Changes in Treatment Patterns and Costs for Lung Cancer Have Not Resulted in Relevant Improvements in Survival: A Population-Based Observational Study in Catalonia
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
- -
- The hospital discharge minimum basic data set, which collects information related to acute hospital care, including surgery;
- -
- The hospital outpatient drugs registry, which contains clinical information on drugs prescribed from different therapeutic areas (not including chemotherapy treatments);
- -
- The Catalan Health Surveillance System, which collects data on health services, including radiotherapy;
- -
- The Datamart Billing Service, which collects all of the specific billing data for hospital outpatient drugs (including chemotherapy treatments);
- -
- The Central Registry of Insured Persons, which collects basic demographic data of insured people covered by CatSalut.
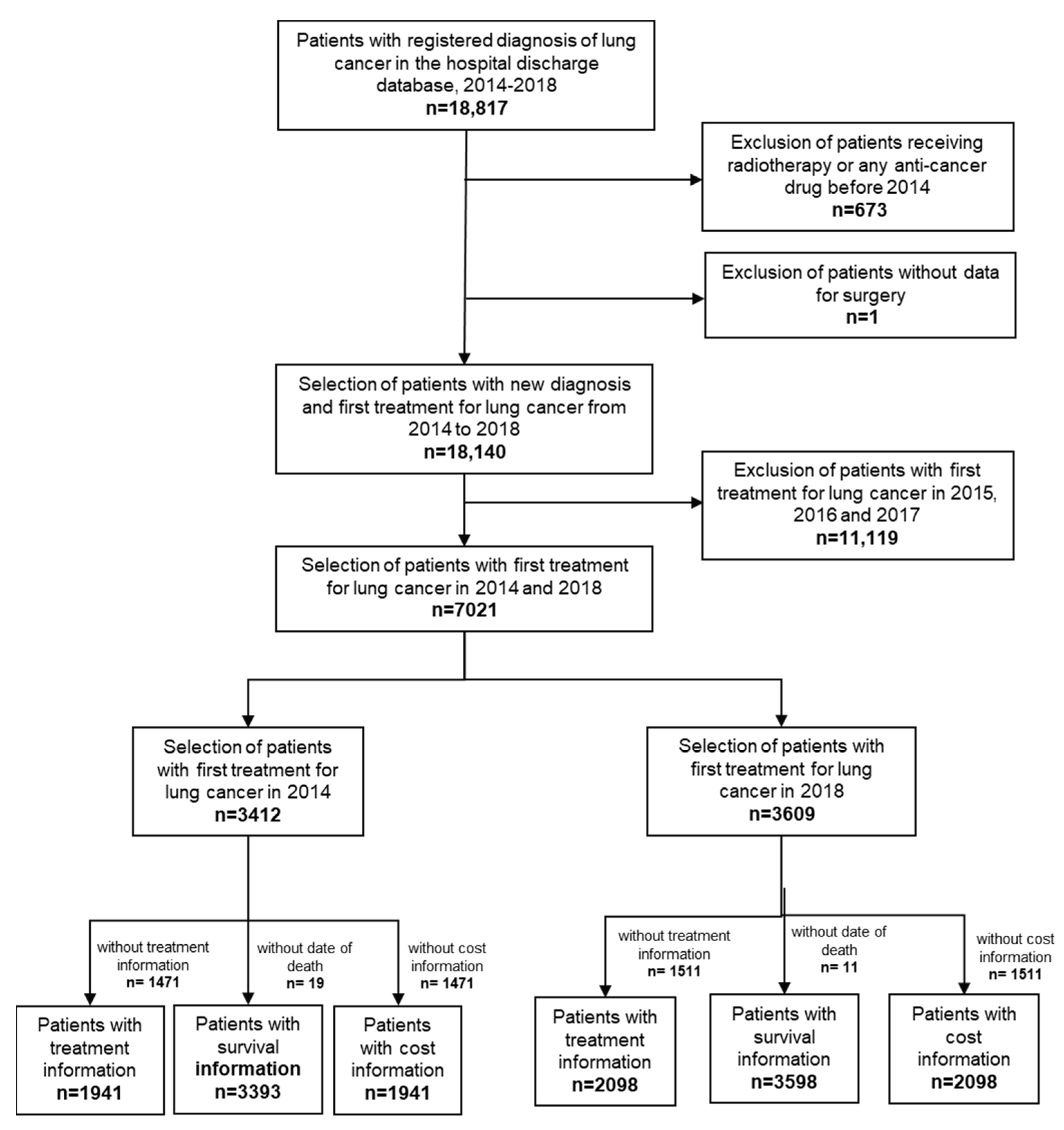
2.2. Study Population
2.3. Outcomes
- -
- NACT ± RT: neoadjuvant treatment, with or without complementary radiotherapy, administered prior to surgery with a curative intent.
