The Analysis of Trends in Survival for Patients with Melanoma Brain Metastases with Introduction of Novel Therapeutic Options before the Era of Combined Immunotherapy—Multicenter Italian–Polish Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
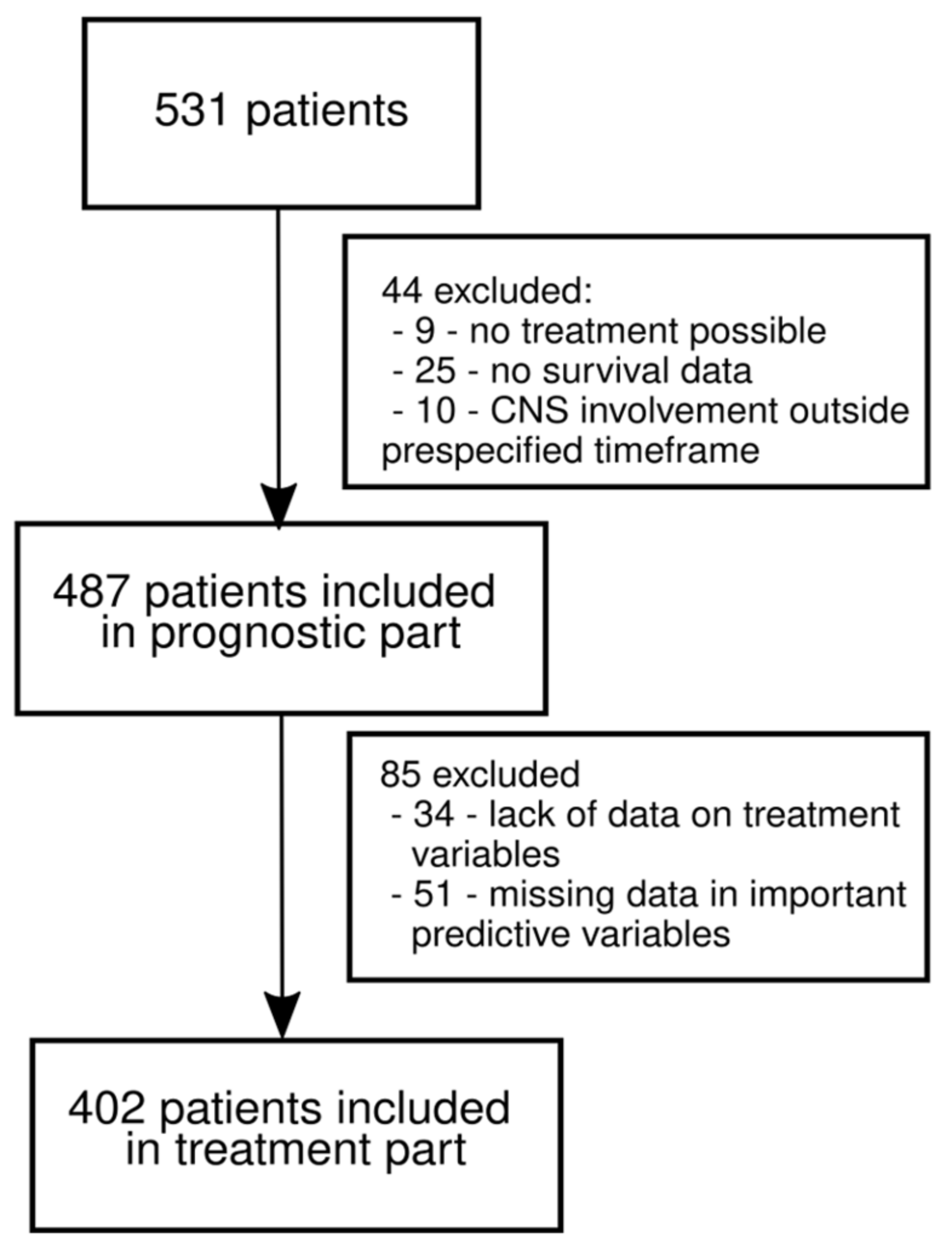
2.1. Patient Cohort and Inclusion Criteria
