Does Bladder Cancer with Inchworm Sign Indicate Better Prognosis after TURBT?
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection and Collection of Clinical Data
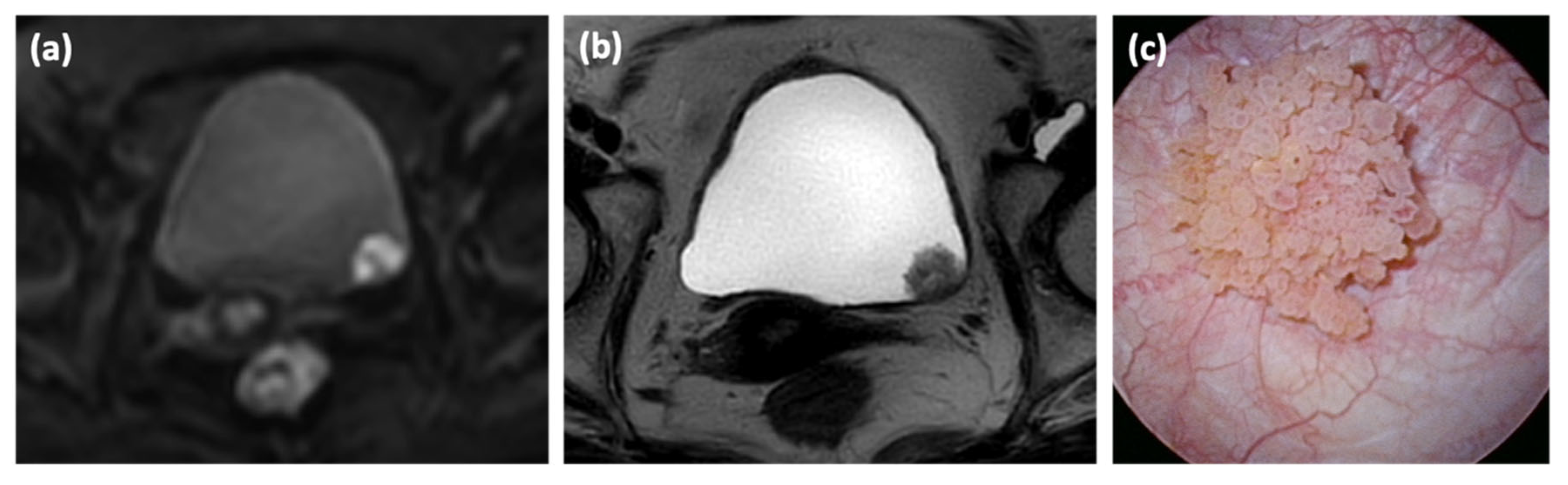
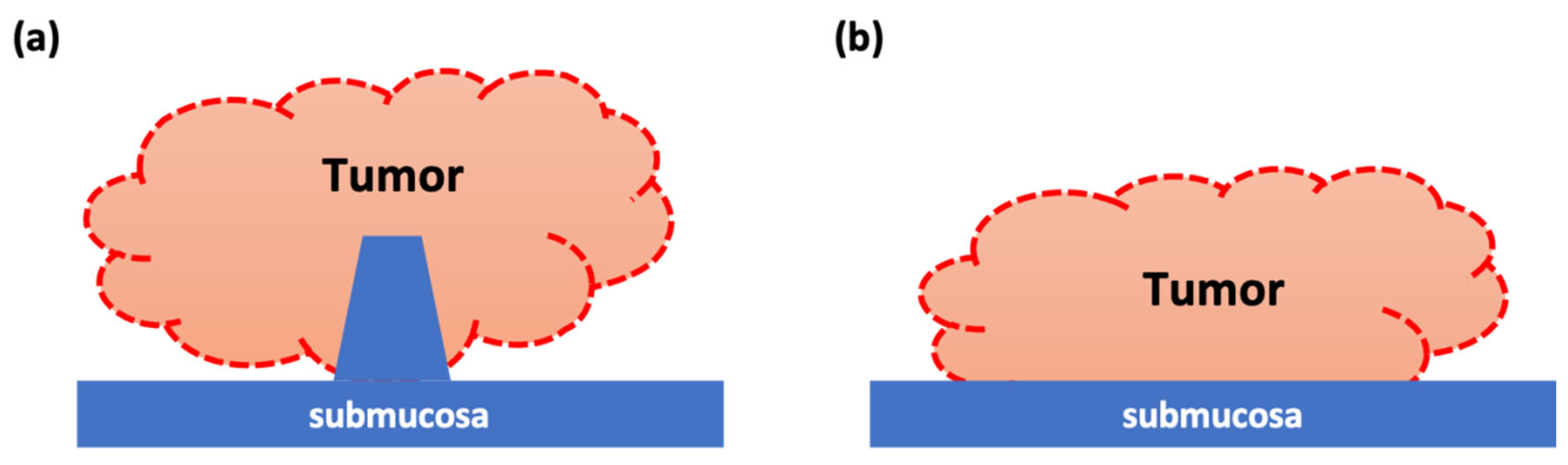
2.2. Image Analysis
2.3. Statistical Analyses
2.4. Ethical Considerations
3. Results
3.1. Patient Characteristics with Inchworm Sign
3.2. Predictors of MIBC in Patients with Inchworm Sign
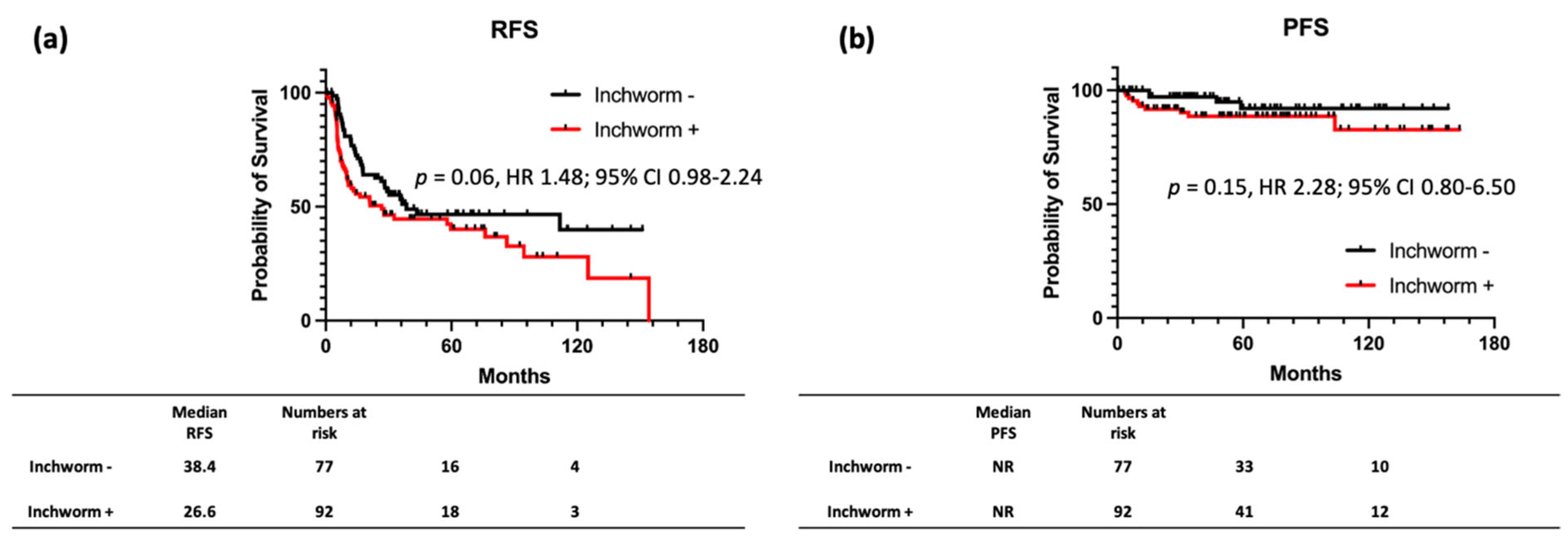
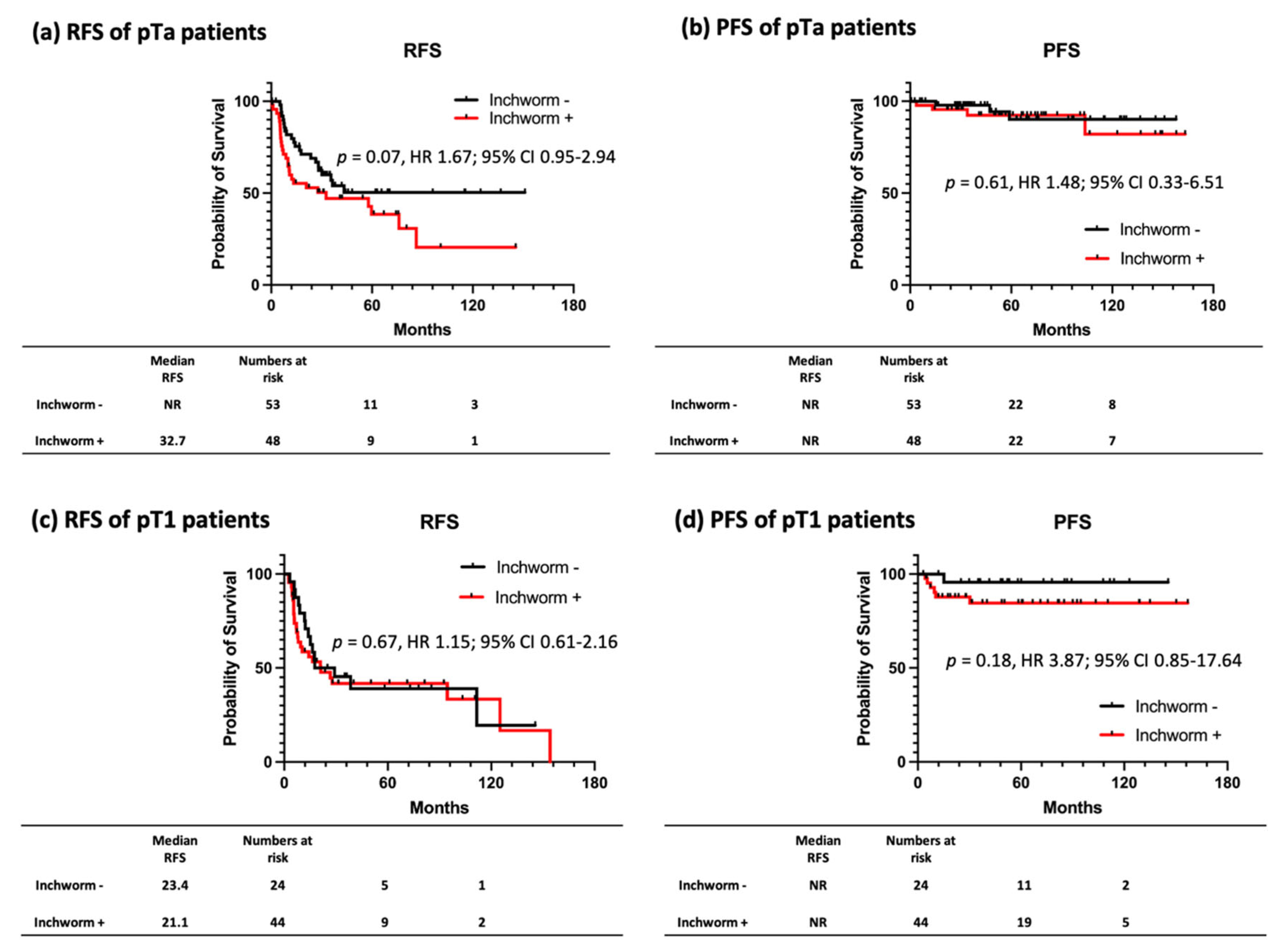
3.3. RFS and PFS Stratified by the Presence of Inchworm Sign on Patients with NMIBC
3.4. Prognostic Factors for RFS and PFS of Patients with NMIBC with/without Inchworm Sign
3.5. Interobserver Variability in Identification of Inchworm Sign
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Haghighi, M.; Horn, J.; Naik, M.; Hardie, A.D.; Somberg, M.B.; Melamed, J.; Xiao, G.Q.; Huang, W.C.; Taouli, B. Utility of quantitative MRI metrics for assessment of stage and grade of urothelial carcinoma of the bladder: Preliminary results. AJR Am. J. Roentgenol. 2013, 201, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Yoshida, S.; Takahara, T.; Kwee, T.C.; Waseda, Y.; Kobayashi, S.; Fujii, Y. DWI as an Imaging Biomarker for Bladder Cancer. AJR Am. J. Roentgenol. 2017, 208, 1218–1228. [Google Scholar] [CrossRef]
- El-Assmy, A.; Abou-El-Ghar, M.E.; Mosbah, A.; El-Nahas, A.R.; Refaie, H.F.; Hekal, I.A.; El-Diasty, T.; Ibrahiem el, H. Bladder tumour staging: Comparison of diffusion- and T2-weighted MR imaging. Eur. Radiol. 2009, 19, 1575–1581. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sasaki, S.; Ito, M.; Okada, S.; Takahashi, S.; Kawai, T.; Suzuki, K.; Oshima, H.; Hara, M.; Shibamoto, Y. Urinary bladder cancer: Diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009, 251, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Saito, W.; Amanuma, M.; Tanaka, J.; Heshiki, A. Histopathological analysis of a bladder cancer stalk observed on MRI. Magn. Reson. Imaging 2000, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Koga, F.; Yoshida, S.; Masuda, H.; Ishii, C.; Tanaka, H.; Komai, Y.; Yokoyama, M.; Saito, K.; Fujii, Y.; et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: Potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur. Radiol. 2011, 21, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Chao, W.M.; Kerr, J. Male pelvic anatomy reconstructed from the visible human data set. J. Urol. 1998, 159, 868–872. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, H.; Li, D.Q.; Zhang, P.; Ma, Q.T.; Zhai, L.D. Muscular structure at the male bladder outlet examined with successive celloidin-embedded slices. Urology 2015, 85, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Q.; Rashid, H. Bladder Neck Urothelial Carcinoma: A Urinary Bladder Subsite Carcinoma With Distinct Clinicopathology. Int. J. Surg. Pathol. 2015, 23, 517–523. [Google Scholar] [CrossRef]
- Wang, H.J.; Pui, M.H.; Guan, J.; Li, S.R.; Lin, J.H.; Pan, B.; Guo, Y. Comparison of Early Submucosal Enhancement and Tumor Stalk in Staging Bladder Urothelial Carcinoma. AJR Am. J. Roentgenol. 2016, 207, 797–803. [Google Scholar] [CrossRef]
