Simple Summary
Chemotherapy administration after surgical resection of pulmonary carcinoid is not well studied. While guidelines state adjuvant therapy may be appropriate in some scenarios, this is not observed clinically. We reviewed the literature to examine if adjuvant chemotherapy in patients with resected pulmonary carcinoid provided any survival benefit. The aim of this review is to aid clinicians when deciding on next steps for these patients after resection.
Abstract
Pulmonary carcinoid tumors are a rare subtype of neuroendocrine cell tumor found in approximately 1–2% of lung cancers. Management is primarily through surgical resection, with limited benefit of adjuvant therapy in the clinical setting. Genomic profiling is in the nascent stages to molecularly classify these tumors, but there are promising insights for future targeted therapy. A total of 80 abstracts were analyzed for further review with 11 included in our final analysis. Only 4 of the 11 reviewed in depth provided statistical analysis. We evaluated PFS, OS, 1- and 5-year survival as mentioned in the studies. Nodal and KI67 status were also analyzed. Based on the current literature, there is no definitive evidence that adjuvant chemotherapy after resection confers a survival benefit in typical or atypical carcinoids.
1. Introduction
Pulmonary carcinoid tumors are a rare type of neuroendocrine tumor (NET) comprising less than 1–2% of all lung cancers [1]. These tumors can be further subclassified into typical carcinoid (TC) (low grade) and atypical carcinoid (AC) (intermediate grade) [2]. Primary treatment consists of surgical resection [3]. Differing surgical techniques are utilized depending on the location and extent of the tumor. Incomplete resection is a poor prognostic indicator and can often lead to the locoregional recurrence of the disease [3,4,5].
The use of cytotoxic agents as adjuvant therapy is mixed and highly debated among clinicians. While guidelines state the use of adjuvant therapy may be appropriate in certain cases, there are a lack of high-quality data due to the rarity of the disease [6]. While there is some evidence for treatment of aggressive metastatic pulmonary carcinoid, the evidence for use after surgical resection is lacking [7]. There are currently no prospective or randomized control trials concerning treatment modalities in regard to pulmonary carcinoid status post-resection. Expert opinion and organizational recommendations concerning individuals with surgical resection are mixed and complicated by a lack of applicable data.
The European Neuroendocrine Tumor Society recommends the use of adjuvant chemotherapy for atypical carcinoids after surgical resection, with Level IV evidence [5]. This is consistent with the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant therapy with a 2B classification of evidence [8]. The North American Neuroendocrine Tumor Society and European Society for Medical Oncology currently have no current recommendations concerning therapy [9,10]. This paper reviews and summarizes the existing literature on survival benefits of individuals who received adjuvant chemotherapy after surgical resection of pulmonary carcinoid.
2. Materials and Methods
MEDLINE/PubMed was utilized in accordance with PRISMA guidelines that identified the studies used to evaluate survival and use of adjuvant chemotherapy in typical and atypical carcinoids. Search parameters were limited from 2010 to 2021 and were evaluated further to meet inclusion and exclusion criteria.
2.1. Inclusion Criteria
- Study contains subjects who were surgically treated for well-differentiated typical or atypical carcinoid.
- Study must contain patients that received chemotherapy.
- Study must give a recommendation, whether qualitative or quantitative, concerning the benefit of chemotherapy if any.
2.2. Study Selection
A total of 80 studies were identified through the search parameters “chemotherapy” AND “pulmonary” AND “carcinoid”. The abstracts of these results were reviewed for possible inclusion in the study. Of the original articles reviewed, only eleven had reference to the survival benefit of our interest. These articles were analyzed in their entirety and of those. Four had statistical data reported to aid in their conclusions. Specific types of chemotherapy regimens used in trials were not necessarily clear and thus not assessed in this review. Of interest was a subgroup analysis of the nodal status and staging in concurrence with adjuvant therapy that was pre-specified before selection, but not an inclusion criterion.
2.3. Data Collection
Due to the heterogeneity of studies, outcomes of interest if applicable were: overall survival (OS), progression-free survival (PFS), median survival, and survival at 1 and 5 years. Exclusion criteria included abstracts, case reports, manuscripts not in English, and articles that did not specify if the primary surgical intervention had been employed before chemotherapy.