2.2. Collected Covariates
2.3. Analysis Plan and Handling of Missing Data
2.4. Statistical Methods
2.5. Ethical Statement
3. Results
3.1. Patient Characteristics
3.2. Prognostic Factors at MBM Diagnosis
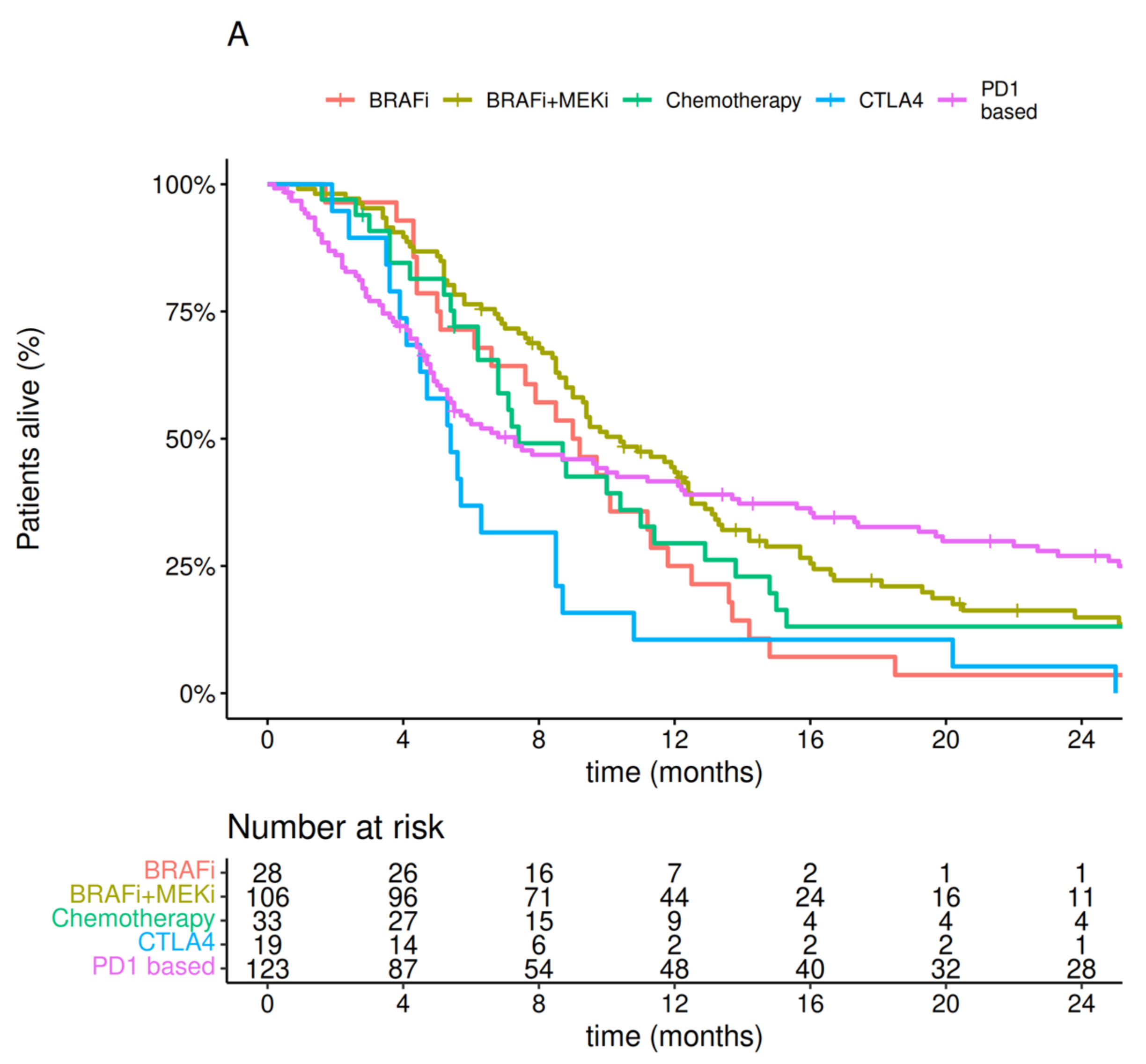
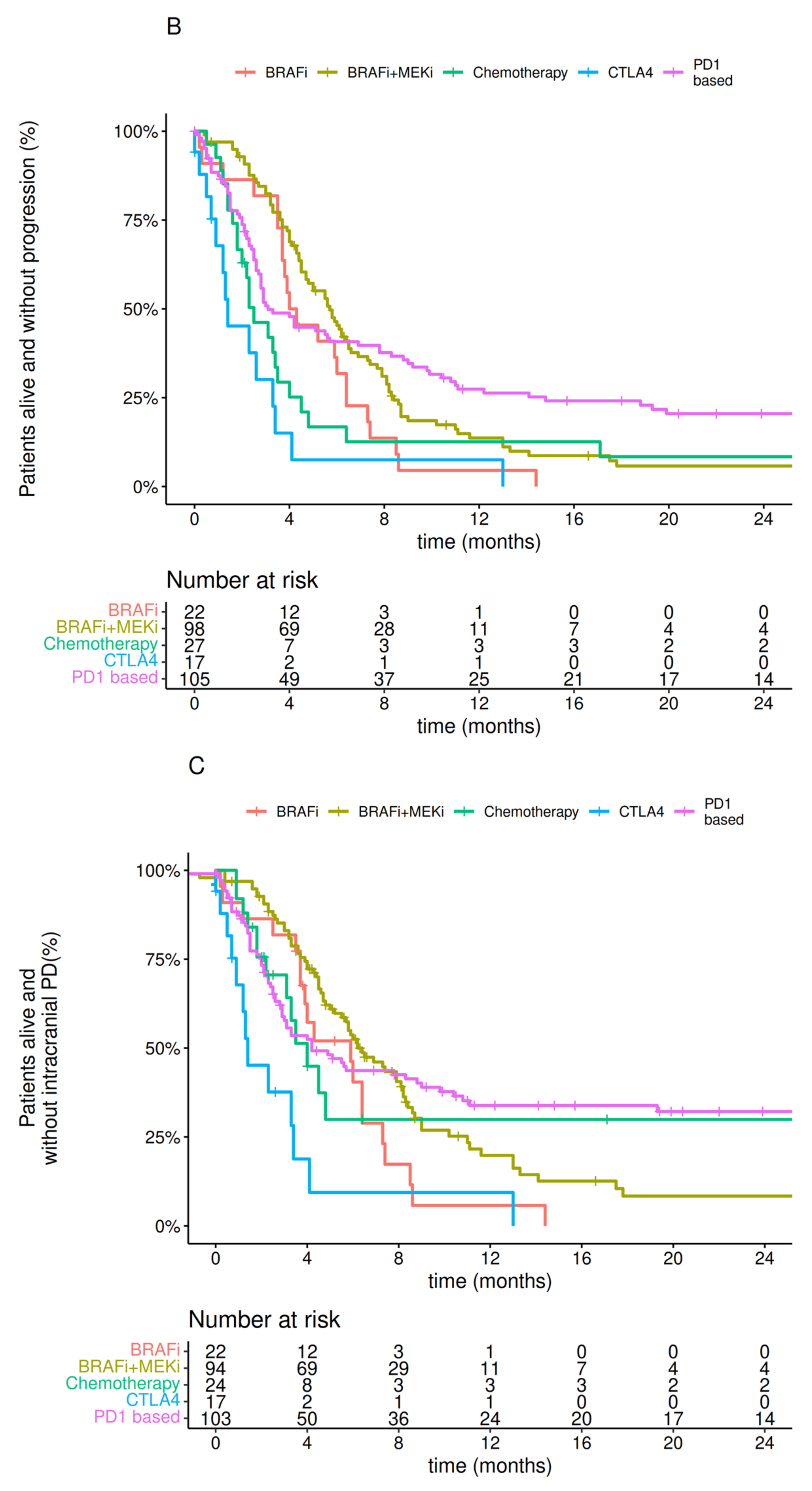
3.3. Treatment
4. Discussion
