Preoperative Cancer Antigen 125 Level as Predictor for Complete Cytoreduction in Ovarian Cancer: A Prospective Cohort Study and Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
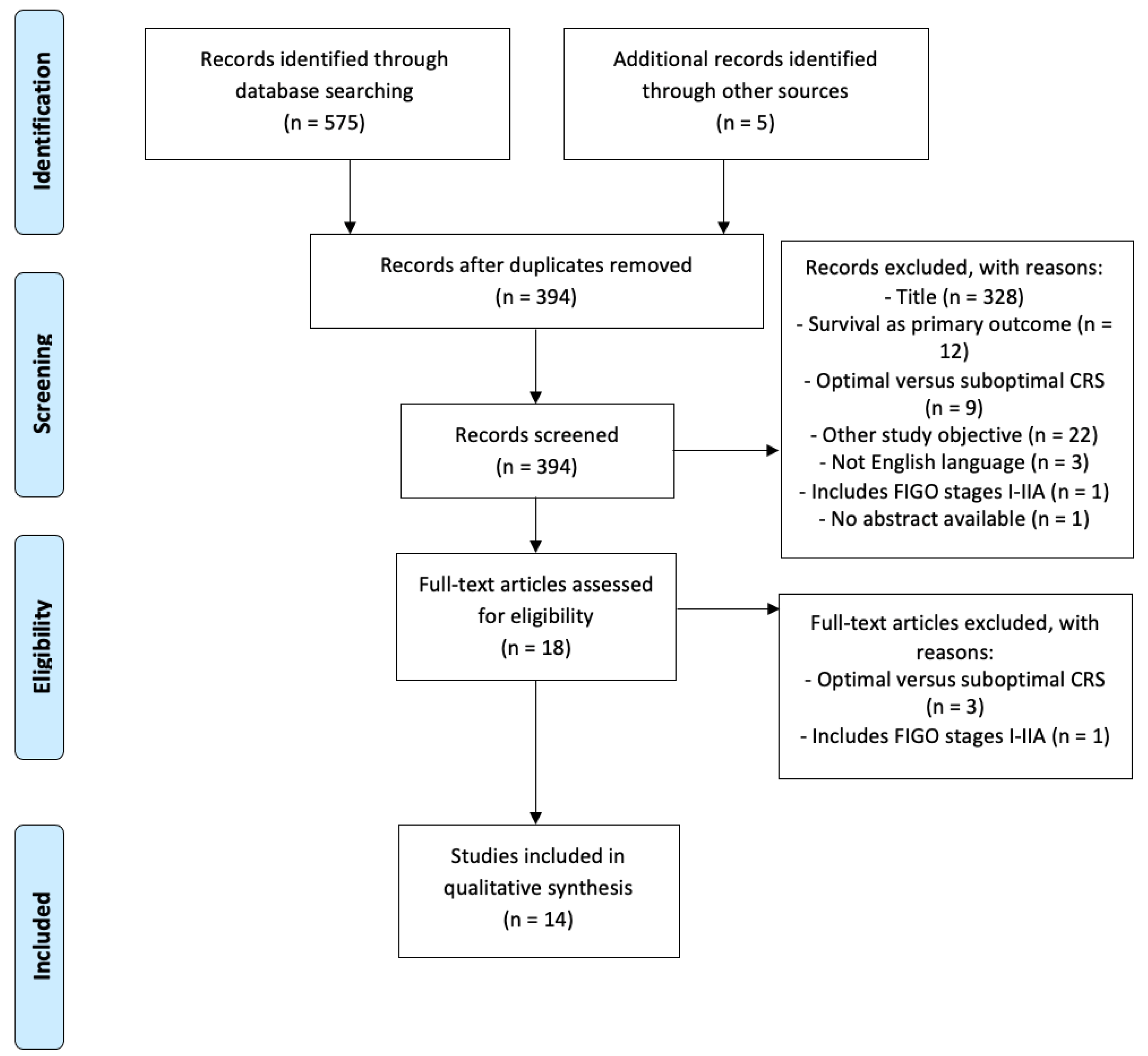
2.1. Systematic Review
2.1.1. Search Strategy
2.1.2. Eligibility Criteria
2.1.3. Data Extraction
2.1.4. Quality Assessment
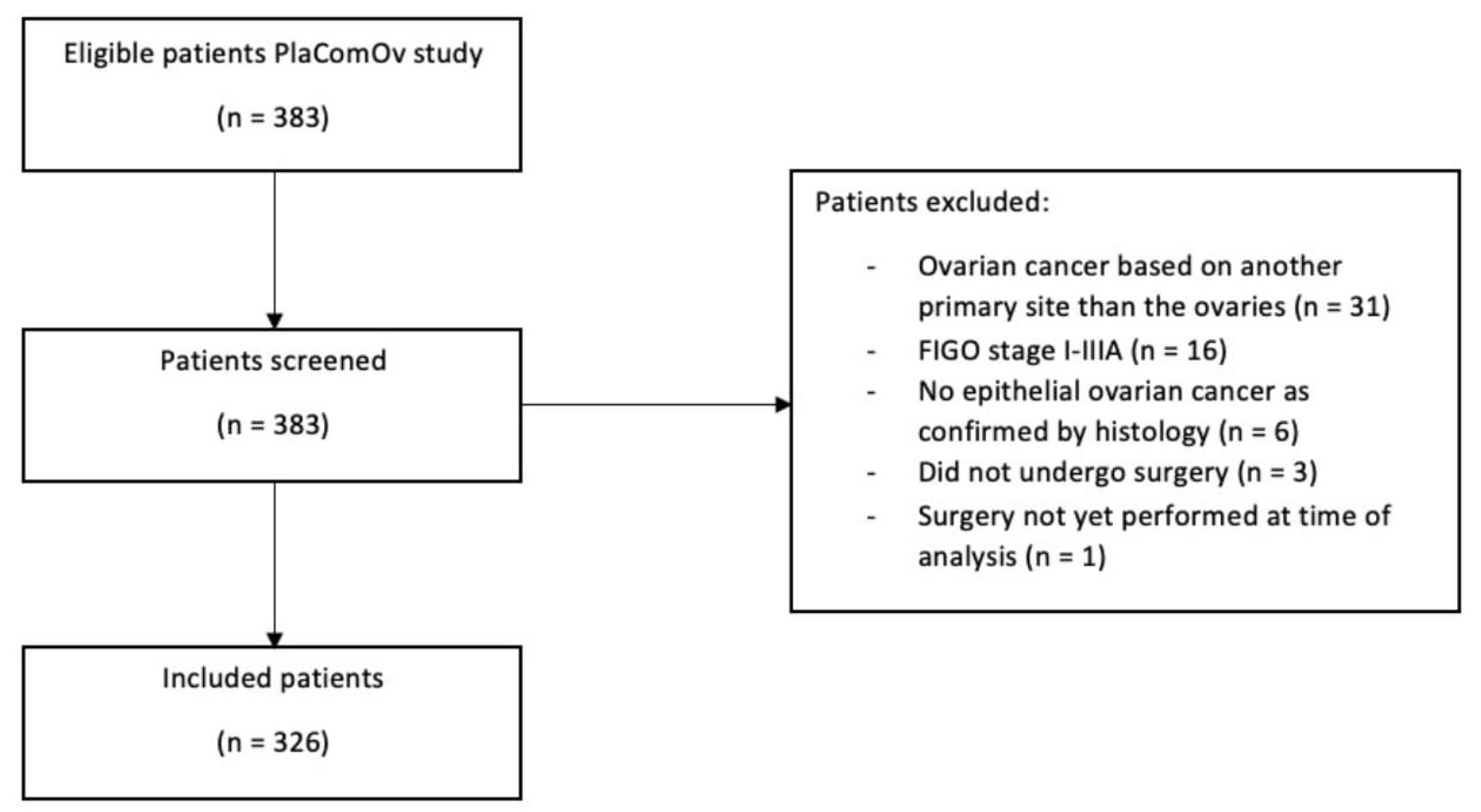
2.2. Prospective Study
2.2.1. Inclusion and Exclusion Criteria
2.2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Systematic Review
3.1.1. General Characteristics of the Studies
3.1.2. Primary Cytoreductive Surgery and CA-125 Level
3.1.3. Interval Cytoreductive Surgery and CA-125 Level
3.2. Prospective Study
3.2.1. Patient Characteristics and Surgical Outcomes
3.2.2. Primary Cytoreductive Surgery and CA-125 Level
3.2.3. Interval Cytoreductive Surgery and CA-125 Level
3.2.4. Reduction of CA-125 Level after NACT
3.2.5. Multivariable Analysis
3.2.6. ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA-125 | Cancer Antigen 125 |
| CRS | Cytoreductive Surgery |
| CT | Computed Tomography |
| EOC | Epithelial Ovarian Cancer |
| FIGO | International Federation of Gynecology and Obstetrics |
| NACT | Neoadjuvant Chemotherapy |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kruitwagen, R.; Van de Vijver, K.; Sonke, G.; Van der Aa, M. Eierstokkanker Integraal Kankercentrum Nederland. Richtlijn Ovariumcarcinoom in Nederland. Available online: https://www.iknl.nl/kankersoorten/eierstokkanker (accessed on 8 October 2022).