- Yajima, S.; Yoshida, S.; Takahara, T.; Arita, Y.; Tanaka, H.; Waseda, Y.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Saito, K.; et al. Usefulness of the inchworm sign on DWI for predicting pT1 bladder cancer progression. Eur. Radiol. 2019, 29, 3881–3888. [Google Scholar] [CrossRef]
- Orsola, A.; Trias, I.; Raventós, C.X.; Español, I.; Cecchini, L.; Búcar, S.; Salinas, D.; Orsola, I. Initial high-grade T1 urothelial cell carcinoma: Feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur. Urol. 2005, 48, 231–238; discussion 238. [Google Scholar] [CrossRef]
- Smits, G.; Schaafsma, E.; Kiemeney, L.; Caris, C.; Debruyne, F.; Witjes, J.A. Microstaging of pT1 transitional cell carcinoma of the bladder: Identification of subgroups with distinct risks of progression. Urology 1998, 52, 1009–1013; discussion 1013–1014. [Google Scholar] [CrossRef]
- Kondylis, F.I.; Demirci, S.; Ladaga, L.; Kolm, P.; Schellhammer, P.F. Outcomes after intravesical bacillus Calmette-Guerin are not affected by substaging of high grade T1 transitional cell carcinoma. J. Urol. 2000, 163, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Jancke, G.; Rosell, J.; Chebil, G.; Jahnson, S. Bladder wash cytology at diagnosis of Ta-T1 bladder cancer is predictive for recurrence and progression. Urology 2012, 80, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshima, K.; Akitake, M.; Shiota, M.; Takeuchi, A.; Takahashi, R.; Inokuchi, J.; Tatsugami, K.; Yokomizo, A.; Eto, M. Prognostic Significance of Preoperative Urine Cytology in Low-grade Non-muscle-invasive Bladder Cancer. Anticancer Res. 2016, 36, 799–802. [Google Scholar] [PubMed]

