Nanoplasmonics Enabling Cancer Diagnostics and Therapy
Abstract
Simple Summary
Abstract
1. Introduction
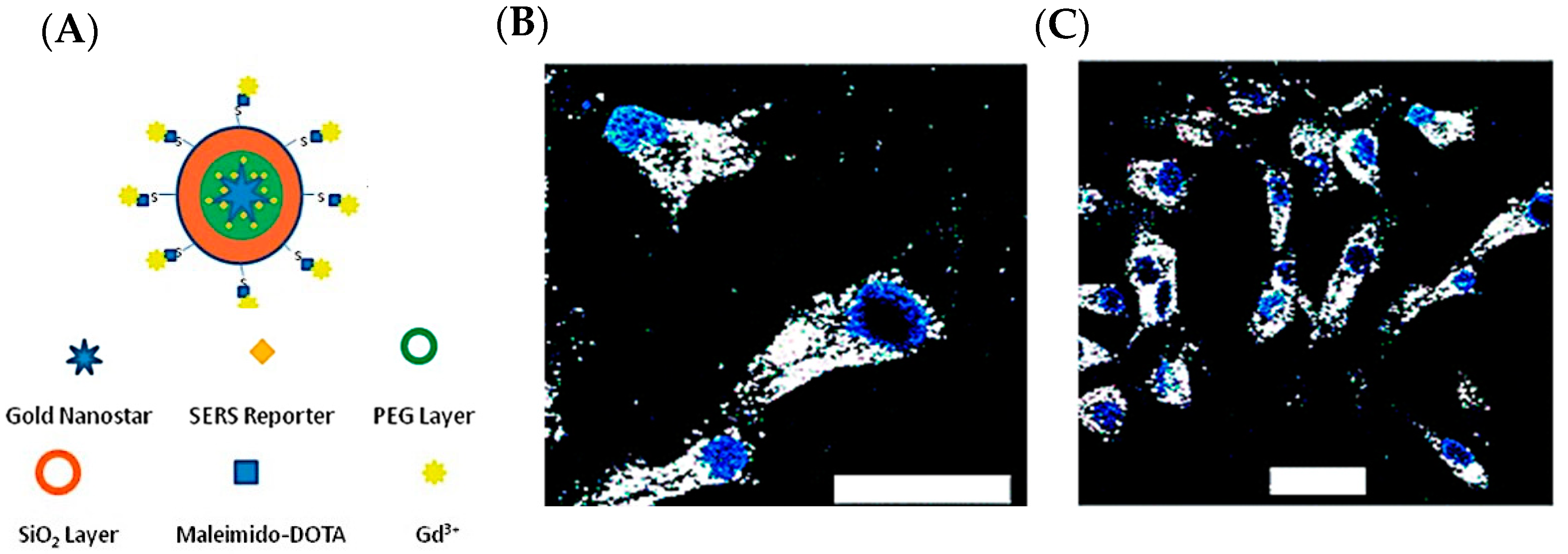
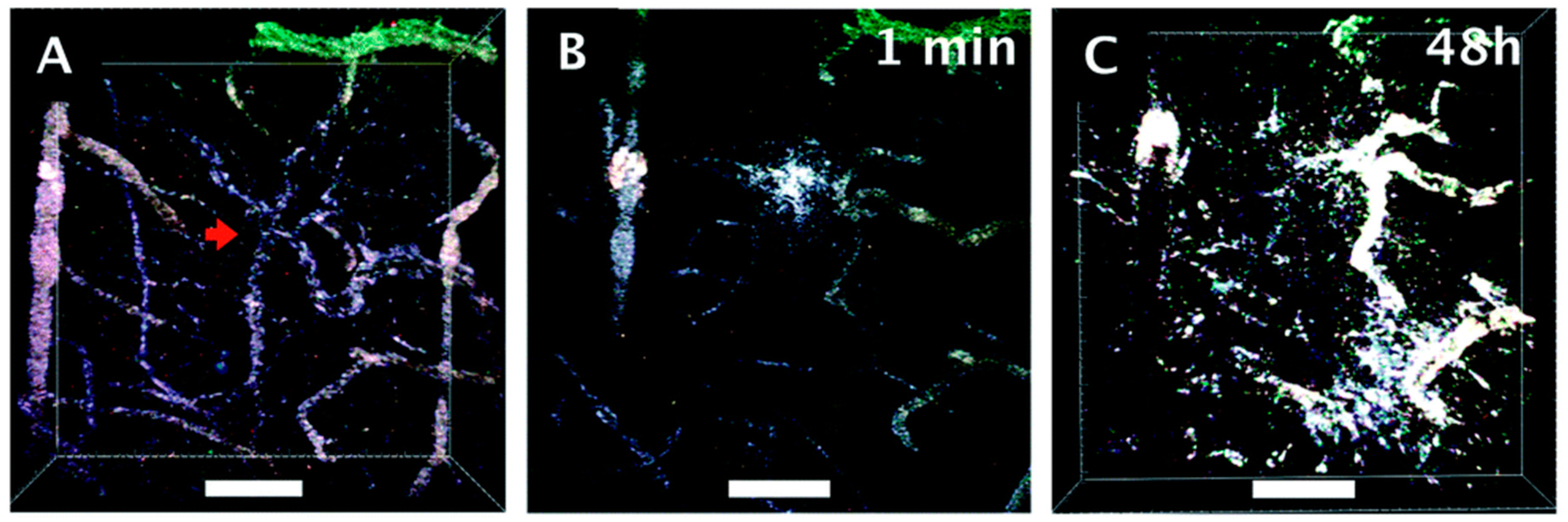
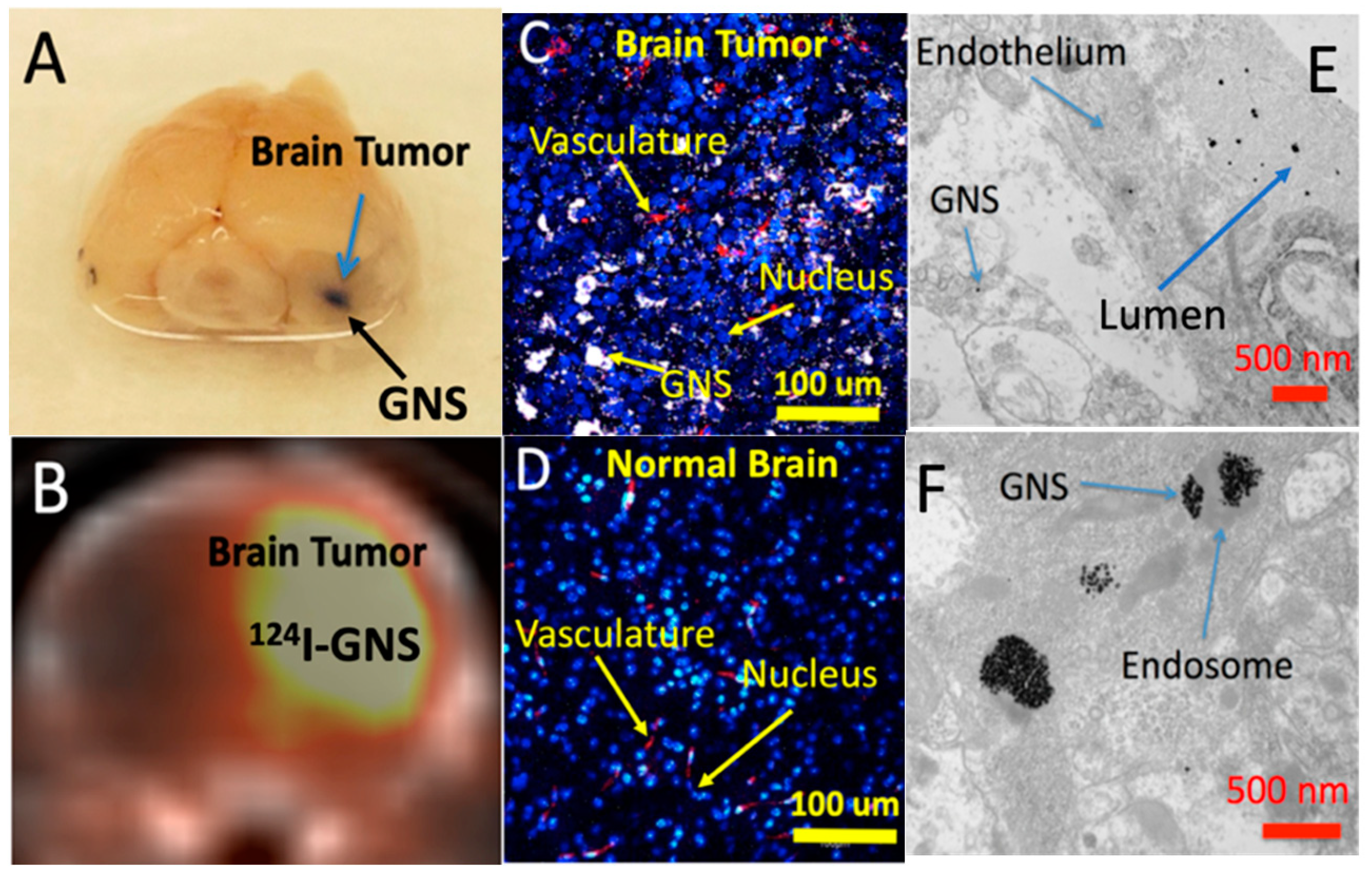
1.1. Plasmonic Nanoparticles for Tumor Imaging and Treatment
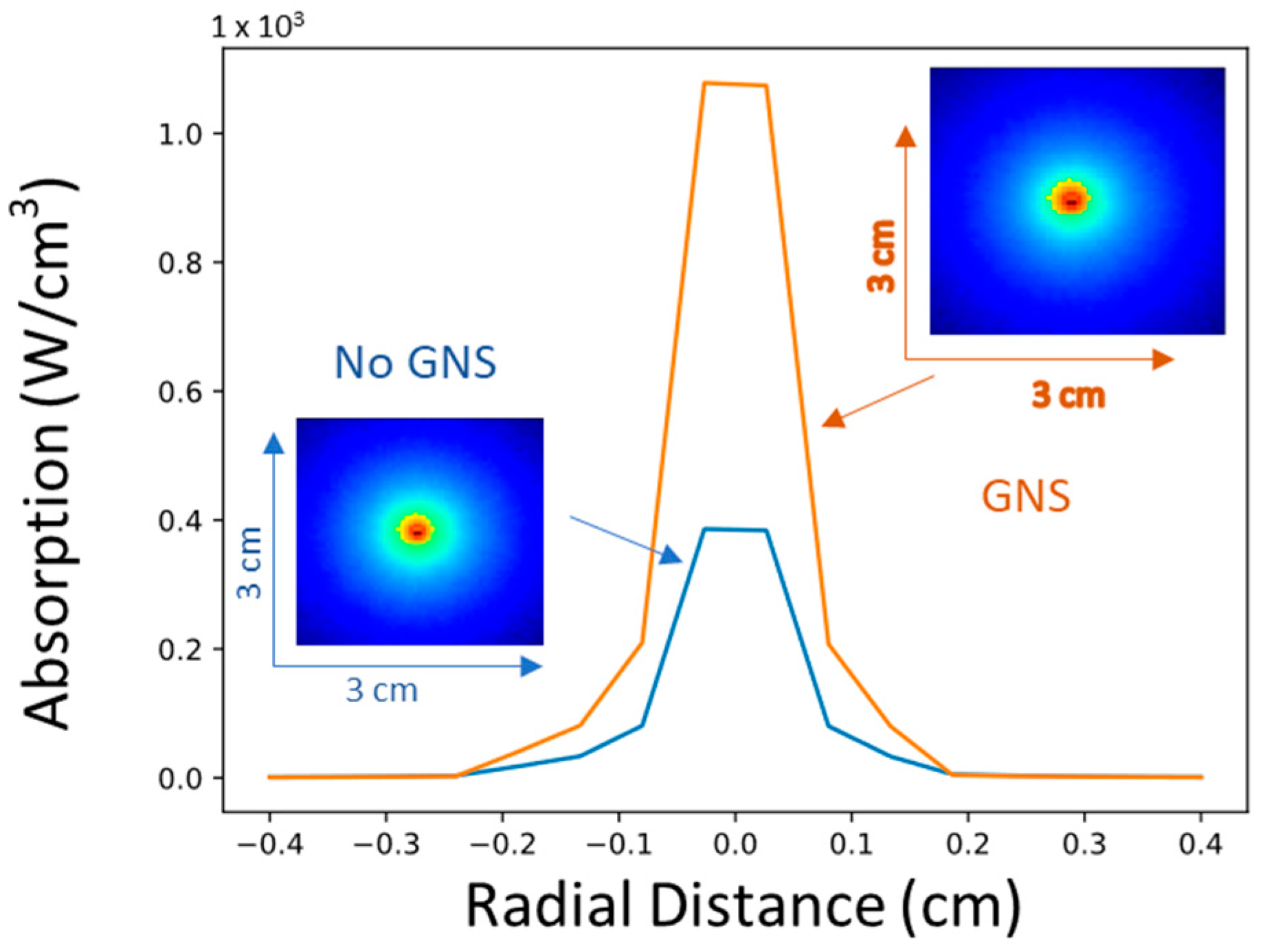
1.2. Photothermal Therapy (PTT)
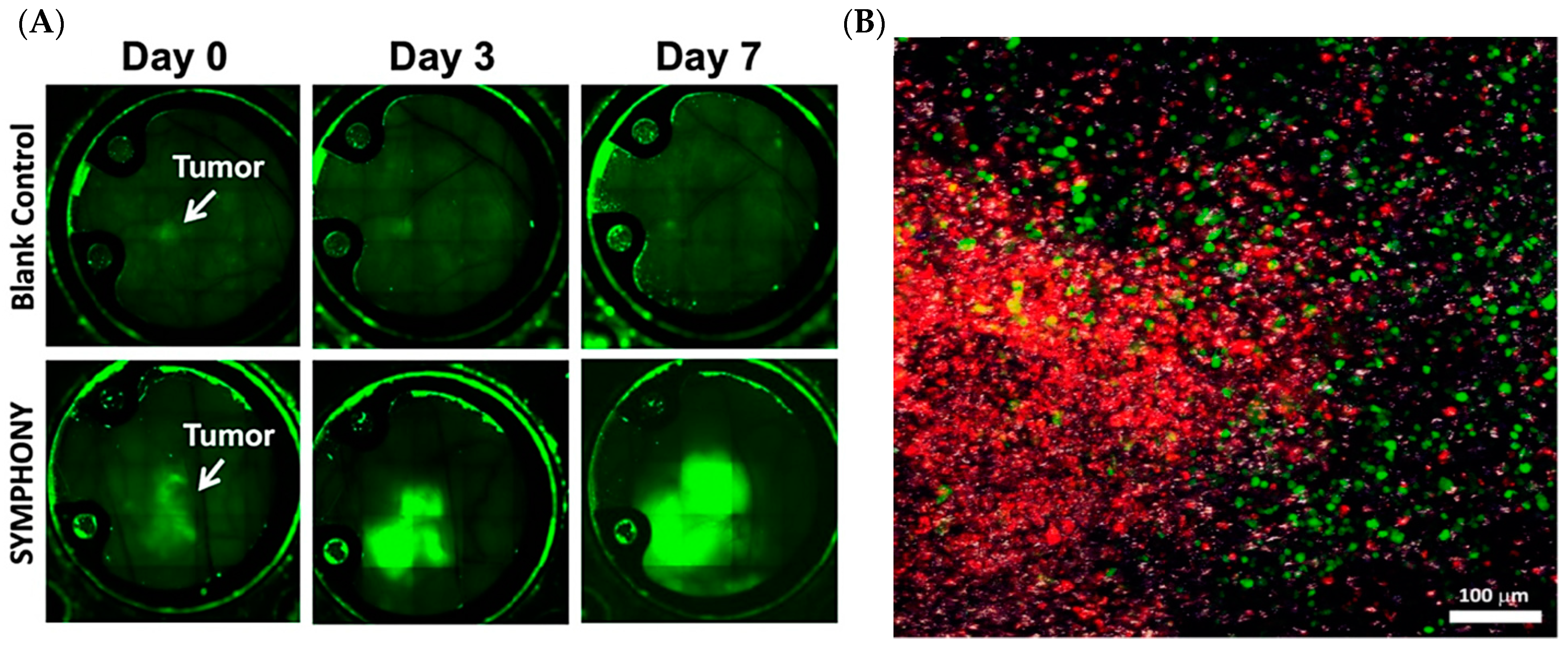
1.3. Synergistic Immuno Photo Nanotherapy (SYMPHONY)
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Filler, A.; Filler, A. The History, Development and Impact of Computed Imaging in Neurological Diagnosis and Neurosurgery: CT, MRI, and DTI. Nat. Preced. 2009. [Google Scholar] [CrossRef]
- Burton, S.; Brown, G.; Daniels, I.R.; Norman, A.R.; Mason, B.; Cunningham, D. MRI Directed Multidisciplinary Team Preoperative Treatment Strategy: The Way to Eliminate Positive Circumferential Margins? Br. J. Cancer 2006, 94, 351–357. [Google Scholar] [CrossRef]
- Perkins, A.; Liu, G. Primary Brain Tumors in Adults: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 211–217. [Google Scholar] [PubMed]
- Kong, S.G.; Martin, M.E.; Vo-Dinh, T. Hyperspectral Fluorescence Imaging for Mouse Skin Tumor Detection. ETRI J. 2006, 28, 770–776. [Google Scholar] [CrossRef]
- Palmer, G.M.; Zhu, C.; Breslin, T.M.; Xu, F.; Gilchrist, K.W.; Ramanujam, N. Comparison of Multiexcitation Fluorescence and Diffuse Reflectance Spectroscopy for the Diagnosis of Breast Cancer (March 2003). IEEE Trans. Biomed. Eng. 2003, 50, 1233–1242. [Google Scholar] [CrossRef]