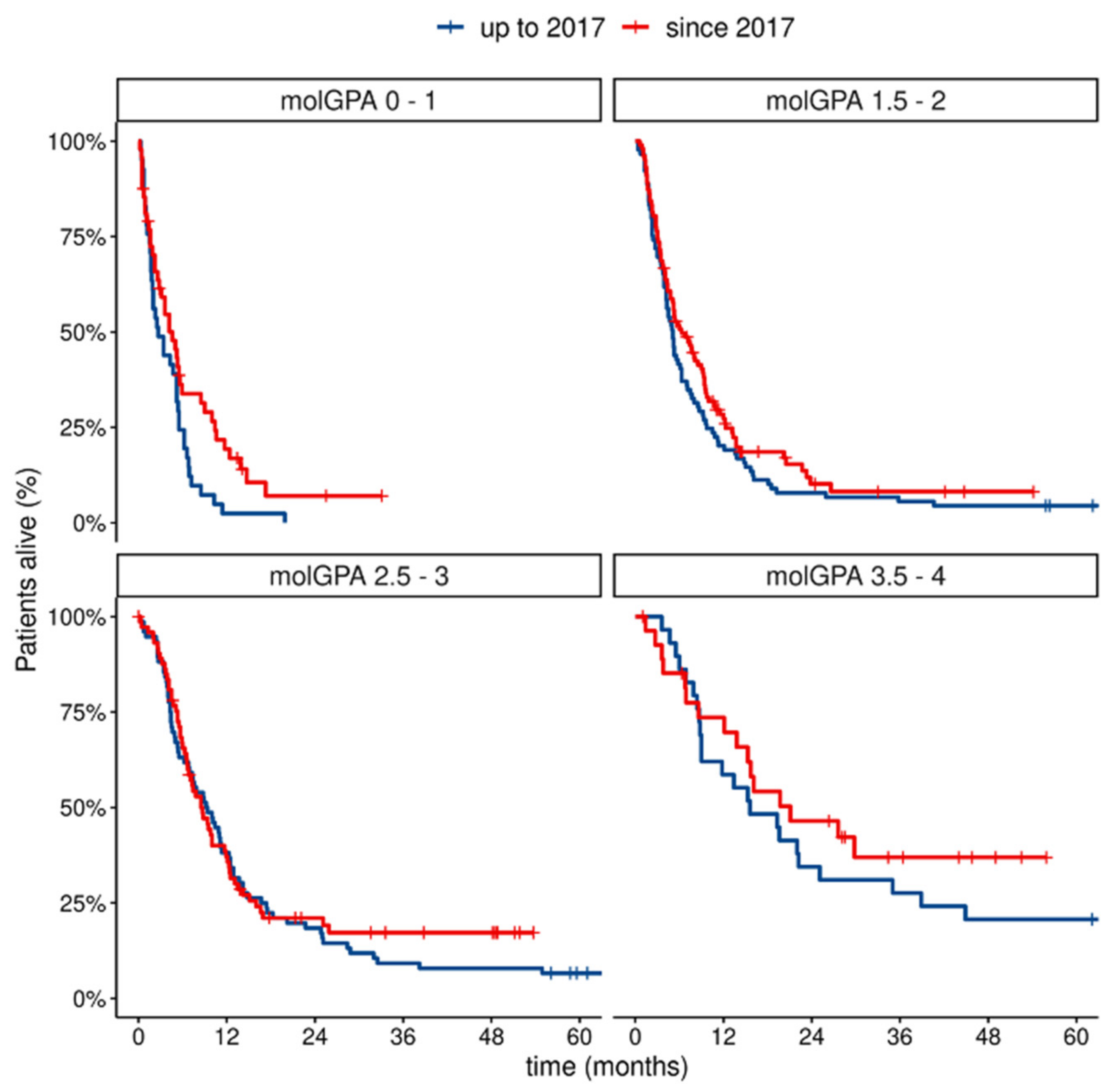
- Patients diagnosed with MBM after 2017 had a better prognosis in our cohort, with a significantly improved median of overall survival after 2017. The biggest change was observed in the poorest prognostic mol-GPA groups, in which survival improved significantly after 2017.