- Tholander, B.; Taube, A.; Lindgren, A.; Sjöberg, O.; Stendahl, U.; Kiviranta, A.; Hallman, K.; Holm, L.; Weiner, E.; Tamsen, L. Pretreatment serum levels of CA-125, carcinoembryonic antigen, tissue polypeptide antigen, and placental alkaline phosphatase, in patients with ovarian carcinoma, borderline tumors, or benign adnexal masses: Relevance for differential diagnosis. Gynecol. Oncol. 1990, 39, 16–25. [Google Scholar] [CrossRef]
- Duffy, M.J.; Bonfrer, J.M.; Kulpa, J.; Rustin, G.J.; Soletormos, G.; Torre, G.C.; Tuxen, M.K.; Zwirner, M. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int. J. Gynecol. Cancer 2005, 15, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, T.J.; Nam, B.H.; Seo, S.S.; Kim, B.G.; Bae, D.S.; Park, S.Y. Preoperative serum CA-125 levels and risk of suboptimal cytoreduction in ovarian cancer: A meta-analysis. J. Surg. Oncol. 2010, 101, 13–17. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Y.X.; Luo, S.J.; Zhou, R.; Jiang, Q.X.; Linghu, H. Serum CA125 levels predict outcome of interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer. Clin. Chim. Acta 2018, 484, 32–35. [Google Scholar] [CrossRef]
- Chi, D.S.; Venkatraman, E.S.; Masson, V.; Hoskins, W.J. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol. Oncol. 2000, 77, 227–231. [Google Scholar] [CrossRef]
- Zeng, J.; Yin, J.; Song, X.; Jin, Y.; Li, Y.; Pan, L. Reduction of CA125 Levels During Neoadjuvant Chemotherapy Can Predict Cytoreduction to No Visible Residual Disease in Patients with Advanced Epithelial Ovarian Cancer, Primary Carcinoma of Fallopian tube and Peritoneal Carcinoma. J. Cancer 2016, 7, 2327–2332. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Nieuwenhuyzen-de Boer, G.M.; Hofhuis, W.; Reesink-Peters, N.; Ewing-Graham, P.C.; Schoots, I.G.; Beltman, J.J.; Piek, J.M.J.; Baalbergen, A.; Kooi, G.S.; van Haaften, A.; et al. Evaluation of effectiveness of the PlasmaJet surgical device in the treatment of advanced stage ovarian cancer (PlaComOv-study): Study protocol of a randomized controlled trial in the Netherlands. BMC Cancer 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Xu, F.J.; Yu, Y.H.; Barnhill, S.; Zhang, Z.; Mills, G.B. CA 125: The past and the future. Int. J. Biol. Markers 1998, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.A.; Fago-Olsen, C.; Hogdall, E.; Schnack, T.H.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Lydolph, M.C.; Engelholm, S.A.; Hogdall, C. A novel index for preoperative, non-invasive prediction of macro-radical primary surgery in patients with stage IIIC-IV ovarian cancer-a part of the Danish prospective pelvic mass study. Tumour Biol. 2016, 37, 12619–12626. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.C.; Kang, S.; Kim, S.C.; Kim, J.W.; Nam, J.H.; Ryu, S.Y.; Seong, S.J.; Kim, B.G. Use of complex surgical procedures, patterns of tumor spread, and CA-125 predicts a risk of incomplete cytoreduction: A Korean Gynecologic Oncology Group study (KGOG-3022). Gynecol. Oncol. 2013, 131, 336–340. [Google Scholar] [CrossRef]
- Eltabbakh, G.H.; Mount, S.L.; Beatty, B.; Simmons-Arnold, L.; Cooper, K.; Morgan, A. Factors associated with cytoreducibility among women with ovarian carcinoma. Gynecol. Oncol. 2004, 95, 377–383. [Google Scholar] [CrossRef]
- Gupta, M.; Patel, S.; Arora, R.; Tiwari, R.; Dave, P.; Desai, A.; Mankad, M. Does preoperative CA-125 cutoff value and percent reduction in CA-125 levels correlate with surgical and survival outcome after neoadjuvant chemotherapy in patients with advanced-stage ovarian cancer?—Our experience from a tertiary cancer institute. South Asian J. Cancer 2020, 9, 30–33. [Google Scholar] [CrossRef]
