A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients
Abstract
Simple Summary
Abstract
1. Introduction
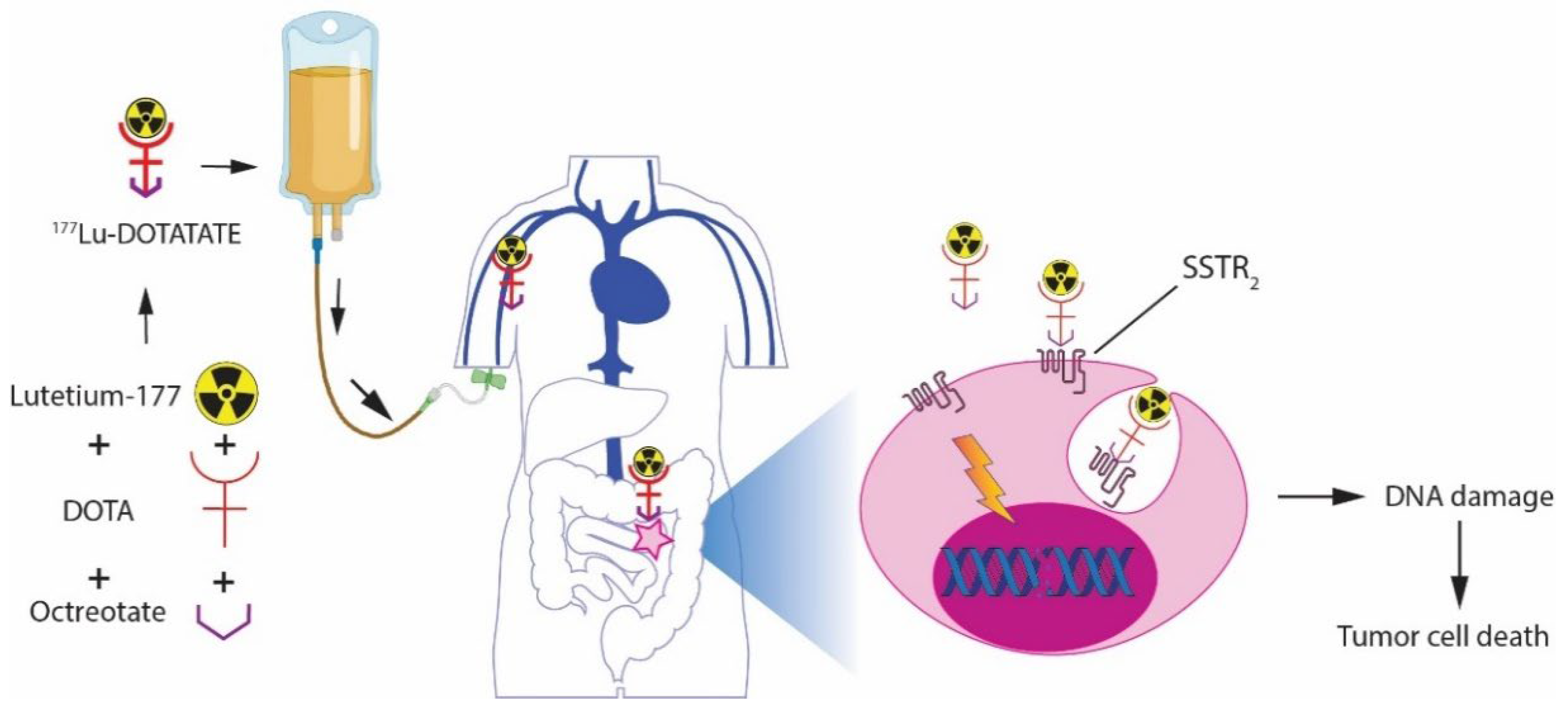
2. Background of PRRT
2.1. Mechanism of Action
2.2. The Choice for 177Lu-DOTATATE
3. Efficacy of PRRT
3.1. Tumor Control
3.2. Symptom Control
3.3. Prediction of Response to PRRT
3.4. Predictive Markers
4. PRRT Protocol
5. Salvage PRRT
6. Patient Selection
7. Eligibility Criteria for PRRT
8. PRRT-Related Toxicity
8.1. Hematological Toxicity
8.2. Nephrotoxicity
8.3. Hepatotoxicity
9. Hormonal Crisis Due to PRRT
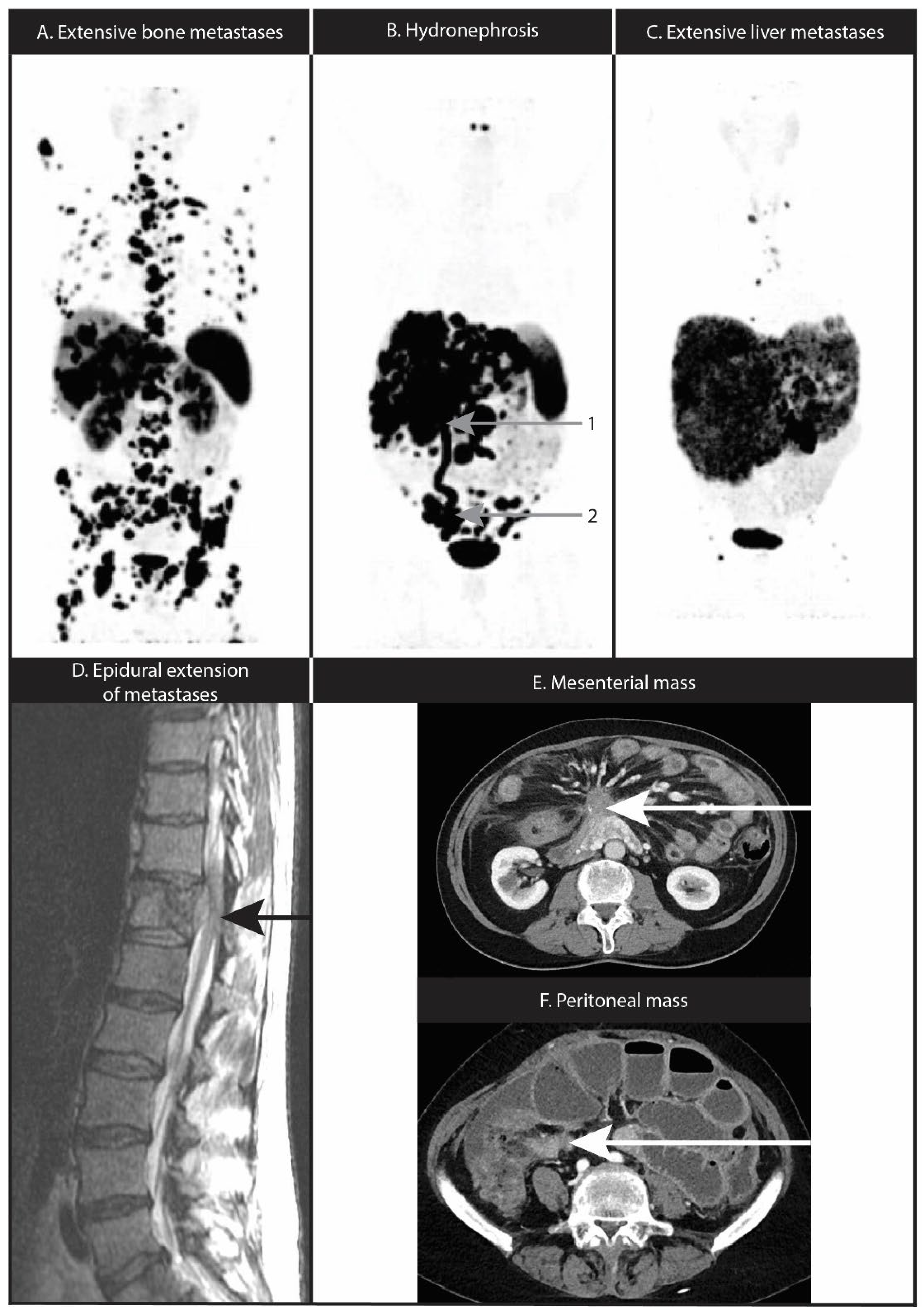
10. Nervous System Location or Compression
11. Mesenteric Fibrosis and Mesenteric or Peritoneal Metastases
12. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Fraenkel, M.; Kim, M.; Faggiano, A.; De Herder, W.W.; Valk, G.D. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr.-Relat. Cancer 2014, 21, R153–R163. [Google Scholar] [CrossRef] [PubMed]
- FDA Letter of Approval for LUTATHERA®. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208700s000lbl.pdf (accessed on 1 January 2022).
- Authorization Details for Lutathera® in Europe. 2018. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera#authorisation-details-section (accessed on 1 January 2022).
