Determination of Interactive States of Immune Checkpoint Regulators in Lung Metastases after Radiofrequency Ablation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population RFA
2.2. Antibodies and Reagents
2.3. Time-Resolved Immune FRET (iFRET) Assay Determined by Frequency-Domain FLIM
2.4. iFRET Labelling Assay for PD-1/PD-L1 and CTLA-4/CD80 in RFA-Treated Lung Metastases
2.5. Intratumoral CD3 and CD8 Labelling
2.6. Statistical Analysis
3. Results
3.1. Patient Population
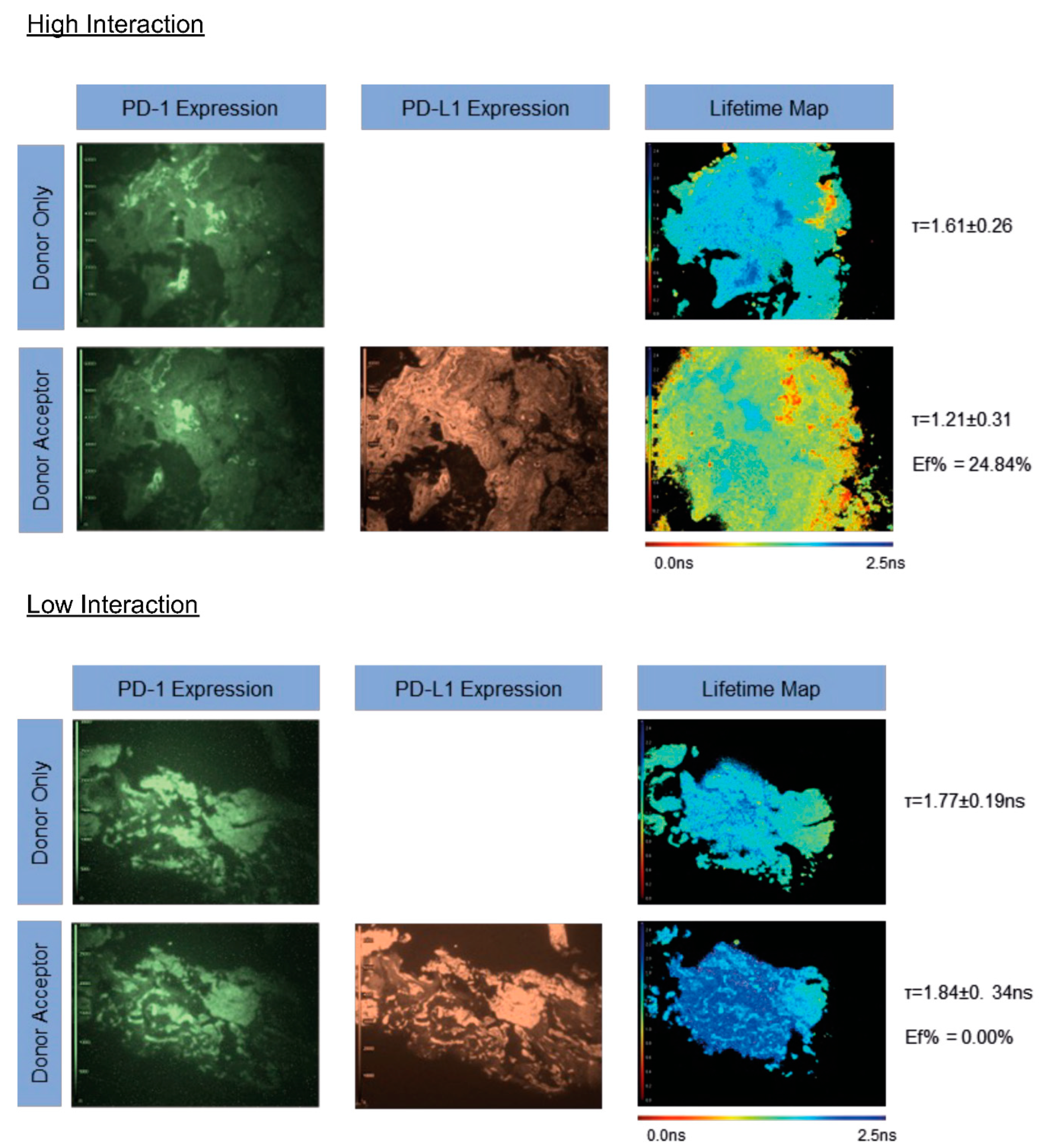
3.2. Validation of CTLA-4/CD80 Quantification in Tissue
3.3. iFRET Quantifies Both PD-1/PD-L1 and CTLA-4/CD80 Interaction States in Pre-RFA Treated Samples
3.4. iFRET Quantifies Both PD-1/PD-L1 and CTLA-4/CD80 Interaction States in Post-RFA-Treated Samples
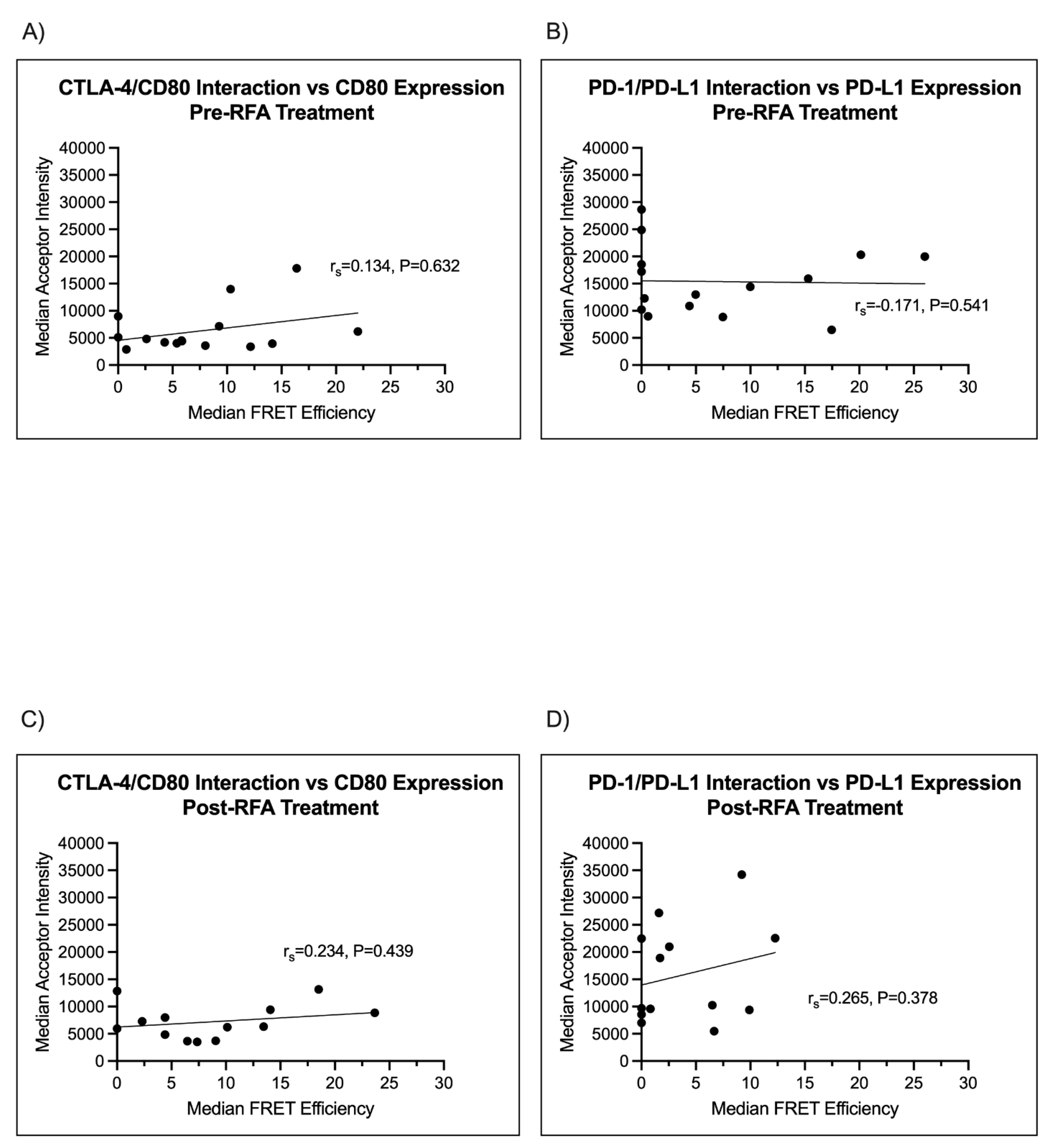
3.5. Immune Checkpoint Ligand Expression Does Not Correlate with Immune Checkpoint Engagement