2.4. Study Risk Bias Assessment
Articles were reviewed by both authors independently and screened for the inclusion criteria aforementioned. Articles that did not meet criteria based on abstract review were excluded from the full analysis. The remaining articles were analyzed and grouped based on qualitative or quantitative. Due to the nature of the studies selected, the authors found it pertinent to include those with and without statistical analysis.
2.5. Additional Analyses
Additional criteria analyzed but not part of the selection criteria were nodal status and tumor stage on survival relative to chemotherapy use.
3. Results
3.1. Main Results
Using the terms “chemotherapy” AND “pulmonary” AND “carcinoid.”, there were 80 studies selected for further analysis for review. The initial review was an abstract review for further evaluation of article consideration. Articles that were not reported in english, and case reports were not included in this systematic review. Out of the initial 80 articles selected for pre-review, 11 were identified to meet the inclusion criteria. Of the 11 cases reviewed, 4 contained some sort of statistical analysis. Many initially reviewed studies did report conclusions and opinions regarding the main objectives, but they did not report findings by outcomes evaluated in this manuscript. Type of chemotherapy was reported in some but not all, of the studies; therefore, individual regimens were not discussed. The sample size in most studies reviewed did not have enough power for statistical analysis. Due to heterogeniety among the studies reviewed, outcomes were reported by: median survival, 1 and 5-year survival, PFS and OS. No study reported a survival benefit with adjuvant chemotherapy, with one reporting worse OS, versus observation in nodal vs. non-nodal disease or based on Ki-67 index.
3.2. Subgroup Analysis
Nodal status and staging appear to confer a disadvantage when adjuvant chemotherapy is employed. One study did demonstrate a percentage overall survival increase at 5 years in Stage III AC with chemotherapy use, but this was not statistically significant (Figure 1).
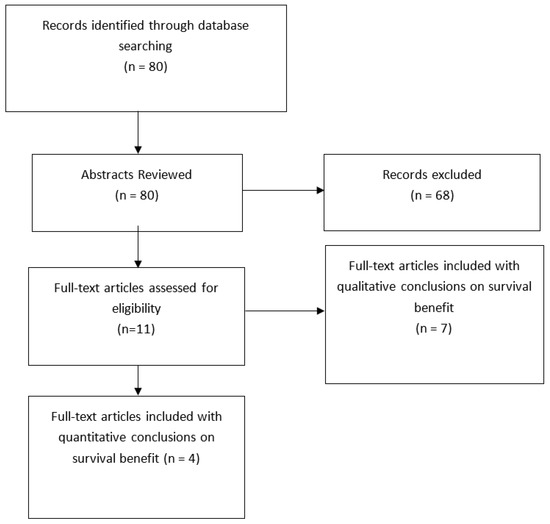
Figure 1.
Study Selection—PRISMA Diagram. The systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol has not been reg-istered.
4. Discussion
4.1. Clinical Implications
4.1.1. Survival Benefit with Adjuvant therapy
Ten of the eleven studies analyzed in this review did not conclude or recommend the use of adjuvant therapy in typical or atypical carcinoids that offered any survival benefit (Table 1). Tumor response was noted in multiple of the articles reviewed, which is supported by the literature. Our review did not directly analyze the different regimens used in the retrospective studies, and in many cases, these were not reported. In the article by Nussbaum et al., there was actually a statistically significant survival disadvantage conferred with adjuvant chemotherapy with typical carcinoids, although when propensity-matched, the statistical significance ceased.

Table 1.
Articles selected assessing survival benefit of individuals with typical and/or atypical carcinoid who underwent surgical resection and received adjuvant chemotherapy. Abbreviations: Progression-Free Survival (PFS); Overall Survival (OS); Median Overall Survival (MOS); Typical Carcinoid (TC); Atypical Carcinoid (AC); S—Surgery; S + CT—Surgery + Chemotherapy.