- Matsuhashi, T.; Takeshita, T.; Yamamoto, A.; Kawase, R.; Yamada, T.; Kurose, K.; Doi, D.; Konnai, K.; Onose, R.; Kato, H. Serum ca 125 level after neoadjuvant chemotherapy is predictive of prognosis and debulking surgery outcomes in advanced epithelial ovarian cancer. J. Nippon Med. Sch. 2017, 84, 170–176. [Google Scholar] [CrossRef][Green Version]
- Pelissier, A.; Bonneau, C.; Chéreau, E.; de La Motte Rouge, T.; Fourchotte, V.; Daraï, E.; Rouzier, R. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol. Oncol. 2014, 135, 542–546. [Google Scholar] [CrossRef]
- Furukawa, N.; Sasaki, Y.; Shigemitsu, A.; Akasaka, J.; Kanayama, S.; Kawaguchi, R.; Kobayashi, H. CA-125 cut-off value as a predictor for complete interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer. J. Gynecol. Oncol. 2013, 24, 141–145. [Google Scholar] [CrossRef][Green Version]
- Rodriguez, N.; Rauh-Hain, J.A.; Shoni, M.; Berkowitz, R.S.; Muto, M.G.; Feltmate, C.; Schorge, J.O.; Del Carmen, M.G.; Matulonis, U.A.; Horowitz, N.S. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol. Oncol. 2012, 125, 362–366. [Google Scholar] [CrossRef]
- Ghisoni, E.; Katsaros, D.; Maggiorotto, F.; Aglietta, M.; Vaira, M.; De Simone, M.; Mittica, G.; Giannone, G.; Robella, M.; Genta, S.; et al. A predictive score for optimal cytoreduction at interval debulking surgery in epithelial ovarian cancer: A two- centers experience. J. Ovarian Res. 2018, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kitahara, Y.; Nishimura, T.; Yamashita, S.; Kigure, K.; Ito, I.; Kanuma, T. Nadir CA-125 serum levels during neoadjuvant chemotherapy and no residual tumor at interval debulking surgery predict prognosis in advanced stage ovarian cancer. World J. Surg. Oncol. 2020, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Risum, S.; Høgdall, E.; Engelholm, S.A.; Fung, E.; Lomas, L.; Yip, C.; Petri, A.L.; Nedergaard, L.; Lundvall, L.; Høgdall, C. A proteomics panel for predicting optimal primary cytoreduction in stage III/IV ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Nagao, S.; Kogiku, A.; Yamamoto, K.; Miwa, M.; Wakahashi, S.; Ichida, K.; Sudo, T.; Yamaguchi, S.; Fujiwara, K. A preoperative low cancer antigen 125 level (≤25.8 mg/dL) is a useful criterion to determine the optimal timing of interval debulking surgery following neoadjuvant chemotherapy in epithelial ovarian cancer. Jpn. J. Clin. Oncol. 2016, 46, 517–521. [Google Scholar] [CrossRef]
- Merlo, S.; Besic, N.; Drmota, E.; Kovacevic, N. Preoperative serum CA-125 level as a predictor for the extent of cytoreduction in patients with advanced stage epithelial ovarian cancer. Radiol. Oncol. 2021, 55, 341–346. [Google Scholar] [CrossRef]
- Nieuwenhuyzen-de Boer, G.M.; Hofhuis, W.; Reesink-Peters, N.; Willemsen, S.; Boere, I.A.; Schoots, I.G.; Piek, J.M.J.; Hofman, L.N.; Beltman, J.J.; van Driel, W.J.; et al. Adjuvant Use of PlasmaJet Device during Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: Results of the PlaComOv-study, a Randomized Controlled Trial in The Netherlands. Ann. Surg. Oncol. 2022, 29, 4833–4843. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; Del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J., Jr. A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome. Am. J. Obstet. Gynecol. 2019, 221, 326.e1–326.e7. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Gerestein, C.G.; Damhuis, R.A.; de Vries, M.; Reedijk, A.; Burger, C.W.; Kooi, G.S. Causes of postoperative mortality after surgery for ovarian cancer. Eur. J. Cancer 2009, 45, 2799–2803. [Google Scholar] [CrossRef]