- De Herder, W.W.; Hofland, L.J.; van der Lely, A.-J.; Lamberts, S.W.J. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr.-Relat. Cancer 2003, 10, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Somatostatin and other Peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 2004, 80 (Suppl. S1), 51–56. [Google Scholar] [CrossRef]
- Hofland, L.J.; Lamberts, S.W.J. The Pathophysiological Consequences of Somatostatin Receptor Internalization and Resistance. Endocr. Rev. 2003, 24, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Shastry, M.; Kayani, I.; Wild, D.; Caplin, M.; Visvikis, D.; Gacinovic, S.; Reubi, J.C.; Bomanji, J.B. Distribution pattern of 68Ga-DOTATATE in disease-free patients. Nucl. Med. Commun. 2010, 31, 1025–1032. [Google Scholar] [CrossRef]
- Siebinga, H.; de Wit-van der Veen, B.J.; Beijnen, J.H.; Stokkel, M.P.M.; Dorlo, T.P.C.; Huitema, A.D.R.; Hendrikx, J. A physiologically based pharmacokinetic (PBPK) model to describe organ distribution of (68)Ga-DOTATATE in patients without neuroendocrine tumors. EJNMMI Res. 2021, 11, 73. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef]
- Tamborino, G.; Nonnekens, J.; De Saint-Hubert, M.; Struelens, L.; Feijtel, D.; de Jong, M.; Konijnenberg, M.W. Dosimetric Evaluation of the Effect of Receptor Heterogeneity on the Therapeutic Efficacy of Peptide Receptor Radionuclide Therapy: Correlation with DNA Damage Induction and In Vivo Survival. J. Nucl. Med. 2022, 63, 100–107. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Mueller-Brand, J.; Paganelli, G.; Anthony, L.B.; Pauwels, S.; Kvols, L.K.; O’Dorisio, T.M.; Valkema, R.; Bodei, L.; Chinol, M. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J. Nucl. Med. 2005, 46 (Suppl. S1), 62S–66S. [Google Scholar]
- Reubi, J.C.; Schar, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Macke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- De Jong, M.; Breeman, W.A.P.; Valkema, R.; Bernard, B.F.; Krenning, E.P. Combination radionuclide therapy using 177Lu-and 90Y-labeled somatostatin analogs. J. Nucl. Med. 2005, 46 (Suppl. S1), 13S–17S. [Google Scholar]
- Breeman, W.A.; Chan, H.S.; de Zanger, R.M.; Konijnenberg, M.K.; de Blois, E. Overview of Development and Formulation of 177Lu-DOTA-TATE for PRRT. Curr. Radiopharm. 2016, 9, 8–18. [Google Scholar] [CrossRef]
- Teunissen, J.J.M.; Kwekkeboom, D.J.; de Jong, M.; Esser, J.-P.; Valkema, R.; Krenning, E.P. Endocrine tumours of the gastrointestinal tract. Peptide receptor radionuclide therapy. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 595–616. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Bakker, W.H.; Kooij, P.P.; Konijnenberg, M.W.; Srinivasan, A.; Erion, J.L.; Schmidt, M.A.; Bugaj, J.L.; de Jong, M.; Krenning, E.P. [177Lu-DOTAOTyr3]octreotate: Comparison with [111In-DTPAo]octreotide in patients. Eur. J. Nucl. Med. 2001, 28, 1319–1325. [Google Scholar] [CrossRef]
- Valkema, R.; De Jong, M.; Bakker, W.H.; Breeman, W.A.; Kooij, P.P.; Lugtenburg, P.J.; De Jong, F.H.; Christiansen, A.; Kam, B.L.; De Herder, W.W.; et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: The Rotterdam experience. Semin. Nucl. Med. 2002, 32, 110–122. [Google Scholar] [CrossRef]
- Anthony, L.B.; Woltering, E.A.; Espenan, G.D.; Cronin, M.D.; Maloney, T.J.; McCarthy, K.E. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin. Nucl. Med. 2002, 32, 123–132. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- D’Arienzo, M. Emission of β+ particles via internal pair production in the 0+–0+ transition of 90Zr: Historical background and current applications in nuclear medicine imaging. Atoms 2013, 1, 2–12. [Google Scholar] [CrossRef]
- Roth, D.; Gustafsson, J.; Warfvinge, C.F.; Sundlöv, A.; Åkesson, A.; Tennvall, J.; Gleisner, K.S. Dosimetric Quantities in Neuroendocrine Tumors over Treatment Cycles with (177)Lu-DOTATATE. J. Nucl. Med. 2022, 63, 399–405. [Google Scholar] [CrossRef]