Overall, there was fewer atypical than typical carcinoids seen throughout the studies. This lines up with current literature on the incidence. Three studies specifically analyzed typical and atypical, respectively [11,12,13]. In all three, there were no survival benefits with therapy, regardless of nodal status. Many studies examined a mixed cohort, and due to their retrospective nature were unable to properly delineate, or did not report, the breakdown between subtypes. Gosain et al. did review TC and AC in the same study and had one of the higher numbers of total individuals analyzed.
4.1.2. Survival Benefit Based on Nodal Status, Ki-67 Index, and Metastasis
Two studies, Nussbaum et al. and Song et al., reported nodal status. In each study, N0 and N+ carcinoids did not differ in a survival advantage [13,14]. For staging, if any difference does exist, could be possibly exist for Stage III AC. This was evidenced by Gosain et al., who showed an OS benefit at 5 years with chemotherapy in Stage III disease [15]. These outcomes are consistent with the current guidelines from NCCN and European Neuroendocrine Tumor Society (ENETS) (Table 2), although it is of note the results were not statistically significant. There was a higher total number of patients analyzed with approximately 800 AC patients compared to many other reviews presented here. This is the most conclusive evidence in our review based on the power of the study.

Table 2.
Recommendations and grade of evidence on adjuvant therapy for pulmonary carcinoid (TC and AC) from: National Comprehensive Cancer Network (NCCN; North American Neuroendocrine Tumor Society (NANETS); European Society of Medical Oncology (ESMO); European Neuroendocrine Tumor Society (ENETS) [5,8,9,10].
Cytotoxic chemotherapy has been shown to give more of a response in pulmonary neuroendocrine tumors (pNETs) than non-pulmonary neuroendocrine tumors (non-pNETs) [22]. Multiple studies demonstrate that having a higher mitotic and Ki-67 index are poor prognostic indicators [23,24,25]. Contrarily, other studies examining Ki-67 index in conjuction with histopathology found no additional value for prognosis, but some value in prediction of recurrence [26,27,28]. Ki-67 may have more of a benefit in TC than AC. [26] Studies assessing Ki-67 as a response to chemotherapy have shown the higher the index the more responsive, in general, the tumor is [29]. These results from the various studies can be seen in Table 3. Overall, survival is not statistically significant for chemotherapy based on low or high-grade mitotic and Ki-67 indexes, and there is limited benefit for prognostication, with some utility in evaluating for recurrence. There is a lack of consensus on the diagnostic and prognostic role of Ki-67 index in pulmonary carcinoids [30,31,32].

Table 3.
Summary of Ki-67 indices in pulmonary and non-pulmonary carcinoids from selected studies.
There is currently not an adequate consensus on the role of surgery in metastatic disease [3,4,5]. The impact of metastatic disease, with or without resection, does not change survival benefits. In our review, one study demonstrated a statistically significant worse prognosis with the use of adjuvant therapy in the metastatic nodal setting with resection [12]. Other studies evaluating the role of adjuvant therapy in metastatic nodal disease, regardless of resection status, did not dictate a difference in outcomes [19,20,21]. This is not to disfavor the use of palliative chemotherapy for the relief of symptoms involved with tumor burden when appropriate.
4.1.3. Current Guideline Based Therapy
Current guidelines do not call for adjuvant therapy in typical carcinoids, consistent with our review overall and one study showing a disadvantage. NCCN guidelines support the use of chemotherapy in atypical carcinoids [8]. These recommendations are partially based on literature showing the response of pulmonary carcinoids treated with any chemotherapy [19,33,34]. The studies cited in guideline recommendations for use of chemotherapy in pulmonary carcinoids are generalized from chemotherapy response in small cell lung cancer (SCLC), per the NCCN [8,35,36,37]. Due to the seldom-seen nature of pulmonary carcinoid, and more specifically atypical carcinoid, performing a prospective trial to find the optimal treatment is difficult and lacking. While histologically and pathologically similar to other neuroendocrine cancers, there is a lack of evidence to demonstrate that regimens extrapolated across cancer types are applicable to improving survival benefits.
Different societies have recommendations for somatostatin analogs. The NCCN and ENETS recommend somatostatin analogs (SSA) use in patients with well-differentiated NETs in certain circumstances if positive on radionucleotide scan [5,8]. ENETS also recommends use in patients with a Ki-67 ≤ 10% along with a positive somatostatin receptor status [5]. ESMO along with NANETS also supports the use of octreotide labeled analogs in symptomatic patients with positive somatostatin receptors [9,10]. Of note, slower more well-differentiated tumors correlated to a longer OS and PFS [3].