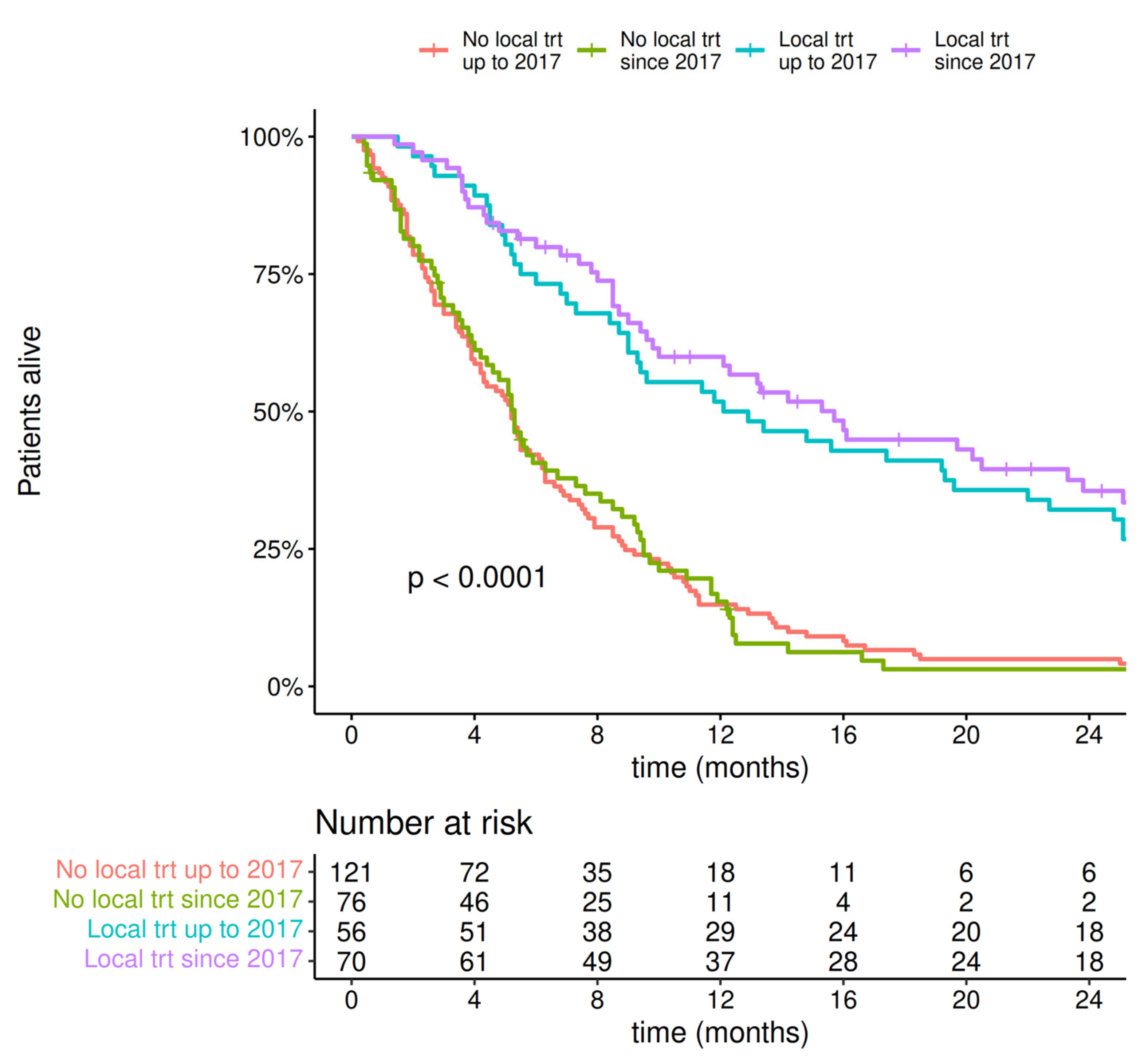
- The outcome of patients with MBM improved when local therapy (neurosurgery ± SRS) was combined with modern systemic therapy. The implementation of systemic therapy and surgery was associated with improved OS regardless of the timing to MBM treatment start (i.e., before or after 30 days from MBM diagnosis).
- The introduction of local therapy for MBM treatment significantly improved survival regardless of the year of diagnosis (treated after or before 2017), with a median survival of more than 12 months.