- -
- ACT ± RT: adjuvant treatment, with or without complementary radiotherapy, initiated for a maximum of 10 weeks after surgery with curative intent [35].
- -
- IND: induction therapy, started prior to the first session of radiotherapy with curative intent.
- -
- CONCO: concomitant treatment, initiated within four weeks of the last session of radiotherapy with curative intent [36].
- -
- SEQ: sequential therapy, started at least four weeks after the last session of radiotherapy with curative intent [36].
- -
- Treatment for recurrent tumor: pharmacological treatment initiated more than 10 weeks after surgery with curative intent; after adjuvant treatment with a different therapeutic regimen; or from 12 weeks after completion of radiotherapy with curative intent [37] or adjuvant treatment [35] (with the same or different therapeutic regimen). Variations in pharmacological treatment that involved changes in active substances were classified as a subsequent line of treatment (second, third and so on).
- -
- Treatments for unresectable tumor: pharmacological treatment in patients that did not receive surgery or radiotherapy with curative intent. Variations in pharmacological treatment that involved changes in active ingredients were classified as a subsequent line of treatment (second, third and so on).
- -
- Patients who did not receive any of the therapeutic approaches above were considered as patients without systemic therapy.
- -
- Surgery: patients receiving surgery with curative intent, with or without complementary preoperative (NACT) and/or postoperative (ACT) pharmacological treatments, complementary preoperative, postoperative or radiotherapy (RT) alone. This category can also include the different lines of treatment for tumor recurrence.
- -
- Radiotherapy: patients receiving radiotherapy with curative intent, with or without pharmacological treatments (IND, CONCO, SEQ). This category can also include different lines of treatment for recurrent tumors.
- -
- Unresectable tumors: patients whose records show only pharmacological treatments with a palliative intent for inoperable disease.
- -
- Without systemic therapy: patients with no record of any of the previous treatments.
2.4. Statistical Analysis
2.5. Ethics
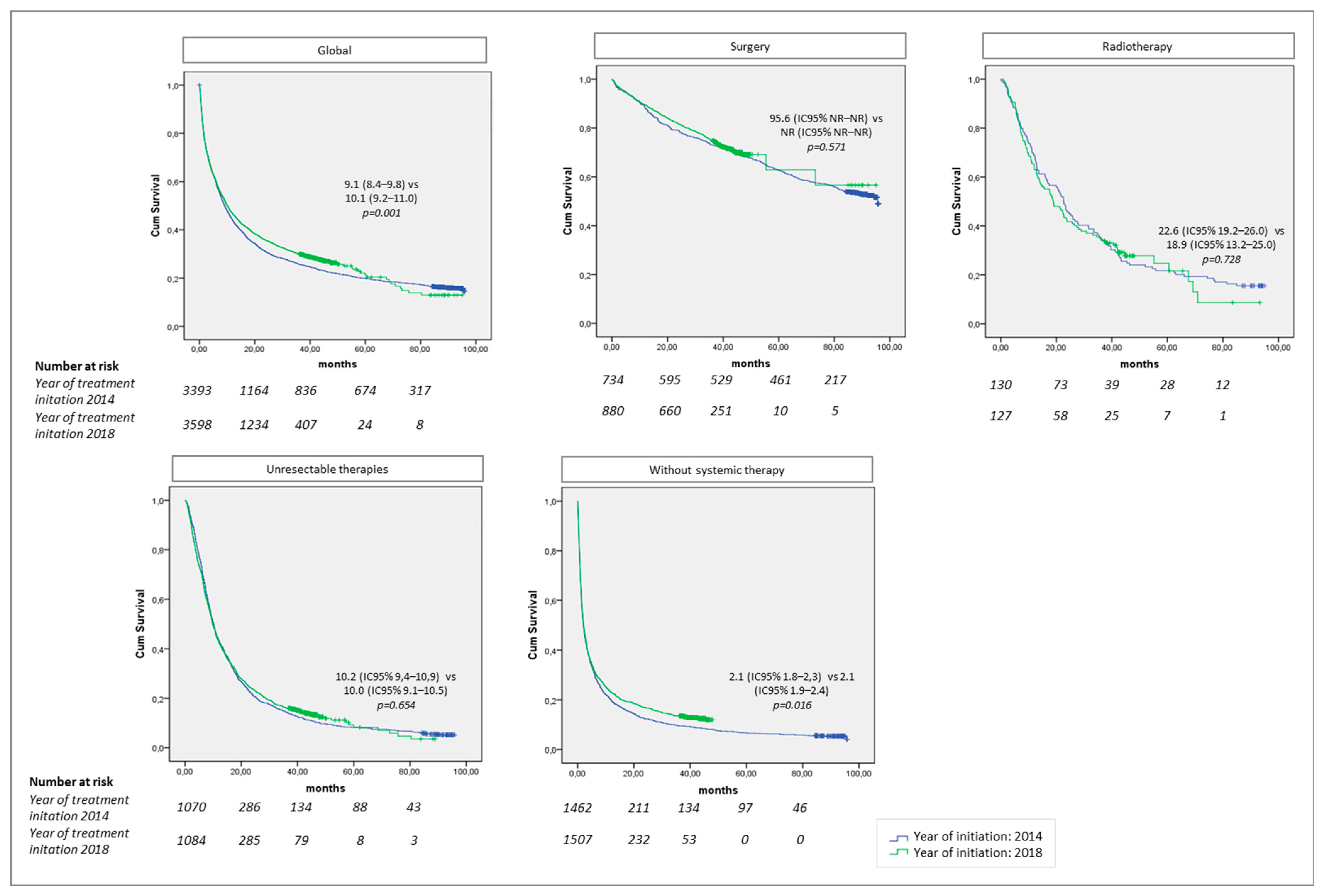
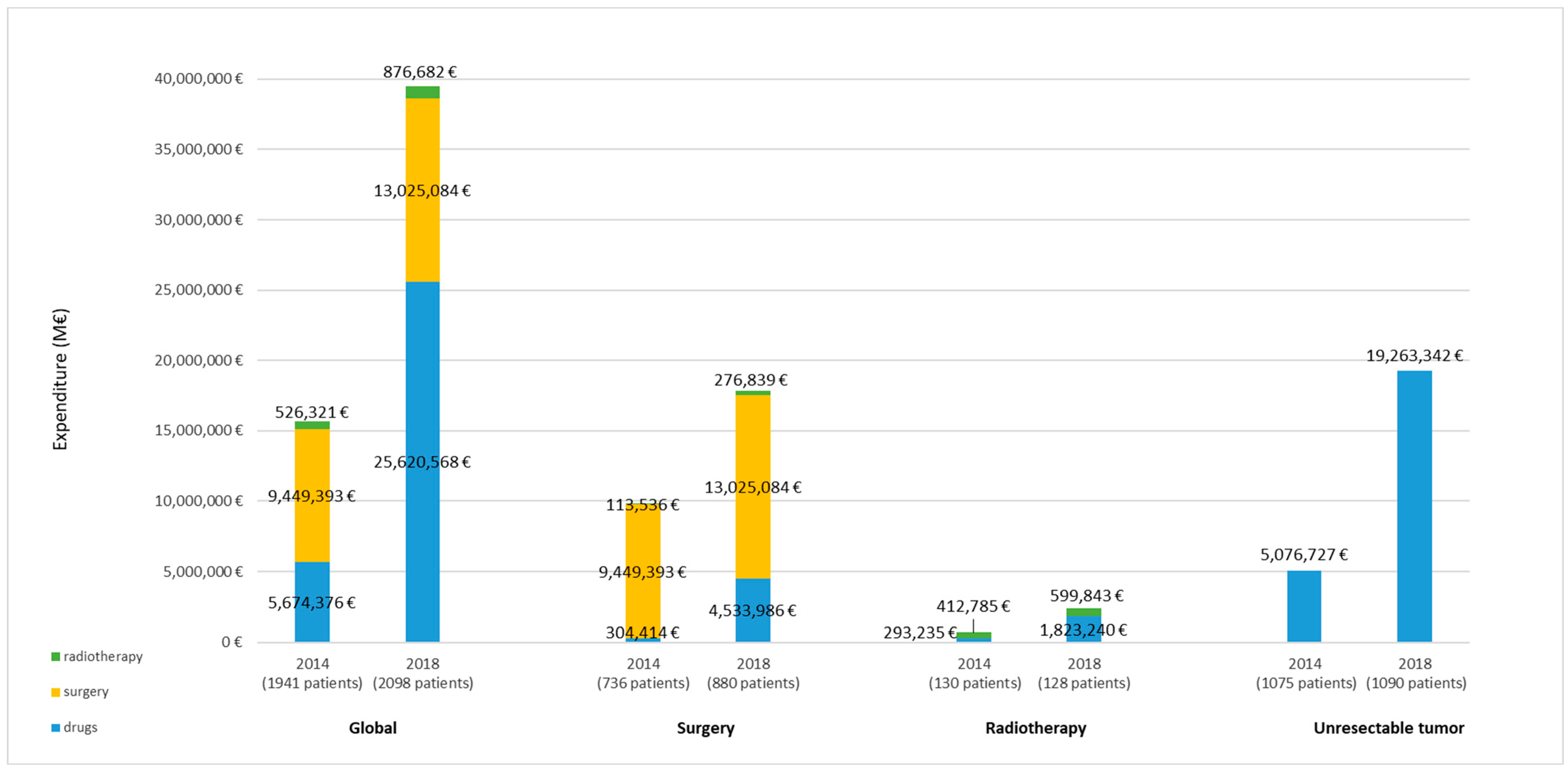
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECIS-European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-AEE$2-All$4-1,2$3-All$6-0,85$5-2008,2008$7-8$CEstByCancer$X0_8-3$CEstRelativeCanc$X1_8-3$X1_9-AE27$CEstBySexByCancer$X2_8-3$X2_-1-1 (accessed on 2 February 2022).
- Corral, J.; Espinàs, J.A.; Cots, F.; Pareja, L.; Solà, J.; Font, R.; Borràs, J.M. Estimation of lung cancer diagnosis and treatment costs based on a patient-level analysis in Catalonia (Spain). BMC Health Serv. Res. 2015, 15, 70. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Oncología Médica. Las Cifras Del Cáncer En España. 2021. Available online: https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf (accessed on 3 May 2022).
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef]
- Van Harten, W.H.; Wind, A.; De Paoli, P.; Saghatchian, M.; Oberst, S. Actual costs of cancer drugs in 15 European countries. Lancet Oncol. 2016, 17, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Sacristan, J.A.; Dilla, T. The Economic Burden of Cancer in Spain: A Literature Review. Health Econ. Outcome Res Open Access 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, 1–21. [Google Scholar] [CrossRef]
- Dingemans, A.M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.T.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 192–237. [Google Scholar] [CrossRef]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W.; World Health Organization. World Cancer Report. In Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; pp. 1–613. [Google Scholar]
- BIFIMED: Buscador de la Información Sobre la Situación de Financiación de los Medicamentos. Available online: https://www.mscbs.gob.es/profesionales/medicamentos.do (accessed on 5 May 2022).