- Di Donato, V.; Kontopantelis, E.; Aletti, G.; Casorelli, A.; Piacenti, I.; Bogani, G.; Lecce, F.; Benedetti Panici, P. Trends in Mortality after Primary Cytoreductive Surgery for Ovarian Cancer: A Systematic Review and Metaregression of Randomized Clinical Trials and Observational Studies. Ann. Surg. Oncol. 2017, 24, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Tankou, J.I.; Foley, O.; Falzone, M.; Kalyanaraman, R.; Elias, K.M. Enhanced recovery after surgery protocols improve time to return to intended oncology treatment following interval cytoreductive surgery for advanced gynecologic cancers. Int. J. Gynecol. Cancer 2021, 31, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; du Bois, A.; Hahmann, M.; Hasenburg, A.; Burges, A.; Loibl, S.; Gropp, M.; Huober, J.; Fink, D.; Schröder, W.; et al. Surgery in recurrent ovarian cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 2006, 13, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Garganese, G.; Vizzielli, G.; Carone, V.; Salerno, M.G.; Scambia, G. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am. J. Obstet. Gynecol. 2008, 199, 642.e1–642.e6. [Google Scholar] [CrossRef] [PubMed]
| Authors | Journal, Publication Year | Sample Size | FIGO Staging | Primary Outcome Measures | Type of CRS | CA-125 Reduction after NACT | Surgical Outcome | Number of NACT Cycles | Results for Preoperative CA-125 | Results for CA-125 Reduction after NACT | Optimal CA-125 Cut-Off Value | Additional Information |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eltabbakh et al. [14] | Gynecol Oncol, 2004 | 72 | 15.3% Stage IIIA 5.6% Stage IIIB 61.1% Stage IIIC 18.1% Stage IV | Relation between preoperative CA-125 and surgical outcome | Primary CRS | N/A | Complete vs. non-complete CRS | N/A | In univariable analysis, preoperative levels of CA-125 predict complete CRS (p < 0.001). In multivariable analysis, preoperative CA-125 was not significantly associated with complete CRS. | N/A | ≤500 kU/L preoperative | No or small amount of ascites significantly correlated with complete CRS (p < 0.001) in univariable analysis. In multivariable analysis, the only independent predictor of complete CRS was p53 expression (p < 0.001). |
| Risum et al. [22] | Int J Gynecol Cancer, 2009 | 75 | 86.7% Stage III 13.3% Stage IV | The predictive value of preoperative CA-125 on incomplete CRS | Primary CRS | N/A | Complete vs. non-complete CRS | N/A | Preoperative CA-125 levels are significantly lower in patients undergoing complete CRS (p = 0.03). | N/A | N/A | Intestinal carcinosis was found in 92% of incomplete CRS and in only 13% of complete CRS. |
| Rodriguez et al. [19] | Gynecol Oncol, 2012 | 103 | 40.5% Stage IIIC 59.5% Stage IV | Relation between change of CA-125 in patients undergoing NACT and surgical outcome | Interval CRS | Overall percentage change, >80% change from presentation to operation and prior to each NACT cycle | Complete vs. optimal CRS | Median: 3 cycles, Range: 1–8 cycles | Lower preoperative CA-125 levels, especially ≤100 kU/L, significantly correlate with complete CRS (p = 0.04). | No significant relation between decrease in CA-125 during NACT and complete CRS. | ≤100 kU/L preoperative | |
| Furukawa et al. [18] | J Gynecol Oncol, 2013 | 75 | 6.7% Stage IIIA 93.3% Stage IIIC | Relation between CA-125 after NACT and surgical outcome Relation between changes of CA-125 levels during NACT and surgical outcome | Interval CRS | Rates of changes prior to each NACT cycle | Complete vs. non-complete CRS | 3 cycles: 100% | Preoperative CA-125 levels are significantly lower in patients undergoing complete CRS (p < 0.001) in both univariable and multivariable analysis. | In univariable analysis, (pre-NACT CA-125—pre-2nd NACT CA-125)/pre-NACT CA-125 (p = 0.01) and (pre-NACT CA-125—pre-3rd NACT CA-125)/pre-NACT CA-125 (p = 0.008) significantly predicted complete CRS. In multivariable analysis, there was no significant relation between decrease in CA-125 during NACT and complete CRS. | ≤20 kU/L preoperative | |