- Liu, Q.; Grant, G.; Li, J.; Zhang, Y.; Hu, F.; Li, S.; Wilson, C.; Chen, K.; Bigner, D.; Vo-Dinh, T. Compact Point-Detection Fluorescence Spectroscopy System for Quantifying Intrinsic Fluorescence Redox Ratio in Brain Cancer Diagnostics. J. Biomed. Opt. 2011, 16, 037004. [Google Scholar] [CrossRef]
- Keller, M.D.; Wilson, R.H.; Mycek, M.A.; Mahadevan-Jansen, A. Monte Carlo Model of Spatially Offset Raman Spectroscopy for Breast Tumor Margin Analysis. Appl. Spectrosc. 2010, 64, 607–614. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.-A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A New Method Using Raman Spectroscopy for in Vivo Targeted Brain Cancer Tissue Biopsy. Sci. Rep. 2018, 8, 1792. [Google Scholar] [CrossRef]
- Caspers, P.J.; Lucassen, G.W.; Puppels, G.J. Combined In Vivo Confocal Raman Spectroscopy and Confocal Microscopy of Human Skin. Biophys. J. 2003, 85, 572–580. [Google Scholar] [CrossRef]
- Everall, N.J. Confocal Raman Microscopy: Common Errors and Artefacts. Analyst 2010, 135, 2512. [Google Scholar] [CrossRef]
- Palmer, G.M.; Ramanujam, N. Monte Carlo-Based Inverse Model for Calculating Tissue Optical Properties. Part I: Theory and Validation on Synthetic Phantoms. Appl. Opt. 2006, 45, 1062–1071. [Google Scholar] [CrossRef]
- Chisanga, M.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Enhancing Disease Diagnosis: Biomedical Applications of Surface-Enhanced Raman Scattering. Appl. Sci. 2019, 9, 1163. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A Plasmonic Gold Nanostar Theranostic Probe for In Vivo Tumor Imaging and Photothermal Therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Z.; Yuan, H.; Fales, A.M.; Vo-Dinh, T. Quintuple-Modality (SERS-MRI-CT-TPL-PTT) Plasmonic Nanoprobe for Theranostics. Nanoscale 2013, 5, 12126. [Google Scholar] [CrossRef]
- Liu, Y.; Carpenter, A.B.; Pirozzi, C.J.; Yuan, H.; Waitkus, M.S.; Zhou, Z.; Hansen, L.; Seywald, M.; Odion, R.; Greer, P.K.; et al. Non-Invasive Sensitive Brain Tumor Detection Using Dual-Modality Bioimaging Nanoprobe. Nanotechnology 2019, 30, 275101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carpenter, A.; Yuan, H.; Zhou, Z.; Zalutsky, M.; Vaidyanathan, G.; Yan, H.; Vo-Dinh, T. Gold Nanostar as Theranostic Probe for Brain Tumor Sensitive PET-Optical Imaging and Image-Guided Specific Photothermal Therapy. Cancer Res. 2016, 76, 4213. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.-M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, H.; Kersey, F.R.; Register, J.K.; Parrott, M.C.; Vo-Dinh, T. Plasmonic Gold Nanostars for Multi-Modality Sensing and Diagnostics. Sensors 2015, 15, 3706–3720. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A First-in-Human Phase 0 Clinical Study of RNA Interference–Based Spherical Nucleic Acids in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Chorniak, E.; Liu, Y.; Odion, R.; Etienne, W.; Canning, A.; Nair, S.K.; Maccarini, P.; Palmer, G.M.; Inman, B.A.; Vo-Dinh, T. Intravital Optical Imaging for Immune Cell Tracking after Photoimmunotherapy with Plasmonic Gold Nanostars. Nanotechnology 2022, 33, 475101. [Google Scholar] [CrossRef]