- Garrido, P.; Conde, E.; De Castro, J.; Gómez-Román, J.J.; Felip, E.; Pijuan, L.; Isla, D.; Sanz, J.; Paz-Ares, L.; López-Ríos, F. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 989–1003. [Google Scholar] [CrossRef]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 expression in lung cancer. J. Thorac. Oncol. 2015, 11, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Pavan, A.; Attili, I.; Bortolami, A.; Bonanno, L.; Menis, J.; Conte, P.; Guarneri, V. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020, 87, 102031. [Google Scholar] [CrossRef] [PubMed]
- Hammers, E.; Kahn, B.; Wagner, A.; Shore, C. Examining the Impact of Real-World Evidence on Medical Product Development; National Academies Press: Washington, DC, USA, 2019; pp. 1–231. [Google Scholar]
- Hilal, T.; Gonzalez-Velez, M.; Prasad, V. Limitations in clinical trials leading to anticancer drug approvals by the US food and drug administration. JAMA Intern. Med. 2020, 180, 1108–1115. [Google Scholar] [CrossRef]
- World Health Organization. Pricing of Cancer Medicines and Its Impacts; World Health Organization (WHO): Geneva, Switzerland, 2018; pp. 1–173. [Google Scholar]
- Garrison, L.P.; Neumann, P.J.; Erickson, P.; Marshall, D.; Mullins, C.D. Using real-world data for coverage and payment decisions: The ISPOR real-world data Task Force report. Value Health 2007, 10, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Prasad, V. Are Observational, Real-World Studies Suitable to Make Cancer Treatment Recommendations? JAMA Netw Open 2020, 3, 2012119. [Google Scholar] [CrossRef]
- Schwarzkopf, L.; Wacker, M.; Holle, R.; Leidl, R.; Günster, C.; Adler, J.B.; Huber, R.M. Cost-components of lung cancer care within the first three years after initial diagnosis in context of different treatment regimens. Lung Cancer 2015, 90, 274–280. [Google Scholar] [CrossRef]
- Helminen, O.; Valo, J.; Andersen, H.; Lautamäki, A.; Vuohelainen, V.; Sihvo, E. Real-world guideline-based treatment of lung cancer improves short- and long-term outcomes and resection rate: A population-based study. Lung Cancer 2020, 140, 1–7. [Google Scholar] [CrossRef]
- Morita, R.; Okishio, K.; Shimizu, J.; Saito, H.; Sakai, H.; Kim, Y.H.; Hataji, O.; Yomota, M.; Nishio, M.; Aoe, K.; et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: A multicenter retrospective observational study in Japan. Lung Cancer 2020, 140, 8–18. [Google Scholar] [CrossRef]
- Levra, M.G.; Cotté, F.E.; Corre, R.; Calvet, C.; Gaudin, A.F.; Penrod, J.R.; Grumberg, V.; Jouaneton, B.; Jolivel, R.; Assié, J.B.; et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer 2020, 140, 99–106. [Google Scholar] [CrossRef]
- Jung, H.A.; Noh, J.M.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Pyo, H.; Ahn, Y.C.; Park, K. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020, 146, 23–29. [Google Scholar] [CrossRef]
- Yao, S.; Wang, R.; Qian, K.; Zhang, Y. Real world study for the concordance between IBM Watson for Oncology and clinical practice in advanced non-small cell lung cancer patients at a lung cancer center in China. Thorac Cancer 2020, 11, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Simeone, J.C.; Nordstrom, B.L.; Patel, K.; Klein, A.B. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol. 2019, 15, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Registre Central de Població del CatSalut. Available online: https://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/central-poblacio/ (accessed on 1 December 2020).
- Generalitat de Catalunya. Departament de Salut. Tecnologies de la Informació i Portal. Available online: https://catsalut.gencat.cat/ca/proveidors-professionals/portal-aplicacions/ (accessed on 1 December 2020).
- Generalitat de Catalunya. Departament de Salut. Registres i Catàlegs. Available online: https://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/ (accessed on 1 December 2020).
- Departament de Salut. Generalitat de Catalunya. CIM-10-MC/SCP. Classificació Internacional de Malalties. 10a Revisió. Modificació Clínica. Available online: http://www.who.int/classifications/icd/en/ (accessed on 1 December 2020).
- Who Health Organization (WHO). Collaborating Centre for Drug Statistics Methodology. Structure and Principles. Available online: www.whocc.no/atc/structure_and_principles/ (accessed on 1 October 2022).
- ICOPraxis Group. ICOPRAXIS Para el Tratamiento Médico del Cáncer de Pulmón de Célula no Pequeña, 2nd ed.; Institut Català d’Oncologia: Barcelona, Spain, 2016; pp. 1–125. [Google Scholar]
- Fournel, P.; Robinet, G.; Thomas, P.; Souquet, P.J.; Léna, H.; Vergnenégre, A.; Delhoume, J.Y.; Le Treut, J.; Silvani, J.A.; Dansin, E.; et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005, 23, 5910–5917. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. NCCN Clinical Practice Guidelines in Oncology. Versión 3.2020. Available online: https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf (accessed on 15 December 2021).