| Variable | ||
|---|---|---|
| No. of patients | 109 | |
| Age (median) | 71 | |
| Sex | ||
| Male (%) | 90 (82.6) | |
| Female (%) | 19 (17.4) | |
| Histology | ||
| UC (%) | 94 (86.2) | |
| UC with squamous differentiation (%) | 10 (9.2) | |
| UC with glandular differentiation (%) | 5 (4.6) | |
| Pathological grade | ||
| 1 (%) | 4 (3.7) | |
| 2 (%) | 65 (59.6) | |
| 3 (%) | 30 (27.5) | |
| Unknown (%) | 10 (9.2) | |
| pT stage | ||
| Tis (%) | 2 (1.8) | |
| Ta (%) | 48 (44.0) | |
| T1 (%) | 44 (40.4) | |
| T2 (%) | 11 (10.1) | |
| T3 (%) | 2 (1.8) | |
| T4 (%) | 2 (1.8) | |
| Tumor size | ||
| 10–30 mm (%) | 71 (65.1) | |
| 30 mm < (%) | 38 (34.9) | |
| Tumor location | ||
| Anterior wall (%) | 13 (11.9) | |
| Posterior wall (%) | 17 (15.6) | |
| Side wall (%) | 59 (54.1) | |
| Dome (%) | 3 (2.8) | |
| Neck (%) | 17 (15.6) | |
| Papillary | ||
| Yes (%) | 92 (84.4) | |
| No (%) | 17 (15.6) | |
| Multiple tumors | ||
| Yes (%) | 46 (42.2) | |
| No (%) | 63 (57.8) | |
| Hematuria | ||
| Yes (%) | 82 (75.2) | |
| No (%) | 26 (23.9) | |
| Unknown (%) | 1 (0.9) | |
| Cytology | ||
| Negative (%) | 36 (33.0) | |
| Positive (%) | 73 (67.0) |
| Variables | Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| p Value | HR | Lower | Upper | p Value | HR | Lower | Upper | |
| Age ≥ 75 (years) | 0.49 | 1.48 | 0.49 | 4.42 | ||||
| Female | 0.72 | 0.75 | 0.15 | 3.65 | ||||
| Cytology positive | 0.15 | 3.12 | 0.65 | 14.89 | ||||
| Hematuria | 0.65 | 1.37 | 0.36 | 5.28 | ||||
| Multiple tumors | 0.04 | 3.22 | 1.02 | 10.19 | 0.09 | 3.38 | 0.83 | 13.69 |