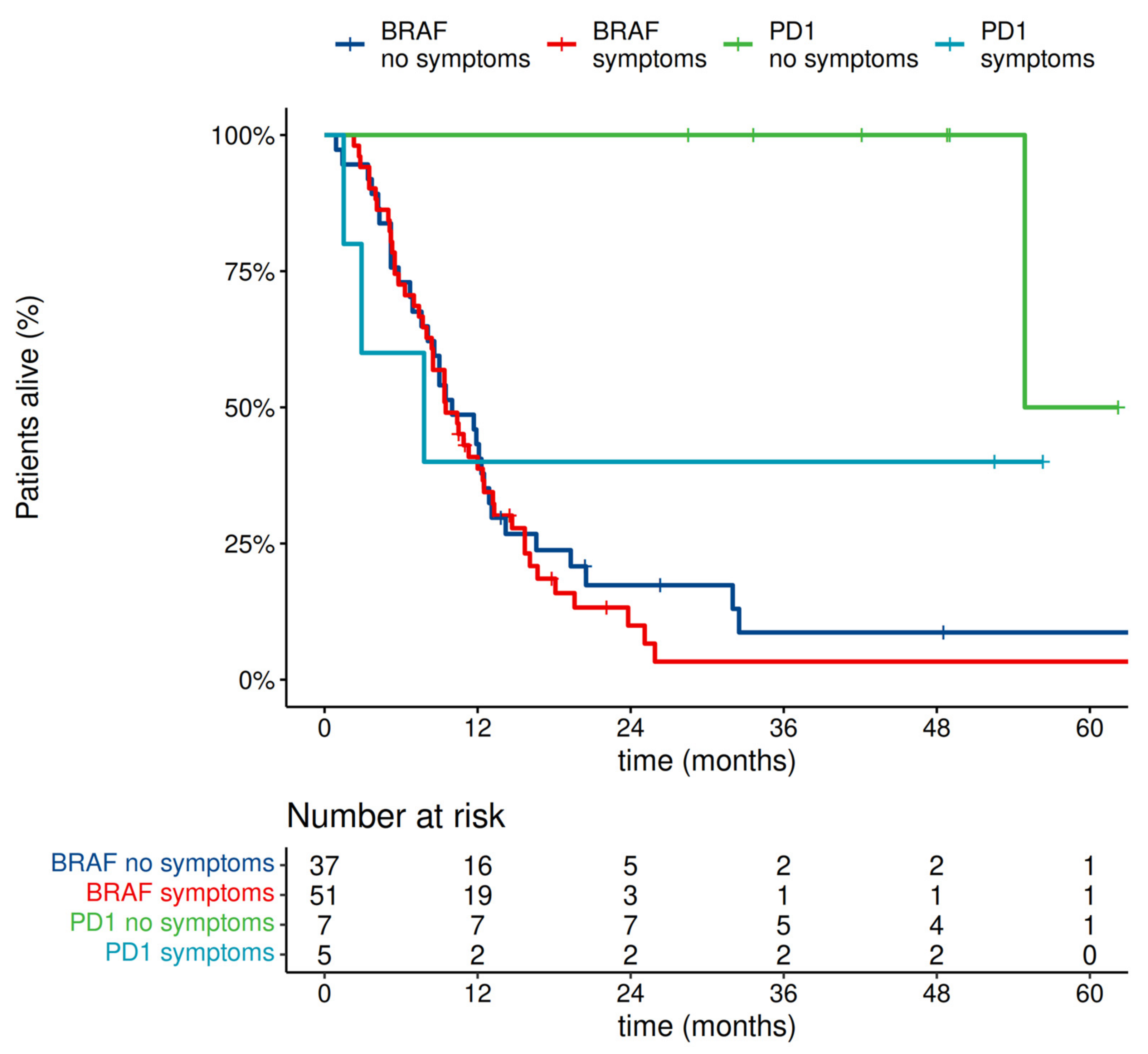
- In our prognostic model, the presence of MBM symptoms without steroid use did not have prognostic significance.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.J.; Hwu, P.; Bedikian, A. Prognostic Factors for Survival in Melanoma Patients with Brain Metastases. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO Consensus Conference Recommendations on the Management of Metastatic Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef]
- NCCN Guidelines; Melanoma; Cutaneus, Version 3. 2022. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 16 May 2022).
- Lin, N.U.; Prowell, T.; Tan, A.R.; Kozak, M.; Rosen, O.; Amiri-Kordestani, L.; White, J.; Sul, J.; Perkins, L.; Beal, K.; et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3760–3773. [Google Scholar] [CrossRef]
- Badakhshi, H.; Engeling, F.; Budach, V.; Ghadjar, P.; Zschaeck, S.; Kaul, D. Are Prognostic Indices for Brain Metastases of Melanoma Still Valid in the Stereotactic Era? Radiat. Oncol. 2018, 13, 3. [Google Scholar] [CrossRef]
- Bander, E.D.; Yuan, M.; Carnevale, J.A.; Reiner, A.S.; Panageas, K.S.; Postow, M.A.; Tabar, V.; Moss, N.S. Melanoma Brain Metastasis Presentation, Treatment, and Outcomes in the Age of Targeted and Immunotherapies. Cancer 2021, 127, 2062–2073. [Google Scholar] [CrossRef]
- Nieder, C.; Marienhagen, K.; Dalhaug, A.; Aandahl, G.; Haukland, E.; Pawinski, A. Prognostic Models Predicting Survival of Patients with Brain Metastases: Integration of Lactate Dehydrogenase, Albumin and Extracranial Organ Involvement. Clin. Oncol. R. Coll. Radiol. 2014, 26, 447–452. [Google Scholar] [CrossRef]
- Lewitzki, V.; Klement, R.J.; Hess, S.; Kosmala, R.; Nieder, C.; Flentje, M. External Validation of a Prognostic Score Predicting Overall Survival for Patients with Brain Metastases Based on Extracranial Factors. Clin. Transl. Radiat. Oncol. 2019, 16, 15–20. [Google Scholar] [CrossRef]
- Dalmasso, C.; Pagès, C.; Chaltiel, L.; Brun, A.; Sibaud, V.; Boulinguez, S.; Chira, C.; Moyal, E.; Lubrano, V.; Meyer, N.; et al. Survival Estimation of Melanoma Patients with Brain Metastasis Using the Melanoma-MolGPA Score: External Validation from a French Cohort. Melanoma Res. 2020, 30, 472–476. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients with Brain Metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; Postow, M.A.; et al. Long-Term Outcomes of Patients with Active Melanoma Brain Metastases Treated with Combination Nivolumab plus Ipilimumab (CheckMate 204): Final Results of an Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Guminski, A.D.; Sandhu, S.K.; Brown, M.P.; Gonzalez, M.; Scolyer, R.A.; Emmett, L.; McArthur, G.A.; et al. Five-Year Overall Survival from the Anti-PD1 Brain Collaboration (ABC Study): Randomized Phase 2 Study of Nivolumab (Nivo) or Nivo+ipilimumab (Ipi) in Patients (Pts) with Melanoma Brain Metastases (Mets). J. Clin. Oncol. 2021, 39, 9508. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.-J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600-Mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in Patients with Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients with Melanoma with Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clin. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Jiang, W.; Brown, P.D.; Braunstein, S.; Sneed, P.; Wattson, D.A.; Shih, H.A.; Bangdiwala, A.; Shanley, R.; Lockney, N.A.; et al. Estimating Survival in Melanoma Patients with Brain Metastases: An Update of the Graded Prognostic Assessment for Melanoma Using Molecular Markers (Melanoma-MolGPA). Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 812–816. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan LD, A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org (accessed on 31 October 2021).