- Thakur, S.K.; Singh, D.P.; Choudhary, J. Lung cancer identification: A review on detection and classification. Cancer Metastasis Rev. 2020, 39, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Li, R.; Kim, J.; Salgia, R.; Hurria, A.; Amini, A. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin. Oncol. 2018, 45, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Soerjomataram, I.; Ferlay, J.; Rutherford, M.; Weiderpass, E.; Bray, F. International trend in lung cancer by histological subtype: Adenocarcinoma stabiliszing in men but still increasing in women. Lung Cancer 2014, 84, 13–22. [Google Scholar] [CrossRef]
- Curado, M.P.; S, B.E.H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. Cancer incidence in five continents.Volum IX. Chem. Eng. J. 2007, 160, 518–525. [Google Scholar]
- Guarga, L.; Ameijide, A.; Marcos-Gragera, R.; Carulla, M.; Delgadillo, J.; Borràs, J.M.; Galceran, J. Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain). Sci. Rep. 2021, 11, 23274. [Google Scholar] [CrossRef] [PubMed]
- Programa d’Harmonització Farmacoterapèutica. Pautes per a l’harmonització Del Tractament Farmacoterapèutic Del Càncer de Pulmó No Microcític Metastàtic. Versió 2.0; Servei Català de la Salut: Barcelona, Spain, 2022; pp. 1–107.
- Carroll, R.; Bortolini, M.; Calleja, A.; Munro, R.; Kong, S.; Daumont, M.J.; Penrod, J.R.; Lakhdari, K.; Lacoin, L.; Cheung, W.Y. Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: A Canadian population-based real-world analysis. BMC Cancer 2022, 22, 255. [Google Scholar] [CrossRef]
- Raphael, J.; Richard, L.; Lam, M.; Blanchette, P.S.; Leighl, N.B.; Rodrigues, G.; Trudeau, M.E.; Krzyzanowska, M.K. Utilization of Immunotherapy in Patients with Cancer Treated in Routine Care Settings: A Population-Based Study Using Health Administrative Data. Oncologist 2022, 27, 675–684. [Google Scholar] [CrossRef] [PubMed]
- European Society for Medical Oncology (ESMO); International Society for Geriatric Oncology (SIOG). ESMO Handbook of Cancer in the Senior Patient, 2nd ed.; European Society for Medical Oncology: Lugano, Switzerland, 2015; pp. 1–167. [Google Scholar]
- Haanen, J.B.A.G.; Califano, R.; Lugowska, I.; Chiara, M. ESMO Handbook of Immuno-Oncology, 1st ed.; European Society for Medical Oncology: Lugano, Switzerland, 2018; pp. 1–389. [Google Scholar]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; Saldaña, J.; Linares, J.; Ruffinelli, J.C.; Palmero, R.; Navarro, A.; Arnaiz, M.D.; Brao, I.; Aso, S.; Padrones, S.; et al. Geriatric assessment may help decision-making in elderly patients with inoperable, locally advanced non-small-cell lung cancer. Br. J. Cancer 2018, 118, 639–647. [Google Scholar] [CrossRef]
- Guzman, R.; Guirao, À.; Vela, E.; Clèries, M.; García-Altés, A.; Sagarra, J.; Magem, D.; Espinas, J.A.; Grau, J.; Nadal, C.; et al. Outcomes and cost of lung cancer patients treated surgically or medically in Catalunya: Cost-benefit implications for lung cancer screening programs. Eur. J. Cancer Prev. 2020, 29, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Modificació de la Instrucció 04/2018, Reordenació de L’atenció Oncològica D’alta Especialització. CatSalut. Available online: https://scientiasalut.gencat.cat/bitstream/handle/11351/1323.3/catsalut_instruccio_01_2019.pdf?sequence=8&isAllowed=y (accessed on 8 June 2022).