4.2. Genomics of Carcinoid Tumors
4.2.1. Profiling of Carcinoid Tumors
Genomic and and immunohistochemical analysis comparing pulmonary NET’s is lacking [38]. Thought to be different histological subtypes by low and high grade (TC and AC vs. LCNEC and SCLC), new literature examining the status of these tumors indicate that rather than distinct etiologies, advanced secondary high-grade NET’s may arise from preexisting carcinoids on a spectrum. This is evident in genomic and transcriptomic data revealing ACs as tumors representing both TCs and LCNECs and SCLCs. Recent multi-omic studies have attempted to molecularly delineate these tumors further than histological types [34,35].
TC and AC display driver mutations in approximately 73% of cases, mainly MEN1 and SWI/SNF complex subunit mutations (ARID1A, SMARC1, SMARCA2, SMRCA5). Less common are RB1 and TP53 mutations [38,39] This correlates with data from other NGS studies of NETs with ACs [40,41,42]. Another analysis classifying 31 TC and 11 AC showed that 40% of sequenced tumors elucidated mutations in histone modifier genes such as: MEN1, ARID1A AND PSIP1 with 20% with SWI/SNF complex mutations, correlating with previously mentioned studies [43]. A large Chinese study with 18 TCs and 24 ACs used a larger 520 gene panel showing LCNEC (12.7 mutations/mb) harbored more mutations than SCLC (11.9 mutations/mb), ACs (7.1 mutations/mb), and TC’s (2.4 mutations/mb). SCLC and ACs displayed 26.3% and 20.8% of driver mutations seen in LCNEC demonstrating increasing evidence for a wide scope of disease and mutations burden between subtypes with similar molecular profiles.
4.2.2. Genomic Spectrum of Carcinoids
Rather than historical classification based off histological subtypes, there is now evidence that a molecular spectrum may help to further subclassify NETs. Work from Simbolo et al. using molecular analysis classified subtypes of pulmary NETs into different clusters which they correlated to the grade C3—low (AC) to C1—high (LCNEC)). The lower C3 cluster profiling mainly histological Acs, demonstrated low MEN1 and high RB1 expression, consistent with the literature. Other studies have shown that while historically TC and AC were considered distinct from higher grade NETs such as LCNEC and SCLC, molecularly ACs tend to behave more LCNEC like in terms of progression of disease [44]. This must be contrasted with analysis showing distinct differences between carcinoids (TC, AC) and SCLC, with little difference between TC and AC [43]. There are still lacking and conflicting data as much of these analyses were limited in their breadth of analysis. There likely are other genomic alterations that would benefit from whole exome sequencing to elicit more targeted molecular therapies.
5. Conclusions
Surgical intervention has been demonstrated and continues to be the primary treatment of pulmonary carcinoids. Based on the available data presented in the literature, there is no current evidence to support the current guidelines of adjuvant chemotherapy after surgical resection of pulmonary carcinoid tumors for typical or atypical carcinoids. In particular cases, SSA use may be appropriate, while in more aggressive and advanced tumors support care can be utilized. Nodal status and tumor staging also do not play a significant role in survival. Due to the uncommon nature of this malignancy, it is difficult to conduct a formal prospective trial. With whole genome sequencing, a logical next step is to identify molecular mutations for a more targeted therapy that could possibly be used in the adjuvant setting. While clinical judgment can be exercised concerning when chemotherapy may be favorable, specifically in palliative care, there is no current literature to support the use of adjuvant chemotherapy versus observation after resection.