| Jung et al. [12] | Gynecol Oncol, 2013 | 358 | 12.1% Stage IIC 71.5% Stage III 15.1% Stage IV 0.3% undocumented | Developing a model to predict non-complete CRS with CA-125, CT scan, age and surgical skill index | Primary CRS | N/A | Complete vs. non-complete CRS | N/A | Higher preoperative CA-125 levels significantly predict non-complete CRS (p = 0.001). | N/A | N/A | CA-125 (p = 0.001), two CT factor scores and surgical skill index were included in the model. |
| Pelissier et al. [17] | Gynecol Oncol, 2014 | 148 | 72.3% Stage IIIC 23.6% Stage IV | Relation between kinetic CA-125 levels during NACT and surgical outcome | Interval CRS | Percentage decrease in CA-125 | Complete vs. non-complete CRS | Median: 6 cycles, Range: 1–9 cycles | In univariable analysis, preoperative CA-125 (p = 0.001) significantly predicts complete CRS, but not according to multivariable analysis. | In univariable analysis, level of CA-125 after 3 cycles of NACT (p = 0.00001), cycle to nadir (p = 0.001) and percentage decrease (p = 0.01) significantly predict complete CRS. Multivariable analysis shows that only CA-125 after 3 cycles of NACT independently predicts complete CRS (p = 0.04). | <75 kU/L after 3 cycles of NACT | |
| Karlsen et al. [11] | Tumor Biol, 2016 | 150 | 78.7% Stage IIIC 21.3% Stage IV | Relation between preoperative CA-125, HE4, age, presence of ascites and performance status and the surgical outcome | Primary CRS | N/A | Complete vs. non-complete CRS | N/A | In univariable analysis, preoperative CA-125 are significantly lower in patients undergoing complete CRS (p = 0.001). | N/A | N/A | CA-125 was excluded from the prediction model (no significant contribution to the model (p = 0.166)). Included in the model were: age, HE4 and performance status. |
| Morimoto et al. [23] | Jpn J Clin Oncol, 2016 | 139 | 59.7% Stage IIIC 40.3% Stage IV (Primary ovarian, fallopian tube and peritoneal) | Relation between CA-125 after NACT, presence of ascites and response rate and the surgical outcome | Interval CRS | N/A | Complete vs. non-complete CRS | Median: 4 cycles Range: 3–6 cycles | Preoperative CA-125 levels are significantly lower in patients undergoing complete CRS (p < 0.001). | N/A | ≤25.8 kU/L preoperative | Presence of preoperative ascites leads to a significant lower complete CRS rate (p < 0.0001). |
| Zeng et al. [8] | J Cancer, 2016 | 118 | 84.7% Stage III 15.3% Stage IV (Primary ovarian, fallopian tube and peritoneal) | Relation between preoperative CA-125 and surgical outcome Relation between changes in CA-125 during NACT and surgical outcome | Interval CRS | Percentage reduction after the first NACT cycle and >30% reduction, overall percentage reduction, >80% reduction after all NACT cycles | Complete vs. non-complete CRS | 1–3 cycles: 97.5% ≥4 cycles: 2.5% | In univariable analysis, preoperative value of CA-125 ≤200 kU/L (p = 0.000) predicts complete CRS. This was also significant in multivariable analysis (p = 0.012). | In univariable analysis, >80% reduction of CA-125 after NACT (p = 0.000) predicts complete CRS. This was not significant in multivariable analysis (p = 0.059). | ≤200 kU/L preoperative | |
| Matsuhashi et al. [16] | J Nippon Med Sch, 2017 | 107 | 55.1% Stage III 44.9% Stage IV | Relation between CA-125 after NACT and surgical outcome | Interval CRS | Number of NACT cycles needed for CA-125 levels to halve or reduce to <35 U/mL | Complete/optimal vs. suboptimal CRS | 6 cycles: >70%, ≤5 cycles: <30% | Lower preoperative CA-125 levels (p = 0.003), especially <35 kU/L (p = 0.0029), significantly correlate with complete/optimal CRS. | No significant difference in frequency of NACT cycles for CA-125 levels to halve or to drop below 35 U/mL between complete/optimal vs. suboptimal CRS (p > 0.05). | <35 kU/L preoperative | |