- Manchon-walsh, P.; Aliste, L.; Espin, J.A. Improving survival and local control in rectal cancer in Catalonia (Spain) in the context of centralisation: A full cycle audit assessment. Eur. J. Surg. Oncol. 2016, 42, 1873–1880. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Passaro, A.; Bestvina, C.; Velez Velez, M.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J. Immunother. Cancer 2021, 9, e002266. [Google Scholar] [CrossRef]
- Gouliaev, A.; Risikesan, J.; Christensen, N.L.; Rasmussen, T.R.; Hilberg, O.; Ibsen, R.; Løkke, A. Direct and indirect economic burden of lung cancer in Denmark a nationwide study. Eur. Clin. Respir. J. 2021, 8, 1951963. [Google Scholar] [CrossRef] [PubMed]
- Migliorino, M.R.; Santo, A.; Romano, G.; Cortinovis, D.; Galetta, D.; Alabiso, O.; Cartenì, G.; Vari, S.; Fasola, G.; Pazzola, A.; et al. Economic burden of patients affected by non-small cell lung cancer (NSCLC): The LIFE study. J. Cancer Res. Clin. Oncol. 2017, 143, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, N.; Bongers, M.L.; Coupé, V.M.H.; Smit, E.F.; Groen, H.J.M.; Welling, A.; Schramel, F.M.N.H.; Uyl-de Groot, C.A. Costs of non-small cell lung cancer in the Netherlands. Lung Cancer 2016, 91, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Verleger, K.; Penrod, J.R.; Daumont, M.M.; Solem, C.; Luo, L.; Macahilig, C.; Hertel, N. Costs and Cost Drivers Associated with Non-Small-Cell Lung Cancer Patients Who Received Two or More Lines of Therapy in Europe. Clin. Outcomes Res. 2020, 12, 23–33. [Google Scholar] [CrossRef] [PubMed]
| Year of Treatment Initiation 2014 (n = 3412) | Year of Treatment Initiation 2018 (n = 3609) | ||||
|---|---|---|---|---|---|
| n | % | n | % | p Value * | |
| Sex | 0.013 | ||||
| Male | 2702 | 79% | 2769 | 77% | |
| Female | 710 | 21% | 840 | 23% | |
| Age groups | 0.000 | ||||
| <60 years | 805 | 24% | 937 | 26% | |
| 60–69 years | 1053 | 31% | 1202 | 33% | |
| 70–79 years | 1035 | 30% | 1039 | 29% | |
| ≥80 years | 519 | 15% | 431 | 12% | |
| 2014 (n = 3412) | 2018 (n = 3609) | |||||
|---|---|---|---|---|---|---|
| Age (Years) | Treatment Patterns | N | % | n | % | p Value * |
| Global | Surgery † | 736 | 21.6% | 880 | 24.4% | 0.047 |
| Radiotherapy ‡ | 130 | 3.8% | 128 | 3.5% | ||
| Unresectable tumor ¶ | 1075 | 31.5% | 1090 | 30.2% | ||
| Without systemic therapy § | 1471 | 43.1% | 1511 | 41.90% | ||
| <60 | Surgery † | 181 | 22.5% | 248 | 26.5% | 0.039 |
| Radiotherapy ‡ | 27 | 3.4% | 35 | 3.7% | ||
| Unresectable tumor ¶ | 371 | 46.1% | 369 | 39.4% | ||
| Without systemic therapy § | 226 | 28.1% | 285 | 30.4% | ||
| 60–69 | Surgery † | 281 | 26.7% | 350 | 29.1% | 0.203 |
| Radiotherapy ‡ | 44 | 4.2% | 39 | 3.2% | ||
| Unresectable tumor ¶ | 382 | 36.3% | 398 | 33.1% | ||
| Without systemic therapy § | 346 | 32.9% | 415 | 34.5% | ||
| 70–79 | Surgery † | 241 | 23.3% | 251 | 24.2% | 0.841 |
| Radiotherapy ‡ | 44 | 4.3% | 49 | 4.7% | ||
| Unresectable tumor ¶ | 269 | 26.0% | 275 | 26.5% | ||
| Without systemic therapy § | 481 | 46.5% | 464 | 44.7% | ||
| ≥80 | Surgery † | 33 | 6.4% | 31 | 7.2% | 0.286 |
| Radiotherapy ‡ | 15 | 2.9% | 5 | 1.2% | ||
| Unresectable tumor ¶ | 53 | 10.2% | 48 | 11.1% | ||
| Without systemic therapy § | 418 | 80.5% | 347 | 80.5% | ||
| 2014 (n = 1358) | 2018 (n = 1347) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Surgery | (n = 207) | (n = 194) | ||