Author Contributions
Conceptualization: P.T.S., A.U. and N.A.K.; Literature Review: P.T.S., A.U. and N.A.K.; Original Draft Preperation: P.T.S., A.U. and N.A.K.; Editing: P.T.S., A.U. and N.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
These data were presented at the 2021 NCCN Conference. https://jnccn.org/view/journals/jnccn/19/3.5/article-pBPI21-009.xml (1 September 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertino, E.M.; Confer, P.D.; Colonna, J.E.; Ross, P.; Otterson, G.A. Pulmonary neuroendocrine/carcinoid tumors: A review article. Cancer 2009, 115, 4434–4441. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Marchevsky, A.M.; Tuli, R. Neuroendocrine tumors of the lung: Current challenges and advances in the diagnosis and management of well-differentiated disease. J. Thorac. Oncol. 2017, 12, 425–436. [Google Scholar] [CrossRef]
- Gosain, R.; Mukherjee, S.; Yendamuri, S.S.; Iyer, R. Management of typical and atypical pulmonary carcinoids based on different established guidelines. Cancers 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Tiensuu Janson, E.; Kianmanesh, R.; Kos-Kudla, B.; et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology 2017, 105, 245–254. [Google Scholar] [CrossRef]
- Noel-Savina, E.; Descourt, R. Focus on treatment of lung carcinoid tumor. OncoTargets Ther. 2013, 6, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, N.; Baxevanos, P.; Aravantinou-Fatorou, E.; Tolia, M.; Galanopoulos, M.; Tsapakidis, K.; Kyrgias, G.; Toumpanakis, C.; Kaltsas, G. Advances on systemic treatment for lung neuroendocrine neoplasms. Ann. Transl. Med. 2018, 6, 146. [Google Scholar] [CrossRef]
- Clark, O.H.; Benson, A.B., III; Berlin, J.D.; Choti, M.A.; Doherty, G.M.; Engstrom, P.F.; Gibbs, J.F.; Heslin, M.J.; Kessinger, A.; Kulke, M.H.; et al. NCCN clinical practice guidelines in oncology: Neuroendocrine tumors. J. Natl. Compr. Cancer Netw. 2009, 7, 712–747. [Google Scholar]
- Phan, A.T.; Oberg, K.; Choi, J.; Harrison, J.; Lynn, H.; Hassan, M.M.; Strosberg, J.R.; Krenning, E.P.; Kocha, W.; Woltering, E.A.; et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010, 39, 784–798. [Google Scholar] [CrossRef]
- Öberg, K.; Hellman, P.; Ferolla, P.; Papotti, M. Neuroendocrine bronchial and thymic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. A7), vii120–vii123. [Google Scholar] [CrossRef]
- Nussbaum, D.P.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; Onaitis, M.W.; D’Amico, T.A.; Berry, M.F. Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors. Ann. Thorac. Surg. 2015, 99, 428–434. [Google Scholar] [CrossRef]
- Anderson, K.L.; Mulvihill, M.S.; Speicher, P.J.; Yerokun, B.A.; Gulack, B.C.; Nussbaum, D.P.; Harpole, D.H.; D’Amico, T.A.; Berry, M.F.; Hartwig, M.G. Adjuvant chemotherapy does not confer superior survival in patients with atypical carcinoid tumors. Ann. Thorac. Surg. 2017, 104, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zang, R.; Liu, L.; Dan, X.; Gao, S. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary atypical carcinoid tumors: Our institutional experience with 68 patients. J. Thorac. Dis. 2018, 10, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Wegner, R.E.; Abel, S.; Hasan, S.; Horne, Z.D.; Colonias, A.; Weksler, B.; Verma, V. The role of adjuvant therapy for atypical bronchopulmonary carcinoids. Lung Cancer 2019, 131, 90–94. [Google Scholar] [CrossRef]
- Gosain, R.; Groman, A.; Yendamuri, S.S.; Iyer, R.; Mukherjee, S. Role of adjuvant chemotherapy in pulmonary carcinoids: An NCDB analysis. Anticancer Res. 2019, 39, 6835–6842. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Lu, T.; Li, M.; Zhao, M.; Yang, X.; Ma, K.; Wang, S.; Zhan, C.; Liu, Y.; et al. Assessment of the prognostic factors in patients with pulmonary carcinoid tumor: A population-based study. Cancer Med. 2018, 7, 2434–2441. [Google Scholar] [CrossRef]