| Ghisoni et al. [20] | J Ovarian Res, 2018 | 93 | 9.7% Stage IIIA 15% Stage IIIB 62.4% Stage IIIC 12.9% Stage IV | Developing a predictive score of cytoreductive outcome | Interval CRS | <96% reduction in CA-125 | Complete vs. non-complete CRS | N/A * | In univariable analysis, preoperative CA-125 >33 kU/L significantly predicted non-complete CRS (p = 0.002). This was not significant in multivariable analysis. | In univariable analysis, <96% reduction in CA-125 after NACT significantly predicted non-complete CRS (p = 0.034). This was not significant in multivariable analysis. | <33 kU/L preoperative >96% reduction | Age >60 years (p = 0.007), CA-125 at diagnosis ≥550 kU/L (p = 0.014) and peritoneal cancer index assessed at laparoscopy of >16 (p < 0.001) were included in the prediction model. |
| Gupta et al. [15] | South Asian J Cancer, 2020 | 406 | 71.5% Stage III 28.5% Stage IV | Relation between preoperative CA-125 and surgical outcome Relation between percent fall of CA-125 after NACT and surgical outcome | Interval CRS | >95% vs. <95% decrease in CA-125 | Complete vs. optimal and vs. suboptimal CRS | <3 cycles: >60%, >3 cycles: <40% | Rate of complete CRS is significantly higher in preoperative levels of CA-125 <100 kU/L (p = 0.00). | Rate of complete CRS is significantly higher in >95% fall of CA-125 after NACT (p = 0.00). | <100 kU/L preoperative >95% decrease in CA-125 after NACT | Mucinous carcinomas were excluded. |
| Nakamura et al. [21] | World J Surg Oncol, 2020 | 63 | 68.3% Stage IIIC 31.7% Stage IV | This study aimed to use CA-125 and CT scanning to generate a model of predicting complete cytoreduction | Interval CRS | N/A | Complete vs. non-complete CRS | Median: 6 cycles Range: 1–14 cycles | Pre-operative levels of CA-125 were significantly lower in patients with complete CRS (p = 0.015). | N/A | N/A | Extra-ovarian implants (p = 0.009) and omental tumors at CT after NACT (p = 0.038) are significantly associated with complete CRS. |
| Merlo et al [24] | Radiol Oncol, 2021 | 253 | Primary CRS: 89.5% Stage IIIC 10.5% Stage IV Interval CRS: 66.6% Stage IIIC 33.4% Stage IV | Relation between pre-operative CA-125 and surgical outcome | Primary CRS and Interval CRS | Percentage reduction | Complete vs. optimal and vs. suboptimal CRS | N/A * | Primary CRS: Lower preoperative levels of CA-125 are associated with complete/optimal CRS. Interval CRS: Lower preoperative levels of CA-125 are associated with complete/optimal CRS (p = 0.020). | The probability of complete/optimal CRS is higher in patients with a CA-125 reduction >96.4%. | <500 kU/L preoperative |
| Characteristics | Complete CRS (n = 234, 71.8%) | Optimal CRS (n = 49, 15.0%) | Suboptimal CRS (n = 16, 4.9%) | Unresectable Disease (n = 27, 8.3%) | p-Value |
|---|---|---|---|---|---|
| Median AGE (IQR), years | 65 (15) | 67 (9) | 66 (18) | 72 (12) | 0.038 |
| Median BMI (IQR), kg/m2 | 24.7 (6.2) | 24.5 (5.6) | 24.5 (3.7) | 22.5 (7.9) | 0.424 |
| WHO performance, n (%) | NS | ||||
| 0 | 130 (55.6) | 26 (53.1) | 7 (43.8) | 9 (33.3) | |
| 1 | 75 (32.1) | 17 (34.7) | 4 (25.0) | 13 (48.2) | |
| ≥2 | 14 (5.9) | 3 (6.1) | 4 (25.0) | 4 (14.8) | |
| TYPE OF SURGERY, N (%) | NS | ||||
| PRIMARY CRS | 33 (14.1) | 8 (16.3) | 4 (25.0) | 0 (0.0) | |
| INTERVAL CRS | 201 (85.9) | 41 (83.7) | 12 (75.0) | 27 (100.0) | |
| FIGO stage, n (%) | 0.082 | ||||
| IIIB/IIIC | 169 (72.2) | 28 (57.1) | 13 (81.3) | 16 (59.3) | |