- Jahn, U.; Ilan, E.; Sandström, M.; Garske-Román, U.; Lubberink, M.; Sundin, A. 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy: Dose Response in Small Intestinal Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske-Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Królicki, L.; Hubalewska-Dydejczyk, A.; Mikołajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Callahan, J.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.; Michael, M.; Eu, P.; Hicks, R.J. High clinical and morphologic response using (90)Y-DOTA-octreotate sequenced with (177)Lu-DOTA-octreotate induction peptide receptor chemoradionuclide therapy (PRCRT) for bulky neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 476–489. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Lin, E.; Chen, T.; Little, A.; Holliday, L.; Roach, P.; Butler, P.; Hosking, E.; Bailey, E.; Elison, B.; Currow, D. Safety and outcomes of (177) Lu-DOTATATE for neuroendocrine tumours: Experience in New South Wales, Australia. Intern. Med. J. 2019, 49, 1268–1277. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cai, L.; Xie, Y.; Chen, Y. The efficacy of (177)Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 1533–1543. [Google Scholar] [CrossRef]
- Hamiditabar, M.; Ali, M.; Roys, J.; Wolin, E.M.; O’Dorisio, T.M.; Ranganathan, D.; Tworowska, I.; Strosberg, J.R.; Delpassand, E.S. Peptide Receptor Radionuclide Therapy With 177Lu-Octreotate in Patients with Somatostatin Receptor Expressing Neuroendocrine Tumors: Six Years’ Assessment. Clin. Nucl. Med. 2017, 42, 436–443. [Google Scholar] [CrossRef]
- Ezziddin, S.; Khalaf, F.; Vanezi, M.; Haslerud, T.; Mayer, K.; Al Zreiqat, A.; Willinek, W.; Biersack, H.J.; Sabet, A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 925–933. [Google Scholar] [CrossRef]
- Sabet, A.; Dautzenberg, K.; Haslerud, T.; Aouf, A.; Sabet, A.; Simon, B.; Mayer, K.; Biersack, H.J.; Ezziddin, S. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1238–1246. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Grana, C.M.; Fazio, N.; Iodice, S.; Baio, S.M.; Bartolomei, M.; Lombardo, D.; Ferrari, M.E.; Sansovini, M.; et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: The IEO phase I-II study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2125–2135. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blazevic, A.; Minczeles, N.S.; Feelders, R.A.; de Herder, W.W.; Hofland, J. Peptide Receptor Radionuclide Therapy With 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e3665–e3672. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated with (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Khan, S.; Krenning, E.P.; van Essen, M.; Kam, B.L.; Teunissen, J.J.; Kwekkeboom, D.J. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J. Nucl. Med. 2011, 52, 1361–1368. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blazevic, A.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; de Herder, W.W. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J. Clin. Endocrinol. Metab. 2019, 104, 1336–1344. [Google Scholar] [CrossRef]
- Seregni, E.; Maccauro, M.; Chiesa, C.; Mariani, L.; Pascali, C.; Mazzaferro, V.; De Braud, F.; Buzzoni, R.; Milione, M.; Lorenzoni, A.; et al. Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 223–230. [Google Scholar] [CrossRef]
- de Herder, W.W. Biochemistry of neuroendocrine tumours. Best. Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 33–41. [Google Scholar] [CrossRef]
- Hofland, J.; Herrera-Martinez, A.D.; Zandee, W.T.; de Herder, W.W. Management of carcinoid syndrome: A systematic review and meta-analysis. Endocr.-Relat. Cancer 2019, 26, R145–R156. [Google Scholar] [CrossRef] [PubMed]
- Grozinsky-Glasberg, S.; Davar, J.; Hofland, J.; Dobson, R.; Prasad, V.; Pascher, A.; Denecke, T.; Tesselaar, M.E.T.; Panzuto, F.; Albåge, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2022 Guidance Paper for Carcinoid Syndrome and Carcinoid Heart Disease. J. Neuroendocrinol. 2022, 34, e13146. [Google Scholar] [CrossRef]