| Tumor size >30 mm | <0.01 | 6.82 | 1.99 | 23.31 | 0.053 | 4.27 | 0.98 | 18.64 |
| Non-papillary tumor | <0.01 | 5.03 | 1.50 | 16.86 | <0.01 | 9.55 | 2.07 | 44.10 |
| Bladder neck | <0.01 | 10.79 | 3.17 | 36.75 | <0.01 | 7.73 | 1.83 | 32.76 |
| Variable | Inchworm Sign − | Inchworm Sign + | p Value | |
|---|---|---|---|---|
| No. of patients | 77 | 92 | ||
| Age (median) | 73 | 71 | 0.35 | |
| Sex | 0.75 | |||
| Male (%) | 65 (84.4) | 76 (82.6) | ||
| Female (%) | 12 (15.6) | 16 (17.4) | ||
| Tumor size | <0.01 | |||
| 10–30 mm (%) | 72 (98.7) | 65 (64.9) | ||
| >30 mm (%) | 5 (1.3) | 27 (35.1) | ||
| Multiple tumors | 0.42 | |||
| Yes (%) | 34 (44.2) | 35 (38.0) | ||
| No (%) | 43 (55.8) | 57 (62.0) | ||
| Histology | 0.66 | |||
| UC (%) | 71 (92.2) | 81 (88.0) | ||
| UC with squamous differentiation (%) | 2 (2.6) | 4 (4.3) | ||
| UC with glandular differentiation (%) | 4 (5.2) | 7 (8.7) | ||
| Pathological grade | 0.11 | |||
| 1 (%) | 9 (11.7) | 4 (4.3) | ||
| 2 (%) | 45 (58.4) | 61 (66.3) | ||
| 3 (%) | 11 (14.3) | 19 (20.7) | ||
| Unknown (%) | 12 (15.6) | 8 (8.7) | ||
| Cytology | 0.03 | |||
| Negative (%) | 38 (49.4) | 34 (37.0) | ||
| Positive (%) | 33 (42.9) | 56 (60.9) | ||
| Unknown (%) | 6 (16.5) | 2 (2.1) | ||
| pT stage | 0.01 | |||
| Ta (%) | 53 (68.8) | 44 (47.8) | ||
| T1 (%) | 24 (31.2) | 48 (52.2) | ||
| BCG treatment | 0.39 | |||
| Yes (%) | 15 (19.5) | 23 (25.0) | ||
| No (%) | 62 (80.5) | 69 (75.0) | ||
| PDD | 0.13 | |||
| Yes (%) | 3 (3.9) | 9 (9.8) | ||
| No (%) | 74 (96.1) | 82 (90.2) |