| IV | 65 (27.8) | 21 (42.9) | 3 (18.7) | 11 (40.7) | |
| Histology, n (%) | NS | ||||
| Serous | 226 (96.6) | 46 (93.9) | 14 (87.5) | 27 (100.0) | |
| Non-serous | 8 (3.4) | 3 (6.1) | 2 (12.5) | 0 (0.0) | |
| Randomization: PlasmaJet, n (%) | 119 (50.9) | 12 (24.5) | 8 (50.0) | N/A | 0.002 |
| Peritoneal carcinomatosis on CT, n (%) | 159 (67.9) | 34 (69.4) | 12 (75.0) | 19 (70.4) | 0.940 |
| Presence of ascites, n (%) | 60 (25.6) | 19 (38.8) | 11 (68.8) | 23 (85.2) | <0.001 |
| Median ascites volume (IQR), mL | 100 (350) | 250 (400) | 300 (450) | 250 (400) | 0.578 |
| Presence of peritoneal carcinomatosis, n (%) | 65 (27.8) | 34 (69.4) | 12 (75.0) | 24 (88.9) | <0.001 |
| Primary CRS | |||||
| Median preoperative CA-125 (IQR), kU/L | 297.4 (602.3) | 171.0 (388.2) | 185.9 (1828.6) | N/A | 0.868 |
| Interval CRS | |||||
| Median preoperative CA-125 (IQR), kU/L | 67.0 (178.0) | 77.5 (166.8) | 252.5 (532.5) | 81.0 (209.0) | 0.026 |
| Median percentage of CA-125 reduction after NACT (IQR), % | 91.1 (21.8) | 89.9 (13.8) | 78.0 (44.0) | 88.6 (40.2) | 0.388 |
| Factor | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR [95%CI] | p-Value | OR [95%CI] | p-Value | |
| Median AGE (IQR), years | 0.99 [0.97–1.01] | 0.417 | 1.00 [0.96–1.03] | 0.768 |
| Median BMI (IQR), kg/m2 | 1.03 [0.98–1.09] | 0.226 | 1.07 [0.99–1.16] | 0.092 |
| WHO performance status, n (%) | ||||
| 0 (ref) | ||||
| 1 | 0.71 [0.42–1.22] | 0.214 | 1.10 [0.53–2.30] | 0.800 |
| ≥2 | 0.41 [0.17–0.98] | 0.044 | 0.54 [0.16–1.80] | 0.318 |
| FIGO stage, n (%) | ||||
| IIIB/IIIC (ref) | ||||
| IV | 0.63 [0.38–1.04] | 0.072 | 0.29 [0.14–0.63] | 0.002 |
| Histology, n (%) | ||||
| Serous (ref) | ||||
| Non-serous | 0.62 [0.20–1.93] | 0.407 | 0.12 [0.02–0.64] | 0.013 |
| Randomization: PlasmaJet, n (%) | 1.47 [0.90–2.40] | 0.121 | 2.26 [1.10–4.61] | 0.026 |
| Presence of peritoneal carcinomatosis on CT, n (%) | 0.88 [0.52–1.49] | 0.636 | 1.05 [0.49–2.27] | 0.893 |
| Presence of ascites, n (%) | 0.25 [0.15–0.42] | <0.001 | 0.26 [0.13–0.55] | <0.001 |
| Presence of peritoneal carcinomatosis, n (%) | 0.11 [0.06–0.19] | <0.001 | 0.10 [0.05–0.23] | <0.001 |
| Median preoperative CA-125, kU/L | 1.00 [1.00–1.00] | 0.990 | 1.00 [1.00–1.00] | 0.628 |
| Preoperative CA-125 ≤ 35 kU/L | 2.79 [1.44–5.41] | 0.002 | 1.74 [0.74–4.09] | 0.207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brons, P.E.; Nieuwenhuyzen-de Boer, G.M.; Ramakers, C.; Willemsen, S.; Kengsakul, M.; van Beekhuizen, H.J. Preoperative Cancer Antigen 125 Level as Predictor for Complete Cytoreduction in Ovarian Cancer: A Prospective Cohort Study and Systematic Review. Cancers 2022, 14, 5734. https://doi.org/10.3390/cancers14235734
Brons PE, Nieuwenhuyzen-de Boer GM, Ramakers C, Willemsen S, Kengsakul M, van Beekhuizen HJ. Preoperative Cancer Antigen 125 Level as Predictor for Complete Cytoreduction in Ovarian Cancer: A Prospective Cohort Study and Systematic Review. Cancers. 2022; 14(23):5734. https://doi.org/10.3390/cancers14235734
Chicago/Turabian StyleBrons, Puck E., Gatske M. Nieuwenhuyzen-de Boer, Christian Ramakers, Sten Willemsen, Malika Kengsakul, and Heleen J. van Beekhuizen. 2022. "Preoperative Cancer Antigen 125 Level as Predictor for Complete Cytoreduction in Ovarian Cancer: A Prospective Cohort Study and Systematic Review" Cancers 14, no. 23: 5734. https://doi.org/10.3390/cancers14235734
APA StyleBrons, P. E., Nieuwenhuyzen-de Boer, G. M., Ramakers, C., Willemsen, S., Kengsakul, M., & van Beekhuizen, H. J. (2022). Preoperative Cancer Antigen 125 Level as Predictor for Complete Cytoreduction in Ovarian Cancer: A Prospective Cohort Study and Systematic Review. Cancers, 14(23), 5734. https://doi.org/10.3390/cancers14235734