- Ramesh, S.; Kudachi, S.; Basu, S. Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE in Carcinoid Heart Disease: A Contraindication or a Promising Treatment Approach Bettering Chances for Corrective Surgery? J. Nucl. Med. Technol. 2018, 46, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Bakker, W.H.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; de Herder, W.W.; Feelders, R.A.; van Eijck, C.H.; de Jong, M.; Srinivasan, A.; et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0),Tyr3]octreotate. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Yalchin, M.; Oliveira, A.; Theocharidou, E.; Pencharz, D.; Navalkissoor, S.; Quigley, A.-M.; Walker, M.; Caplin, M.; Toumpanakis, C. The Impact of Radiological Response to Peptide Receptor Radionuclide Therapy on Overall Survival in Patients with Metastatic Midgut Neuroendocrine Tumors. Clin. Nucl. Med. 2017, 42, e135–e141. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grünwald, F.; Guhlke, S.; Biersack, H.-J.; Sabet, A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Minczeles, N.S.; de Herder, W.W.; Feelders, R.A.; Verburg, F.A.; Hofland, J.; Brabander, T. Long-term outcomes of submaximal activities of peptide receptor radionuclide therapy with (177)Lu-DOTATATE in neuroendocrine tumour patients. J. Nucl. Med. 2022, 62, 11. [Google Scholar]
- Magi, L.; Prosperi, D.; Lamberti, G.; Marasco, M.; Ambrosini, V.; Rinzivillo, M.; Campana, D.; Gentiloni, G.; Annibale, B.; Signore, A.; et al. Role of [(18)F]FDG PET/CT in the management of G1 gastro-entero-pancreatic neuroendocrine tumors. Endocrine 2022, 76, 484–490. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Chan, D.L.; Ulaner, G.A.; Pattison, D.; Wyld, D.; Ladwa, R.; Kirchner, J.; Li, B.T.; Lai, W.V.; Pavlakis, N.; Roach, P.J.; et al. Dual PET Imaging in Bronchial Neuroendocrine Neoplasms: The NETPET Score as a Prognostic Biomarker. J. Nucl. Med. 2021, 62, 1278–1284. [Google Scholar] [CrossRef]
- Arnold, R.; Wilke, A.; Rinke, A.; Mayer, C.; Kann, P.H.; Klose, K.J.; Scherag, A.; Hahmann, M.; Muller, H.H.; Barth, P. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin. Gastroenterol. Hepatol. 2008, 6, 820–827. [Google Scholar] [CrossRef]
- Fross-Baron, K.; Garske-Roman, U.; Welin, S.; Granberg, D.; Eriksson, B.; Khan, T.; Sandstrom, M.; Sundin, A. 177Lu-DOTATATE Therapy of Advanced Pancreatic Neuroendocrine Tumors Heavily Pretreated with Chemotherapy: Analysis of Outcome, Safety, and Their Determinants. Neuroendocrinology 2021, 111, 330–343. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; de Herder, W.W.; Feelders, R.A.; Krenning, E.P.; Kwekkeboom, D.J. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [(177)Lu-DOTA(0),Tyr(3)]octreotate. Endocr.-Relat. Cancer 2017, 24, 243–251. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; Van Der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Cwikla, J.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1155–1169. [Google Scholar] [CrossRef]
- Kidd, M.; Drozdov, I.; Modlin, I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr.-Relat. Cancer 2015, 22, 561–575. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; Zaknun, J.J. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Syguła, A.; Ledwon, A.; Hasse-Lazar, K.; Jurecka-Lubieniecka, B.; Michalik, B.; Paliczka-Cieślik, E.; Zeman, M.; Chmielik, E.; Sczasny, J.; Jarzab, B. In patients with well-differentiated neuroendocrine tumours, there is no apparent benefit of somatostatin analogues after disease control by peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Kim, Y.I. Salvage peptide receptor radionuclide therapy in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Nucl. Med. Commun. 2021, 42, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; de Herder, W.W. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Pusceddu, S.; Prinzi, N.; Tafuto, S.; Ibrahim, T.; Filice, A.; Brizzi, M.P.; Panzuto, F.; Baldari, S.; Grana, C.M.; Campana, D.; et al. Association of Upfront Peptide Receptor Radionuclide Therapy with Progression-Free Survival among Patients with Enteropancreatic Neuroendocrine Tumors. JAMA Netw. Open 2022, 5, e220290. [Google Scholar] [CrossRef]
- Baudin, E.; Walter, T.; Docao, C.; Haissaguerre, M.; Hadoux, J.; Taieb, D.; Ansquer, C.; Dierickx, L.; De Mestier, L.; Deshayes, E.; et al. First multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionuclide therapy with 177Lutetium—Octreotate (OCLU) in unresectable progressive neuroendocrine pancreatic tumor: Results of the OCLURANDOM trial, on behalf of the ENDOCAN RENATEN network and GTE. Ann. Endocrinol. 2022, 83, 289–290. [Google Scholar]
- Merola, E.; Alonso Gordoa, T.; Zhang, P.; Al-Toubah, T.; Pellè, E.; Kolasińska-Ćwikła, A.; Zandee, W.; Laskaratos, F.; Mestier, L.; Lamarca, A. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is ≥ 10%? Oncologist 2021, 26, 294–301. [Google Scholar] [CrossRef]