- Tancredi, A.; Muscarella, L.; la Torre, A.; Scaramuzzi, R.; Valori, V.; Fazio, V.; Scaramuzzi, G. The post-surgical long-term behaviour of lung carcinoid tumours. Indian J. Surg. 2015, 77, 481–485. [Google Scholar] [CrossRef]
- Furqan, M.; Tien, Y.; Schroeder, M.C.; Parekh, K.R.; Keech, J.; Allen, B.G.; Thomas, A.; Zhang, J.; Clamon, G.; Abu Hejleh, T. Lobar versus sub-lobar surgery for pulmonary typical carcinoid, a population-based analysis. J. Thorac. Dis. 2018, 10, 5850–5859. [Google Scholar] [CrossRef]
- Chong, C.R.; Wirth, L.J.; Nishino, M.; Chen, A.B.; Sholl, L.M.; Kulke, M.H.; Mcnamee, C.J.; Jänne, P.A.; Johnson, B.E. Chemotherapy and irradiation for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014, 86, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Hooker, C.M.; Boikos, S.A.; Petrini, I.; Giaccone, G.; Rudin, C.M.; Yang, S.C.; Illei, P.B.; Hann, C.L.; Ettinger, D.S.; et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: A brief report. J. Thorac. Oncol. 2014, 9, 414–418. [Google Scholar] [CrossRef]
- Robelin, P.; Hadoux, J.; Forestier, J.; Planchard, D.; Hervieu, V.; Berdelou, A.; Scoazec, J.; Valette, P.; Leboulleux, S.; Ducreux, M.; et al. Characterization, prognosis, and treatment of patients with metastatic lung carcinoid tumors. J. Thorac. Oncol. 2019, 14, 993–1002. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A review of neuroendocrine tumors and carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.M.; Kroneman, T.N.; Jenkins, S.M.; Simone, B.S.P.; Terra, M.D.; Xie, H.; Molina, J.; Mounajjed, T.; Roden, A.C. Ki-67 labeling index in pulmonary carcinoid tumors: Comparison between small biopsy and resection using tumor tracing and hot spot methods. Arch. Pathol. Lab. Med. 2020, 144, 982–990. [Google Scholar] [CrossRef]
- Marchiò, C.; Gatti, G.; Massa, F.; Bertero, L.; Filosso, P.; Pelosi, G.; Cassoni, P.; Volante, M.; Papotti, M. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch. 2017, 471, 713–720. [Google Scholar] [CrossRef]
- Chirieac, L.R. Ki-67 expression in pulmonary tumors. Transl. Lung Cancer Res. 2016, 5, 547–551. [Google Scholar] [CrossRef]
- Swarts, D.R.A.; Rudelius, M.; Claessen, S.M.H.; Cleutjens, J.P.; Seidl, S.; Volante, M.; Ramaekers, F.C.S.; Speel, E.J.M. Limited additive value of the ki-67 proliferative index on patient survival in world health organization-classified pulmonary carcinoids. Histopathology 2017, 70, 412–422. [Google Scholar] [CrossRef]
- Dermawan, J.; Farver, C. The role of histologic grading and ki-67 index in predicting outcomes in pulmonary carcinoid tumors. Am. J. Surg. Pathol. 2020, 44, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Walts, A.E.; Ines, D.; Marchevsky, A.M. Limited role of ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod. Pathol. 2012, 25, 1258–1264. [Google Scholar] [CrossRef]
- Childs, A.; Kirkwood, A.; Edeline, J.; Luong, T.V.; Watkins, J.; Lamarca, A.; Alrifai, D.; Nsiah-Sarbeng, P.; Gillmore, R.; Mayer, A.; et al. Ki-67 index and response to chemotherapy in patients with neuroendocrine tumours. Endocr. Relat. Cancers 2016, 23, 563–570. [Google Scholar] [CrossRef]
- Skov, B.G.; Holm, B.; Erreboe, A.; Skov, T.; Mellemgaard, A. ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: Distribution and impact on survival. J. Thorac. Oncol. 2010, 5, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, F.; Muser, D.; Beltrami, C.A.; Machin, P.; Morelli, A.; Pizzolitto, S.; Talmassons, G.; Marciello, F.; Colao, A.A.L.; Monaco, R.; et al. Partitioning of bronchopulmonary carcinoids in two different prognostic categories by ki-67 score. Front. Endocrinol. 2011, 2, 20. [Google Scholar] [CrossRef]