| Pre-surgery and neoadjuvant therapies | ||||
| Platinum + pyrimidine analogues | 22 | 43% | 18 | 39% |
| Platinum + taxane | 4 | 8% | 9 | 19% |
| Platinum | 13 | 25% | 5 | 11% |
| Pyrimidine analogues | 6 | 12% | 4 | 9% |
| Other regimens * | 6 | 12% | 10 | 22% |
| Post-surgery and adjuvant therapies | ||||
| Platinum + pyrimidine analogs | 109 | 64% | 130 | 71% |
| Pyrimidine analogues | 37 | 22% | 8 | 4% |
| Platinum + antifolate | 4 | 2% | 17 | 9% |
| Platinum | 10 | 6% | 6 | 3% |
| Other regimens * | 11 | 6% | 24 | 13% |
| Radiotherapy | (n = 76) | (n = 63) | ||
| Pre-radiotherapy and induction therapies | ||||
| Platinum | 33 | 44% | 5 | 8% |
| Vinca alkaloids | 14 | 18% | 8 | 13% |
| Platinum + vinca alkaloids | 7 | 9% | 17 | 27% |
| Taxane | 8 | 11% | 4 | 6% |
| Other regimens * | 14 | 18% | 29 | 46% |
| Post-radiotherapy and sequential therapies | ||||
| Taxane | 1 | 100% | 1 | 100% |
| First-line treatment for unresectable tumors | (n = 1075) | (n = 1090) | ||
| Total chemotherapy | 974 | 91% | 893 | 82% |
| Antifolate + platinum | 181 | 17% | 211 | 19% |
| Platinum | 153 | 14% | 64 | 6% |
| Antifolate | 140 | 13% | 42 | 4% |
| Pyrimidine analogs | 142 | 13% | 54 | 5% |
| Platinum + pyrimidine analogs | 132 | 12% | 149 | 14% |
| Taxane | 123 | 11% | 30 | 3% |
| Platinum + podophyllotoxin derivative | 1 | 0% | 186 | 17% |
| Other chemotherapy regimens * | 102 | 9% | 157 | 14% |
| Immune checkpoint inhibitor | 0 | 0% | 98 | 9% |
| EGFR-TKI | 76 | 7% | 64 | 6% |
| ALK/ROS1-TKI | 5 | 0% | 13 | 1% |
| Other non-chemotherapy regimens * | 20 | 2% | 22 | 2% |
| 2014 | 2018 | ||||||
|---|---|---|---|---|---|---|---|
| (n = 1941) | (n = 2098) | ||||||
| Treatment Pattern | n | Mean | SD | n | Mean | SD | p Value ± |
| Surgery † | 736 | 14,123 | 4327 | 880 | 14,550 | 3880 | 0.003 |
| Radiotherapy ‡ | 130 | 4655 | 3540 | 128 | 5873 | 6455 | 0.051 |
| Unresectable tumor ¶ | 1075 | 4723 | 7003 | 1090 | 6458 | 10116 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarga, L.; Paco, N.; Vela, E.; Clèries, M.; Corral, J.; Delgadillo, J.; Pontes, C.; Borràs, J.M. Changes in Treatment Patterns and Costs for Lung Cancer Have Not Resulted in Relevant Improvements in Survival: A Population-Based Observational Study in Catalonia. Cancers 2022, 14, 5791. https://doi.org/10.3390/cancers14235791
Guarga L, Paco N, Vela E, Clèries M, Corral J, Delgadillo J, Pontes C, Borràs JM. Changes in Treatment Patterns and Costs for Lung Cancer Have Not Resulted in Relevant Improvements in Survival: A Population-Based Observational Study in Catalonia. Cancers. 2022; 14(23):5791. https://doi.org/10.3390/cancers14235791
Chicago/Turabian StyleGuarga, Laura, Noelia Paco, Emili Vela, Montse Clèries, Julieta Corral, Joaquim Delgadillo, Caridad Pontes, and Josep Maria Borràs. 2022. "Changes in Treatment Patterns and Costs for Lung Cancer Have Not Resulted in Relevant Improvements in Survival: A Population-Based Observational Study in Catalonia" Cancers 14, no. 23: 5791. https://doi.org/10.3390/cancers14235791
APA StyleGuarga, L., Paco, N., Vela, E., Clèries, M., Corral, J., Delgadillo, J., Pontes, C., & Borràs, J. M. (2022). Changes in Treatment Patterns and Costs for Lung Cancer Have Not Resulted in Relevant Improvements in Survival: A Population-Based Observational Study in Catalonia. Cancers, 14(23), 5791. https://doi.org/10.3390/cancers14235791