- Minczeles, N.S.; van Eijck, C.H.J.; van Gils, M.J.; van Velthuysen, M.F.; Nieveen van Dijkum, E.J.M.; Feelders, R.A.; de Herder, W.W.; Brabander, T.; Hofland, J.l. Induction therapy with (177)Lu-DOTATATE procures long-term survival in locally advanced or oligometastatic pancreatic neuroendocrine neoplasm patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3203–3214. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Rubin, D.; Yao, J.C.; Kulke, M.H.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: Final analysis of efficacy and evaluation of MGMT (ECOG-ACRIN E2211). J. Clin. Oncol. 2022, 40, 4004. [Google Scholar] [CrossRef]
- Sorbye, H.; Baudin, E.; Borbath, I.; Caplin, M.; Chen, J.; Cwikla, J.B.; Frilling, A.; Grossman, A.; Kaltsas, G.; Scarpa, A.; et al. Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology 2019, 108, 54–62. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Vélayoudom-Céphise, F.-L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T. Are G3 ENETS neuroendocrine neoplasms heterogeneous. Endocr.-Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Watkins, J.; Marelli, L.; Khan, K.; Caplin, M.E. Expression of Somatostatin and Dopamine 2 Receptors in Neuroendocrine Tumours and the Potential Role for New Biotherapies. Neuroendocrinology 2009, 89, 308–314. [Google Scholar] [CrossRef]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr.-Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- Raj, N.; Valentino, E.; Capanu, M.; Tang, L.H.; Basturk, O.; Untch, B.R.; Allen, P.J.; Klimstra, D.S.; Reidy-Lagunes, D. Treatment Response and Outcomes of Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology: Well Differentiated Versus Poorly Differentiated. Pancreas 2017, 46, 296–301. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr.-Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. (111)In-Pentetreotide Scintigraphy Versus (68)Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019, 60, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Krenning, E.P. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2016, 30, 179–191. [Google Scholar] [CrossRef]
- Minczeles, N.S.; de Herder, W.W.; Konijnenberg, M.W.; Feelders, R.A.; Brabander, T.; Hofland, J. Dose-Limiting Bone Marrow Toxicities after Peptide Receptor Radionuclide Therapy Are More Prevalent in Women Than in Men. Clin. Nucl. Med. 2022, 47, 599–605. [Google Scholar] [CrossRef]
- Brabander, T.; Hofland, H. Radionuclide therapy in the time of COVID-19. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2066–2067. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Singh, A.; Mencia-Trinchant, N.; Griffiths, E.A.; Altahan, A.; Swaminathan, M.; Gupta, M.; Gravina, M.; Tajammal, R.; Faber, M.G.; Yan, L.; et al. Mutant PPM1D- and TP53-Driven Hematopoiesis Populates the Hematopoietic Compartment in Response to Peptide Receptor Radionuclide Therapy. JCO Precis. Oncol. 2022, 6, e2100309. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Samarghandi, A.; Zamanian, S.; Wolin, E.M.; Hamiditabar, M.; Espenan, G.D.; Erion, J.L.; O’Dorisio, T.M.; Kvols, L.K.; Simon, J.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: The first US phase 2 experience. Pancreas 2014, 43, 518–525. [Google Scholar] [CrossRef]
- Bergsma, H.; Konijnenberg, M.W.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; de Herder, W.W.; Franssen, G.J.; van Eijck, C.H.; Krenning, E.P.; Kwekkeboom, D.J. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: Prognostic factors, incidence and course. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 453–463. [Google Scholar] [CrossRef]
- Geenen, L.; Nonnekens, J.; Konijnenberg, M.; Baatout, S.; De Jong, M.; Aerts, A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [(177)Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl. Med. Biol. 2021, 102, 1–11. [Google Scholar] [CrossRef]
- Rolleman, E.J.; Valkema, R.; de Jong, M.; Kooij, P.P.; Krenning, E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef]
- De Jong, M.; Krenning, E. New advances in peptide receptor radionuclide therapy. J. Nucl. Med. 2002, 43, 617–620. [Google Scholar]
