, 288, C1451–C1460. [Google Scholar] [CrossRef]
- Sukhorukov, V.L.; Imes, D.; Woellhaf, M.W.; Andronic, J.; Kiesel, M.; Shirakashi, R.; Zimmermann, U.; Zimmermann, H. Pore Size of Swelling-Activated Channels for Organic Osmolytes in Jurkat Lymphocytes, Probed by Differential Polymer Exclusion. Biochim. Biophys. Acta 2009, 1788, 1841–1850. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Xi, Y.; Wang, F.; Sha, H.; Luo, L.; Zhu, Y.; Hong, X.; Bu, S. High Glucose Induces Epithelial-Mesenchymal Transition and Results in the Migration and Invasion of Colorectal Cancer Cells. Exp. Ther. Med. 2018, 16, 222–230. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lee, C.-H.; Huang, C.-C.; Lee, S.-T.; Guo, H.-R.; Su, S.-B. Impact of High Glu-cose on Metastasis of Colon Cancer Cells. World J. Gastroenterol. 2015, 21, 2047–2057. [Google Scholar] [CrossRef]
- Santos, J.M.; Hussain, F. Higher Glucose Enhances Breast Cancer Cell Aggressiveness. Nutr. Cancer 2020, 72, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-F.; Chen, L.-Y.; Cheng, C.-S.; Chen, H.; Meng, Z.-Q.; Chen, Z. SLC5A1 Promotes Growth and Proliferation of Pancreatic Carcinoma via Glucose-Dependent AMPK/MTOR Signaling. Cancer Manag. Res. 2019, 11, 3171–3185. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Monteith, G.R. Plasma Membrane Ion Channels and Epithelial to Mesenchymal Transition in Cancer Cells. Endocr. -Relat. Cancer 2016, 23, R517–R525. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Li, N.Y.; Wai, P.Y.; Kuo, P.C. Epithelial-Mesenchymal Transition, TGF-β, and Osteopontin in Wound Healing and Tissue Remodeling After Injury. J. Burn Care Res. 2012, 33, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Schwab, A.; Stock, C. Ion Channels and Transporters in Tumour Cell Migration and Invasion. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130102. [Google Scholar] [CrossRef]
- Chen, X.-M.; O’Hara, S.P.; Huang, B.Q.; Splinter, P.L.; Nelson, J.B.; LaRusso, N.F. Localized Glucose and Water Influx Facilitates Cryptosporidium Parvum Cellular Invasion by Means of Modulation of Host-Cell Membrane Protrusion. Proc. Natl. Acad. Sci. USA 2005, 102, 6338–6343. [Google Scholar] [CrossRef]
- Huebert, R.C.; Vasdev, M.M.; Shergill, U.; Das, A.; Huang, B.Q.; Charlton, M.R.; LaRusso, N.F.; Shah, V.H. Aquaporin-1 Facilitates Angiogenic Invasion in the Pathological Neovasculature That Accompanies Cirrhosis. Hepatology 2010, 52, 238–248. [Google Scholar] [CrossRef]
- Taloni, A.; Kardash, E.; Salman, O.U.; Truskinovsky, L.; Zapperi, S.; La Porta, C.A.M. Volume Changes During Active Shape Fluctuations in Cells. Phys. Rev. Lett. 2015, 114, 208101. [Google Scholar] [CrossRef]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer Cell Motility: Lessons from Migration in Confined Spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef]
- Kirk, K. Swelling-Activated Organic Osmolyte Channels. J. Membr. Biol. 1997, 158, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.W.; Pedersen, S.F.; Christensen, S.T.; Lambert, I.H. Regulation of the Expression and Subcellular Localization of the Taurine Transporter TauT in Mouse NIH3T3 Fibroblasts. Eur. J. Biochem. 2004, 271, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Parikh, V.; Xi, L.; Chu, S.; Montrose, M.H. Subcellular Redistribution of the Renal Betaine Transporter during Hypertonic Stress. Am. J. Physiol. -Cell Physiol. 2003, 285, C1091–C1100. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Thorens, B. The SLC2 Family of Facilitated Hexose and Polyol Transporters. Pflug. Arch.–Eur. J. Physiol. 2004, 447, 480–489. [Google Scholar] [CrossRef]
- Eiden, L.E.; Schäfer, M.K.-H.; Weihe, E.; Schütz, B. The Vesicular Amine Transporter Family (SLC18): Amine/Proton Antiporters Required for Vesicular Accumulation and Regulated Exocytotic Secretion of Monoamines and Acetylcholine. Pflug. Arch-Eur. J. Physiol. 2004, 447, 636–640. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Microtubules in Cell Migration. Annu. Rev. Cell Dev. Biol. 2013, 29, 471–499. [Google Scholar] [CrossRef]
- Watanabe, T.; Noritake, J.; Kaibuchi, K. Regulation of Microtubules in Cell Migration. Trends Cell Biol. 2005, 15, 76–83. [Google Scholar] [CrossRef]
- Kaverina, I.; Straube, A. Regulation of Cell Migration by Dynamic Microtubules. Semin. Cell Dev. Biol. 2011, 22, 968–974. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brosch, P.K.; Korsa, T.; Taban, D.; Eiring, P.; Kreisz, P.; Hildebrand, S.; Neubauer, J.; Zimmermann, H.; Sauer, M.; Shirakashi, R.; et al. Glucose and Inositol Transporters, SLC5A1 and SLC5A3, in Glioblastoma Cell Migration. Cancers 2022, 14, 5794. https://doi.org/10.3390/cancers14235794
Brosch PK, Korsa T, Taban D, Eiring P, Kreisz P, Hildebrand S, Neubauer J, Zimmermann H, Sauer M, Shirakashi R, et al. Glucose and Inositol Transporters, SLC5A1 and SLC5A3, in Glioblastoma Cell Migration. Cancers. 2022; 14(23):5794. https://doi.org/10.3390/cancers14235794
Chicago/Turabian StyleBrosch, Philippa K., Tessa Korsa, Danush Taban, Patrick Eiring, Philipp Kreisz, Sascha Hildebrand, Julia Neubauer, Heiko Zimmermann, Markus Sauer, Ryo Shirakashi, and et al. 2022. "Glucose and Inositol Transporters, SLC5A1 and SLC5A3, in Glioblastoma Cell Migration" Cancers 14, no. 23: 5794. https://doi.org/10.3390/cancers14235794
APA StyleBrosch, P. K., Korsa, T., Taban, D., Eiring, P., Kreisz, P., Hildebrand, S., Neubauer, J., Zimmermann, H., Sauer, M., Shirakashi, R., Djuzenova, C. S., Sisario, D., & Sukhorukov, V. L. (2022). Glucose and Inositol Transporters, SLC5A1 and SLC5A3, in Glioblastoma Cell Migration. Cancers, 14(23), 5794. https://doi.org/10.3390/cancers14235794