- Liu, Y.; Maccarini, P.; Palmer, G.M.; Etienne, W.; Zhao, Y.; Lee, C.-T.; Ma, X.; Inman, B.A.; Vo-Dinh, T. Synergistic Immuno Photothermal Nanotherapy (SYMPHONY) for the Treatment of Unresectable and Metastatic Cancers. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Harris, N.; Ford, M.J.; Cortie, M.B. Optimization of Plasmonic Heating by Gold Nanospheres and Nanoshells. J. Phys. Chem. B 2006, 110, 10701–10707. [Google Scholar] [CrossRef]
- Near, R.D.; Hayden, S.C.; Hunter, R.E.; Thackston, D.; El-Sayed, M.A. Rapid and Efficient Prediction of Optical Extinction Coefficients for Gold Nanospheres and Gold Nanorods. J. Phys. Chem. C 2013, 117, 23950–23955. [Google Scholar] [CrossRef]
- Liu, J.; Ong, W.; Román, E.; Lynn, M.J.; Kaifer, A.E. Cyclodextrin-Modified Gold Nanospheres. Langmuir 2000, 16, 3000–3002. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Appak-Baskoy, S.; Berndl, E.; Kolios, M.C. Laser Activatable Perfluorocarbon Bubbles for Imaging and Therapy through Enhanced Absorption from Coupled Silica Coated Gold Nanoparticles. RSC Adv. 2021, 11, 4906–4920. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Fernandes, D.D.; Malik, A.; Gomes, G.-N.W.; Appak-Baskoy, S.; Berndl, E.; Gradinaru, C.C.; Kolios, M.C. Multifunctional Nanoparticles as Theranostic Agents for Therapy and Imaging of Breast Cancer. J. Photochem. Photobiol. B 2021, 218, 112110. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Universal Scaling of Plasmon Coupling in Metal Nanostructures: Extension from Particle Pairs to Nanoshells. Nano Lett. 2007, 7, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.J.; Vo-Dinh, T. Plasmon Resonances of Nanoshells of Spheroidal Shape. IEEE Trans. Nanotechnol. 2007, 6, 627–638. [Google Scholar] [CrossRef]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars For Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Silica-Coated Gold Nanostars for Combined Surface-Enhanced Raman Scattering (SERS) Detection and Singlet-Oxygen Generation: A Potential Nanoplatform for Theranostics. Langmuir 2011, 27, 12186–12190. [Google Scholar] [CrossRef] [PubMed]
- De Silva Indrasekara, A.S.; Johnson, S.F.; Odion, R.A.; Vo-Dinh, T. Manipulation of the Geometry and Modulation of the Optical Response of Surfactant-Free Gold Nanostars: A Systematic Bottom-Up Synthesis. ACS Omega 2018, 3, 2202–2210. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Dhawan, A.; Norton, S.J.; Khoury, C.G.; Wang, H.-N.; Misra, V.; Gerhold, M.D. Plasmonic Nanoparticles and Nanowires: Design, Fabrication and Application in Sensing. J. Phys. Chem. C 2010, 114, 7480–7488. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Hiromoto, M.Y.K.; Begun, G.M.; Moody, R.L. Surface-Enhanced Raman Spectrometry for Trace Organic Analysis. Anal. Chem. 1984, 56, 1667–1670. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Surface-Enhanced Raman Spectroscopy Using Metallic Nanostructures. TrAC Trends Anal. Chem. 1998, 17, 557–582. [Google Scholar] [CrossRef]