- Moll, S.; Nickeleit, V.; Mueller-Brand, J.; Brunner, F.P.; Maecke, H.R.; Mihatsch, M.J. A new cause of renal thrombotic microangiopathy: Yttrium 90-DOTATOC internal radiotherapy. Am. J. Kidney Dis. 2001, 37, 847–851. [Google Scholar] [CrossRef]
- Bergsma, H.; Konijnenberg, M.W.; van der Zwan, W.A.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; Mauff, K.A.; Krenning, E.P.; Kwekkeboom, D.J. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1802–1811. [Google Scholar] [CrossRef]
- Stolniceanu, C.R.; Nistor, I.; Bilha, S.C.; Constantin, V.; Simona, V.; Matovic, M.; Stefanescu, C.; Covic, A. Nephrotoxicity/renal failure after therapy with 90Yttrium- and 177Lutetium-radiolabeled somatostatin analogs in different types of neuroendocrine tumors: A systematic review. Nucl. Med. Commun. 2020, 41, 601–617. [Google Scholar] [CrossRef]
- Daskalakis, K.; Karakatsanis, A.; Stålberg, P.; Norlén, O.; Hellman, P. Clinical signs of fibrosis in small intestinal neuroendocrine tumours. J. Br. Surg. 2017, 104, 69–75. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J. (Eds.) Gallium-68 EDTA PET/CT for Renal Imaging. Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Van Binnebeek, S.; Baete, K.; Vanbilloen, B.; Terwinghe, C.; Koole, M.; Mottaghy, F.M.; Clement, P.M.; Mortelmans, L.; Haustermans, K.; Van Cutsem, E.; et al. Individualized dosimetry-based activity reduction of 90Y-DOTATOC prevents severe and rapid kidney function deterioration from peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1141–1157. [Google Scholar] [CrossRef][Green Version]
- Krajewski, W.; Wojciechowska, J.; Dembowski, J.; Zdrojowy, R.; Szydełko, T. Hydronephrosis in the course of ureteropelvic junction obstruction: An underestimated problem? Current opinions on the pathogenesis, diagnosis and treatment. Adv. Clin. Exp. Med. 2017, 26, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.L.; Nijsen, J.F.; van den Bosch, M.A.; Lam, M.G.; Vente, M.A.; Mali, W.P.; van Het Schip, A.D.; Zonnenberg, B.A. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): A phase 1, dose-escalation study. Lancet Oncol. 2012, 13, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases after Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Intervent. Radiol. 2020, 43, 246–253. [Google Scholar]
- Ezziddin, S.; Meyer, C.; Kahancova, S.; Haslerud, T.; Willinek, W.; Wilhelm, K.; Biersack, H.-J.; Ahmadzadehfar, H. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J. Nucl. Med. 2012, 53, 1663–1669. [Google Scholar] [CrossRef]
- De Keizer, B.; van Aken, M.O.; Feelders, R.A.; de Herder, W.W.; Kam, B.L.; van Essen, M.; Krenning, E.P.; Kwekkeboom, D.J. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 749–755. [Google Scholar] [CrossRef]
- Del Olmo-García, M.I.; Muros, M.A.; López-de-la-Torre, M.; Agudelo, M.; Bello, P.; Soriano, J.M.; Merino-Torres, J.F. Prevention and Management of Hormonal Crisis during Theragnosis with LU-DOTA-TATE in Neuroendocrine Tumors. A Systematic Review and Approach Proposal. J. Clin. Med. 2020, 9, 2203. [Google Scholar] [CrossRef]
- Hlatky, R.; Suki, D.; Sawaya, R. Carcinoid metastasis to the brain. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 101, 2605–2613. [Google Scholar] [CrossRef]
- Pavel, M.; Grossman, A.; Arnold, R.; Perren, A.; Kaltsas, G.; Steinmüller, T.; De Herder, W.; Nikou, G.; Plöckinger, U.; Lopes, J.M. ENETS consensus guidelines for the management of brain, cardiac and ovarian metastases from neuroendocrine tumors. Neuroendocrinology 2010, 91, 326–332. [Google Scholar] [CrossRef]
- Scharf, M.; Petry, V.; Daniel, H.; Rinke, A.; Gress, T.M. Bone metastases in patients with neuroendocrine neoplasm: Frequency and clinical, therapeutic, and prognostic relevance. Neuroendocrinology 2018, 106, 30–37. [Google Scholar] [CrossRef]
- Cecchin, D.; Schiavi, F.; Fanti, S.; Favero, M.; Manara, R.; Fassina, A.; Briani, C.; Allegri, V.; Sansovini, M.; Bui, F.; et al. Peptide receptor radionuclide therapy in a case of multiple spinal canal and cranial paragangliomas. J. Clin. Oncol. 2011, 29, e171–e174. [Google Scholar] [CrossRef]