- Zach. How to Calculate AIC in R (Including Examples). Statology. 2021. Available online: https://www.statology.org (accessed on 31 October 2021).
- Liu, J.L.; Walker, E.V.; Paudel, Y.R.; Davis, F.G.; Yuan, Y. Brain Metastases among Cancer Patients Diagnosed from 2010–2017 in Canada: Incidence Proportion at Diagnosis and Estimated Lifetime Incidence. Curr. Oncol. 2022, 29, 2091–2105. [Google Scholar] [CrossRef]
- Steindl, A.; Brunner, T.J.; Heimbach, K.; Schweighart, K.; Moser, G.M.; Niziolek, H.M.; Moor, E.; Kreminger, J.; Starzer, A.M.; Dieckmann, K.; et al. Changing Characteristics, Treatment Approaches and Survival of Patients with Brain Metastasis: Data from Six Thousand and Thirty-One Individuals over an Observation Period of 30 Years. Eur. J. Cancer 2022, 162, 170–181. [Google Scholar] [CrossRef]
- Dalmasso, C.; Pagès, C.; Chaltiel, L.; Sibaud, V.; Moyal, E.; Chira, C.; Sol, J.C.; Latorzeff, I.; Meyer, N.; Modesto, A. Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy. Cancers 2021, 13, 4493. [Google Scholar] [CrossRef]
- van Zeijl, M.C.T.; Ismail, R.K.; de Wreede, L.C.; van den Eertwegh, A.J.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Boers-Sonderen, M.J.; et al. Real-World Outcomes of Advanced Melanoma Patients Not Represented in Phase III Trials. Int. J. Cancer 2020, 147, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Mehta, M.P. Prognostic Indices for Brain Metastases--Usefulness and Challenges. Radiat. Oncol. 2009, 4, 10. [Google Scholar] [CrossRef]
- OncLive Management of Brain Metastases in Melanoma. Available online: https://www.onclive.com/view/management-of-brain-metastases-in-melanoma (accessed on 3 June 2022).
- Staudt, M.; Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Bamberg, M.; Tatagiba, M.; Brossart, P.; Garbe, C. Determinants of Survival in Patients with Brain Metastases from Cutaneous Melanoma. Br. J. Cancer 2010, 102, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.; Kiecker, F.; Schaefer, S.; Stege, H.; Kaehler, K.; Terheyden, P.; Gesierich, A.; Gutzmer, R.; Haferkamp, S.; Uttikal, J.; et al. Combined Immunotherapy with Nivolumab and Ipilimumab with and without Local Therapy in Patients with Melanoma Brain Metastasis: A DeCOG* Study in 380 Patients. J. Immunother. Cancer 2020, 8, e000333. [Google Scholar] [CrossRef] [PubMed]
- Paderi, A.; Gambale, E.; Botteri, C.; Giorgione, R.; Lavacchi, D.; Brugia, M.; Mazzoni, F.; Giommoni, E.; Bormioli, S.; Amedei, A.; et al. Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis. Molecules 2021, 26, 5789. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Melanoma Metastasized to the Brain and Steroids (MEMBRAINS). Available online: https://clinicaltrials.gov/ct2/show/NCT03563729 (accessed on 29 September 2022).