- Yan, F.; Wabuyele, M.B.; Griffin, G.D.; Vass, A.A.; Vo-Dinh, T. Surface-Enhanced Raman Scattering Detection of Chemical and Biological Agent Simulants. IEEE Sens. J. 2005, 5, 665–670. [Google Scholar] [CrossRef]
- Chen, K.; Leona, M.; Vo-Dinh, T. Surface-enhanced Raman Scattering for Identification of Organic Pigments and Dyes in Works of Art and Cultural Heritage Material. Sens. Rev. 2007, 27, 109–120. [Google Scholar] [CrossRef]
- Yuan, H.; Wilson, C.M.; Xia, J.; Doyle, S.L.; Li, S.; Fales, A.M.; Liu, Y.; Ozaki, E.; Mulfaul, K.; Hanna, G.; et al. Plasmonics-Enhanced and Optically Modulated Delivery of Gold Nanostars into Brain Tumor. Nanoscale 2014, 6, 4078–4082. [Google Scholar] [CrossRef] [PubMed]
- Odion, R.; Liu, Y.; Vo-Dinh, T. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic Cell Death, DAMPs and Anticancer Therapeutics: An Emerging Amalgamation. Biochim. Biophys. Acta BBA—Rev. Cancer 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Chemo-, Immuno-, and Combined Therapy: Review [Invited]. Biomed. Opt. Express 2019, 10, 3152. [Google Scholar] [CrossRef]
- Liu, Y.; Chongsathidkiet, P.; Crawford, B.M.; Odion, R.; Dechant, C.A.; Kemeny, H.R.; Cui, X.; Maccarini, P.F.; Lascola, C.D.; Fecci, P.E.; et al. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy for Brain Tumor Ablation and Immunologic Memory. Immunotherapy 2019, 11, 1293–1302. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P.; Chen, L.; Baird, J.R.