- De Bruin, C.; Hofland, L.J.; Nieman, L.K.; van Koetsveld, P.M.; Waaijers, A.M.; Sprij-Mooij, D.M.; van Essen, M.; Lamberts, S.W.; de Herder, W.W.; Feelders, R.A. Mifepristone effects on tumor somatostatin receptor expression in two patients with Cushing’s syndrome due to ectopic adrenocorticotropin secretion. J. Clin. Endocrinol. Metab. 2012, 97, 455–462. [Google Scholar] [CrossRef]
- Blažević, A.; Hofland, J.; Hofland, L.J.; Feelders, R.A.; de Herder, W.W. Small intestinal neuroendocrine tumours and fibrosis: An entangled conundrum. Endocr.-Relat. Cancer 2018, 25, R115–R130. [Google Scholar] [CrossRef]
- Merola, E.; Prasad, V.; Pascher, A.; Pape, U.-F.; Arsenic, R.; Denecke, T.; Fehrenbach, U.; Wiedenmann, B.; Pavel, M.E. Peritoneal carcinomatosis in gastro-entero-pancreatic neuroendocrine neoplasms: Clinical impact and effectiveness of the available therapeutic options. Neuroendocrinology 2020, 110, 517–524. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Al-Toubah, T.; Pellè, E.; Smith, J.; Haider, M.; Hutchinson, T.; Fleming, J.B.; El-Haddad, G. Risk of Bowel Obstruction in Patients with Mesenteric or Peritoneal Disease Receiving Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2021, 62, 69–72. [Google Scholar] [CrossRef]
- Paganelli, G.; Sansovini, M.; Ambrosetti, A.; Severi, S.; Monti, M.; Scarpi, E.; Donati, C.; Ianniello, A.; Matteucci, F.; Amadori, D. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: Results from a phase II study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1845–1851. [Google Scholar] [CrossRef]
- Blažević, A.; Brabander, T.; Zandee, W.T.; Hofland, J.; Franssen, G.J.H.; van Velthuysen, M.-L.F.; Feelders, R.A.; De Herder, W.W. Evolution of the mesenteric mass in small intestinal neuroendocrine tumours. Cancers 2021, 13, 443. [Google Scholar] [CrossRef]
- Nonnekens, J.; van Kranenburg, M.; Beerens, C.E.; Suker, M.; Doukas, M.; van Eijck, C.H.; de Jong, M.; van Gent, D.C. Potentiation of Peptide Receptor Radionuclide Therapy by the PARP Inhibitor Olaparib. Theranostics 2016, 6, 1821–1832. [Google Scholar] [CrossRef]
- Hofving, T.; Sandblom, V.; Arvidsson, Y.; Shubbar, E.; Altiparmak, G.; Swanpalmer, J.; Almobarak, B.; Elf, A.-K.; Johanson, V.; Elias, E. 177Lu-octreotate therapy for neuroendocrine tumours is enhanced by Hsp90 inhibition. Endocr.-Relat. Cancer 2019, 26, 437–449. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of 212Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model212Pb-DOTAMTATE for Treatment of Neuroendocrine Tumors. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
| Patients, n | NET Subtype | PFS (Months) | mOS (Months) | ORR | SD | PD | mTTD QOL Global Health (Months) | Overall Symptom Improvement | |
|---|---|---|---|---|---|---|---|---|---|
| Strosberg et al. [27,30,38] | 101 | SI-NET | 28 | 48 | 18% | 66% * | 16% | 29 m | |
| Control group SSA | 100 | SI-NET | 8.5 | 36 | 3% | 41% | 56% | 6.1 m | |
| Brabander et al. [29] | 443 | GEP and bronchial NET | 29 | 63 | 39% | 43% | 12% | ||
| Bodei et al. [36] | 51 | GEP and bronchial NET | 36 | 68% at 36 months | 55% | 27% | 18% | ||
| Ezziddin et al. [34] | 68 | GEP-NET | 34 | 53 | 72% | 13% | 15% | ||
| Sabet et al. [35] | 61 | SI-NET | 44% | 48% | 8% | ||||
| Hamiditabar et al. [33] | 143 | NET of all origins | 8% | 46% | 38% | 47% | |||
| Khan et al. [39] | 265 | GEP and bronchial NET | 38–90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becx, M.N.; Minczeles, N.S.; Brabander, T.; de Herder, W.W.; Nonnekens, J.; Hofland, J. A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients. Cancers 2022, 14, 5792. https://doi.org/10.3390/cancers14235792
Becx MN, Minczeles NS, Brabander T, de Herder WW, Nonnekens J, Hofland J. A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients. Cancers. 2022; 14(23):5792. https://doi.org/10.3390/cancers14235792
Chicago/Turabian StyleBecx, Morticia N., Noémie S. Minczeles, Tessa Brabander, Wouter W. de Herder, Julie Nonnekens, and Johannes Hofland. 2022. "A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients" Cancers 14, no. 23: 5792. https://doi.org/10.3390/cancers14235792
APA StyleBecx, M. N., Minczeles, N. S., Brabander, T., de Herder, W. W., Nonnekens, J., & Hofland, J. (2022). A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients. Cancers, 14(23), 5792. https://doi.org/10.3390/cancers14235792