- Banks, P.D.; Lasocki, A.; Lau, P.K.H.; Sandhu, S.; McArthur, G.; Shackleton, M. Bevacizumab as a Steroid-Sparing Agent during Immunotherapy for Melanoma Brain Metastases: A Case Series. Health Sci. Rep. 2019, 2, e115. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.; Thomas, R.P.; Glaspy, J.A.; et al. Safety and Efficacy of the Combination of Nivolumab plus Ipilimumab in Patients with Melanoma and Asymptomatic or Symptomatic Brain Metastases (CheckMate 204). Neuro-Oncol. 2021, 23, 1961–1973. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Stephen Hodi, F.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’Reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An Inflammation-Based Prognostic Score (MGPS) Predicts Cancer Survival Independent of Tumour Site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Shinyashiki, Y.; Ito, T.; Akagi, M. Characterizing Inflammatory Markers in Highly Aggressive Soft Tissue Sarcomas. Medicine 2022, 101, e30688. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Gao, H.; Zhao, W.; Zhou, Y.; Yang, J. Development and Validation of an Immune Gene Set-Based Prognostic Signature in Cutaneous Melanoma. Future Oncol. 2021, 17, 4115–4129. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Ricken, G.; Widhalm, G.; Rajky, O.; Dieckmann, K.; Birner, P.; Bartsch, R.; Höller, C.; Preusser, M. Tumour-Infiltrating Lymphocytes and Expression of Programmed Death Ligand 1 (PD-L1) in Melanoma Brain Metastases. Histopathology 2015, 66, 289–299. [Google Scholar] [CrossRef]
- Levatić, J.; Salvadores, M.; Fuster-Tormo, F.; Supek, F. Mutational Signatures Are Markers of Drug Sensitivity of Cancer Cells. Nat. Commun. 2022, 13, 2926. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, M.; Chung, Y.-J. Two Subtypes of Cutaneous Melanoma with Distinct Mutational Signatures and Clinico-Genomic Characteristics. Front. Genet. 2022, 13, 987205. [Google Scholar] [CrossRef]
| Prognostic Part | Treatment Part | ||||
|---|---|---|---|---|---|
| Data Analyzed | Variable | Overall | Missing | Overall | Missing |
| number of pts (%) | % | number of pts (%) | % | ||
| 487 | 402 | ||||
| Sex | female | 204 (41.9) | 0 | 169 (42.0) | 0 |
| male | 283 (58.1) | 233 (58.0) | |||
| CNS involvement type | both | 14 (2.9) | 0 | 9 (2.2) | 0 |
| brain | 468 (96.1) | 391 (97.3) | |||
| meninges | 5 (1.0) | 2 (0.5) | |||
| BRAF mutation status | v600 | 316 (64.9) | 0 | 255 (63.4) | 0 |
| wt | 171 (35.1) | 147 (36.6) | |||
| Presence of lung metastases | no | 217 (44.6) | 0 | 175 (43.5) | 0 |
| yes | 270 (55.4) | 227 (56.5) | |||
| Presence of visceral metastases | no | 270 (55.4) | 0 | 231 (57.5) | 0 |
| yes | 217 (44.6) | 171 (42.5) | |||
| No. of MBM | 1 | 131 (26.9) | 0 | 95 (23.6) | 0 |
| 2 | 58 (11.9) | 50 (12.4) | |||
| 3 | 44 (9.0) | 39 (9.7) | |||
| 4 | 15 (3.1) | 11 (2.7) | |||
| 5+ | 239 (49.1) | 207 (51.5) | |||
| Diameter of MBM (mm) (median (IQR)) | 16.00 [9.00, 27.00] | 28.1 | 16.00 [10.00, 27.00] | 25.9 | |
| Karnofsky performance status | <70 | 119 (24.4) | 0 | 52 (12.9) | 0 |
| 100 | 53 (10.9) | 43 (10.7) | |||
| 70 | 84 (17.2) | 83 (20.6) | |||
| 80 | 98 (20.1) | 95 (23.6) | |||
| 90 | 133 (27.3) | 129 (32.1) | |||
| Presence of CNS symptoms | no | 177 (40.7) | 10.7 | 163 (42.7) | 5 |
| yes | 258 (59.3) | 219 (57.3) | |||
| Use of GCS | no | 110 (22.6) | 0 | 106 (26.4) | 0 |
| unknown | 102 (20.9) | 63 (15.7) | |||
| yes | 275 (56.5) | 233 (58.0) | |||
| Use of previous treatment | 0 | 306 (62.8) | 0 | 252 (62.7) | 0 |
| 1 | 128 (26.3) | 102 (25.4) | |||
| 2+ | 53 (10.9) | 48 (11.9) | |||
| Extracranial involvement | no | 237 (48.7) | 0 | 183 (45.5) | 0 |
| yes | 250 (51.3) | 219 (54.5) | |||
| Age (median (IQR)) | 56.00 [44.00, 65.00] | 0 | 56.00 [44.00, 65.00] | 0 | |
| Previous chemotherapy | no | 451 (92.6) | 0 | 368 (91.5) | 0 |
| yes | 36 (7.4) | 34 (8.5) | |||
| Previous BRAF/MEK inhibitors | no | 451 (92.6) | 0 | 372 (92.5) | 0 |
| yes | 36 (7.4) | 30 (7.5) | |||
| Previous BRAF inhibitors | no | 453 (93.0) | 0 | 372 (92.5) | 0 |
| yes | 34 (7.0) | 30 (7.5) | |||
| Previous ipilimumab | no | 460 (94.5) | 0 | 377 (93.8) | 0 |
| yes | 27 (5.5) | 25 (6.2) | |||
| Previous anti-pd1 antibodies | no | 460 (94.5) | 0 | 377 (93.8) | 0 |
| yes | 27 (5.5) | 25 (6.2) | |||