| (a) RFS | (b) PFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | p Value | HR | 95% CI | Upper | Variables | p Value | HR | 95% CI | Upper |
| Lower | Lower | ||||||||
| Age ≥ 75 (years) | 0.39 | 0.78 | 0.44 | 1.38 | Age ≥ 75 (years) | 0.72 | 1.27 | 0.36 | 4.53 |
| Male | 0.67 | 1.18 | 0.55 | 2.52 | Male | 0.43 | 2.29 | 0.29 | 18.09 |
| Tumor size ≥ 30 mm | 0.81 | 1.08 | 0.6 | 1.93 | Tumor size ≥ 30 mm | 0.15 | 2.51 | 0.73 | 8.72 |
| Bladder neck | 0.92 | 0.95 | 0.38 | 2.4 | Bladder neck | 0.5 | 0.04 | >0.01 | 440.34 |
| Non-papillary tumor | 0.41 | 0.61 | 0.19 | 1.97 | Non-papillary tumor | 0.52 | 0.04 | >0.01 | 573.38 |
| Multiple tumors | 0.29 | 0.74 | 0.42 | 1.3 | Multiple tumors | 0.58 | 0.68 | 0.18 | 2.64 |
| Hematuria | 0.66 | 1.15 | 0.61 | 2.17 | Hematuria | 0.25 | 3.38 | 0.43 | 26.8 |
| Positive cytology | 0.04 | 1.9 | 1.04 | 3.48 | Positive cytology | 0.15 | 47.15 | 0.25 | 9005.87 |
| BCG treatment | 0.82 | 0.93 | 0.48 | 1.8 | BCG treatment | 0.16 | 2.48 | 0.7 | 8.8 |
| PDD | 0.27 | 0.45 | 0.11 | 1.86 | PDD | 0.58 | 0.04 | >0.01 | 2761.93 |
| (a) RFS | (b) PFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | p Value | HR | 95% CI | Upper | Variables | p Value | HR | 95% CI | Upper |
| Lower | Lower | ||||||||
| Age ≥ 75 (years) | 0.13 | 1.64 | 0.86 | 3.14 | Age ≥ 75 (years) | 0.65 | 0.6 | 0.06 | 5.71 |
| Male | 0.28 | 1.78 | 0.63 | 5.07 | Male | 0.7 | 0.64 | 0.07 | 6.27 |
| Tumor size ≥ 30 mm | 0.09 | 2.83 | 0.85 | 9.34 | |||||
| Bladder neck | 0.47 | 0.65 | 0.2 | 2.12 | |||||
| Non-papillary tumor | 0.33 | 1.51 | 0.66 | 3.48 | Non-papillary tumor | 0.55 | 0.04 | >0.01 | 1955.36 |