- Rindi, G.; Klersy, C.; Inzani, F.; Fellegara, G.; Ampollini, L.; Ardizzoni, A.; Campanini, N.; Carbognani, P.; De Pas, T.M.; Galetta, D.; et al. Grading the neuroendocrine tumors of the lung: An evidence-based proposal. Endocr.-Relat. Cancer 2014, 21, 1–16. [Google Scholar] [CrossRef]
- Wirth, L.J.; Carter, M.R.; Jänne, P.A.; Johnson, B.E. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer 2004, 44, 213–220. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet (Br. Ed.) 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Lassen, U.; Kristjansen, P.E.G.; Østerlind, K.; Bergman, B.; Sigsgaard, T.C.; Hirsch, F.R.; Hansen, M.; Dombernowsky, P.; Hansen, H.H. Superiority of cisplatin or carboplatin in combination with teniposide and vincristine in the induction chemotherapy of small-cell lung cancer. A randomized trial with 5 years follow up. Ann. Oncol. 1996, 7, 365–372. [Google Scholar] [CrossRef]
- Catherine Pietanza, M.; Kadota, K.; Chan, T.A.; Rizvi, N.A.; Azzoli, C.G.; Riely, G.J.; Kris, M.G.; Krug, L.M.; Huberman, K.; Sima, C.S.; et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin. Cancer Res. 2012, 18, 1138–1145. [Google Scholar] [CrossRef]
- Zauderer, M.G.; Drilon, A.; Kadota, K.; Huberman, K.; Sima, C.S.; Bergagnini, I.; Sumner, D.K.; Travis, W.D.; Heguy, A.; Ginsberg, M.S.; et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014, 86, 237–240. [Google Scholar] [CrossRef]
- Pelosi, G.; Sonzogni, A.; Harari, S.; Albini, A.; Bresaola, E.; Marchiò, C.; Massa, F.; Righi, L.; Gatti, G.; Papanikolaou, N.; et al. Classification of pulmonary neuroendocrine tumors: New insights. Transl. Lung Cancer Res. 2017, 6, 513–529. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Peifer, M.; Lu, X.; Sun, R.; Ozretić, L.; Seidal, D.; Zander, T.; Leenders, F.; George, J.; Müller, C.; et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat. Commun. 2014, 5, 3518. [Google Scholar] [CrossRef]
- Simbolo, M.; Barbi, S.; Fassan, M.; Mafficini, A.; Ali, G.; Vicentini, C.; Sperandio, N.; Corbo, V.; Rusev, B.; Mastracci, L.; et al. Gene expression profiling of lung atypical carcinoids and large cell neuroendocrine carcinomas identifies three transcriptomic subtypes with specific genomic alterations. J. Thorac. Oncol. 2019, 14, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chung, Y.S.; Kim, K.A.; Shim, H.S. Genomic profiling and clinicopathological characteristics of neuroendocrine tumors of the lung in east asian patients. In Vivo 2020, 34, 3375–3385. [Google Scholar] [CrossRef]
- Rekhtman, N.; Desmeules, P.; Litvak, A.M.; Pietanza, M.C.; Santos-Zabala, M.L.; Ni, A.; Montecalvo, J.; Chang, J.C.; Beras, A.; Preeshagul, I.R.; et al. Stage IV lung carcinoids: Spectrum and evolution of proliferation rate, focusing on variants with elevated proliferation indices. Mod. Pathol. 2019, 32, 1106–1122. [Google Scholar] [CrossRef]
- Asiedu, M.K.; Thomas, J.; Charles, F.; Dong, J.; Schulte, S.C.; Khadka, P.; Sun, Z.; Kosari, F.; Jen, J.; Molina, J.; et al. Pathways impacted by genomic alterations in pulmonary carcinoid tumors. Clin. Cancer Res. 2018, 24, 1691–1704. [Google Scholar] [CrossRef]
- Alcala, N.; Leblay, N.; Gabriel, A.A.G.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.S.; Ferrari, A.; Derks, J.; Ghantous, A.; et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat. Commun. 2019, 10, 3407. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).