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Fiering, S. Local Hyperthermia Treatment of Tumors Induces CD8+ T Cell-Mediated Resistance against Distal and Secondary Tumors. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1273–1285. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in Oncology. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2001, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and New Facts about Hyperthermia-Induced Modulations of the Immune System. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Norton, S.J.; Vo-Dinh, T. Photothermal Effects of Plasmonic Metal Nanoparticles in a Fluid. J. Appl. Phys. 2016, 119, 083105. [Google Scholar] [CrossRef]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Biomedical Photonics Handbook: Biomedical Diagnostics; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1-4200-8515-8. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odion, R.A.; Liu, Y.; Vo-Dinh, T. Nanoplasmonics Enabling Cancer Diagnostics and Therapy. Cancers 2022, 14, 5737. https://doi.org/10.3390/cancers14235737
Odion RA, Liu Y, Vo-Dinh T. Nanoplasmonics Enabling Cancer Diagnostics and Therapy. Cancers. 2022; 14(23):5737. https://doi.org/10.3390/cancers14235737
Chicago/Turabian StyleOdion, Ren A., Yang Liu, and Tuan Vo-Dinh. 2022. "Nanoplasmonics Enabling Cancer Diagnostics and Therapy" Cancers 14, no. 23: 5737. https://doi.org/10.3390/cancers14235737
APA StyleOdion, R. A., Liu, Y., & Vo-Dinh, T. (2022). Nanoplasmonics Enabling Cancer Diagnostics and Therapy. Cancers, 14(23), 5737. https://doi.org/10.3390/cancers14235737