| Multiple tumors | >0.01 | 3.04 | 1.56 | 5.9 | Multiple tumors | 0.5 | 0.46 | 0.05 | 4.39 |
| Hematuria | 0.7 | 1.14 | 0.59 | 2.17 | Hematuria | 0.99 | 0.98 | 0.14 | 7 |
| Positive cytology | 0.19 | 1.6 | 0.8 | 3.22 | Positive cytology | 0.21 | 4.28 | 0.44 | 41.43 |
| BCG treatment | 0.46 | 1.33 | 0.63 | 2.82 | BCG treatment | 0.82 | 1.31 | 0.14 | 12.63 |
| PDD | 0.88 | 1.12 | 0.27 | 4.69 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, R.; Izumi, K.; Naito, R.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; Yoshida, K.; et al. Does Bladder Cancer with Inchworm Sign Indicate Better Prognosis after TURBT? Cancers 2022, 14, 5767. https://doi.org/10.3390/cancers14235767
Nakagawa R, Izumi K, Naito R, Kadomoto S, Iwamoto H, Yaegashi H, Kawaguchi S, Nohara T, Shigehara K, Yoshida K, et al. Does Bladder Cancer with Inchworm Sign Indicate Better Prognosis after TURBT? Cancers. 2022; 14(23):5767. https://doi.org/10.3390/cancers14235767
Chicago/Turabian StyleNakagawa, Ryunosuke, Kouji Izumi, Renato Naito, Suguru Kadomoto, Hiroaki Iwamoto, Hiroshi Yaegashi, Shohei Kawaguchi, Takahiro Nohara, Kazuyoshi Shigehara, Kotaro Yoshida, and et al. 2022. "Does Bladder Cancer with Inchworm Sign Indicate Better Prognosis after TURBT?" Cancers 14, no. 23: 5767. https://doi.org/10.3390/cancers14235767
APA StyleNakagawa, R., Izumi, K., Naito, R., Kadomoto, S., Iwamoto, H., Yaegashi, H., Kawaguchi, S., Nohara, T., Shigehara, K., Yoshida, K., Kadono, Y., & Mizokami, A. (2022). Does Bladder Cancer with Inchworm Sign Indicate Better Prognosis after TURBT? Cancers, 14(23), 5767. https://doi.org/10.3390/cancers14235767