| No. of pts with MBM diagnosis by year | <2017 | 235 (48.3) | 0 | 207 (51.5) | 0 |
| ≥2017 | 252 (51.7) | 195 (48.5) | |||
| Analyzed Variables | Full Model | Reduced Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Term | HR | Lower 95 | Upper 95 | p-Value | HR | Lower 95 | Upper 95 | p-Value |
| Sex male | 1.16 | 0.95 | 1.42 | 0.139 | ||||
| Age (per 1 year change) | 1.01 | 1 | 1.02 | 0.0056 | 1.01 | 1 | 1.02 | 0.0048 |
| BRAF status (WT vs. mutated) | 1.31 | 1.05 | 1.64 | 0.0159 | 1.33 | 1.07 | 1.66 | 0.0114 |
| Lung metastases (yes vs. no) | 1.24 | 1.01 | 1.54 | 0.0446 | 1.25 | 1.01 | 1.54 | 0.0375 |
| Visceral metastases (yes vs. no) | 1.3 | 1.05 | 1.61 | 0.0168 | 1.27 | 1.03 | 1.57 | 0.0239 |
| Other extracranial metastases (yes vs. no) | 0.98 | 0.8 | 1.21 | 0.8665 | ||||
| No. of CNS metastases = 1 | Reference | Reference | ||||||
| No. of CNS metastases = 2–4 | 1.65 | 1.23 | 2.21 | 0.0009 | 1.6 | 1.19 | 2.13 | 0.0017 |
| No. of CNS metastases >5 | 2.1 | 1.61 | 2.73 | <0.0001 | 2.08 | 1.61 | 2.7 | <0.0001 |
| Diameter of biggest CNS meta (per 1mm change) | 1 | 0.99 | 1.01 | 0.3625 | ||||
| Year of MBM diagnosis (per 1 year change since 2014) | 0.91 | 0.85 | 0.97 | 0.0038 | 0.91 | 0.86 | 0.97 | 0.0045 |
| Karnofsky performance status score (per 10 pts change) | 0.9 | 0.83 | 0.98 | 0.011 | 0.9 | 0.83 | 0.97 | 0.009 |
| No CNS symptoms, no GCS use | Reference | Reference | ||||||
| No CNS symptoms, GCS due to other causes | 1.42 | 1 | 2.03 | 0.0525 | 1.42 | 0.99 | 2.02 | 0.0549 |
| CNS symptoms, no GKS use | 1.16 | 0.8 | 1.69 | 0.4309 | 1.22 | 0.85 | 1.76 | 0.2832 |
| CNS symptoms requiring GCS | 1.63 | 1.23 | 2.16 | 0.0007 | 1.69 | 1.29 | 2.21 | 0.0001 |
| No previous systemic treatment | Reference | Reference | ||||||
| 1 line of previous systemic treatment | 1.65 | 1.31 | 2.08 | <0.0001 | 1.65 | 1.31 | 2.09 | <0.0001 |
| >1 line of previous systemic treatment | 1.62 | 1.16 | 2.27 | 0.0051 | 1.57 | 1.14 | 2.16 | 0.0062 |
| Term | HR | Lower_95 | Upper_95 | p-Value |
|---|---|---|---|---|
| molGPA 3.5–4 | Reference | |||
| molGPA 2.5–3 | 1.88 | 1.31 | 2.7 | 0.0007 |
| molGPA 1.5–2 | 2.83 | 1.98 | 4.03 | <0.0001 |
| molGPA 0–1 | 4.84 | 3.26 | 7.17 | <0.0001 |
| Factor | HR | p-Value | Lower 95 | Upper 95 |
|---|---|---|---|---|
| for no symptoms and no GCs as a reference group | ||||
| pts with no symptoms and on GCS | 1.81 | 0.0006 | 1.29 | 2.53 |
| pts with symptoms without GCS | 1.26 | 0.1767 | 0.90 | 1.78 |
| pts with symptoms on GCS | 1.98 | 0.0000 | 1.56 | 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placzke, J.; Teterycz, P.; Quaglino, P.; Cybulska-Stopa, B.; Tucci, M.; Rubatto, M.; Skora, T.; Interno, V.; Rosinska, M.; Borkowska, A.; et al. The Analysis of Trends in Survival for Patients with Melanoma Brain Metastases with Introduction of Novel Therapeutic Options before the Era of Combined Immunotherapy—Multicenter Italian–Polish Report. Cancers 2022, 14, 5763. https://doi.org/10.3390/cancers14235763
Placzke J, Teterycz P, Quaglino P, Cybulska-Stopa B, Tucci M, Rubatto M, Skora T, Interno V, Rosinska M, Borkowska A, et al. The Analysis of Trends in Survival for Patients with Melanoma Brain Metastases with Introduction of Novel Therapeutic Options before the Era of Combined Immunotherapy—Multicenter Italian–Polish Report. Cancers. 2022; 14(23):5763. https://doi.org/10.3390/cancers14235763
Chicago/Turabian StylePlaczke, Joanna, Paweł Teterycz, Pietro Quaglino, Bozena Cybulska-Stopa, Marco Tucci, Marco Rubatto, Tomasz Skora, Valeria Interno, Magdalena Rosinska, Aneta Borkowska, and et al. 2022. "The Analysis of Trends in Survival for Patients with Melanoma Brain Metastases with Introduction of Novel Therapeutic Options before the Era of Combined Immunotherapy—Multicenter Italian–Polish Report" Cancers 14, no. 23: 5763. https://doi.org/10.3390/cancers14235763
APA StylePlaczke, J., Teterycz, P., Quaglino, P., Cybulska-Stopa, B., Tucci, M., Rubatto, M., Skora, T., Interno, V., Rosinska, M., Borkowska, A., Szumera-Cieckiewicz, A., Mandala, M., & Rutkowski, P. (2022). The Analysis of Trends in Survival for Patients with Melanoma Brain Metastases with Introduction of Novel Therapeutic Options before the Era of Combined Immunotherapy—Multicenter Italian–Polish Report. Cancers, 14(23), 5763. https://doi.org/10.3390/cancers14235763