3.6. Intratumoral CD3 and CD8 Densities Negatively Correlate with PD-1/PD-L1 Interaction
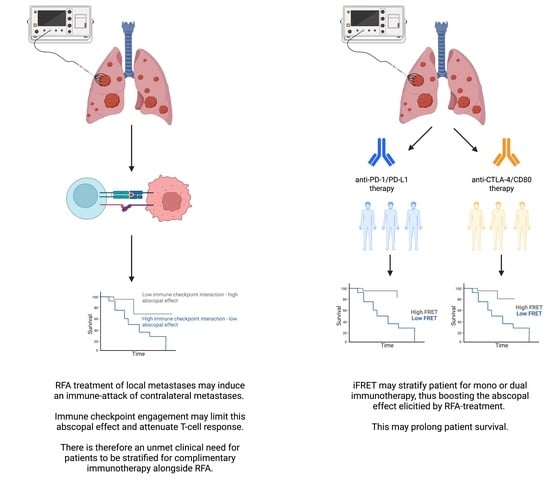
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Baère, T.; Aupérin, A.; Deschamps, F.; Chevallier, P.; Gaubert, Y.; Boige, V.; Fonck, M.; Escudier, B.; Palussiére, J. Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Ann. Oncol. 2015, 26, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Palussière, J.; Chomy, F.; Savina, M.; Deschamps, F.; Gaubert, J.Y.; Renault, A.; Bonnefoy, O.; Laurent, F.; Meunier, C.; Bellera, C.; et al. Radiofrequency ablation of stage IA non–small cell lung cancer in patients ineligible for surgery: Results of a prospective multicenter phase II trial. J. Cardiothorac. Surg. 2018, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Escudier, B.; de Baere, T. Spontaneous Regression of Multiple Pulmonary Metastases After Radiofrequency Ablation of a Single Metastasis. Cardiovasc. Interv. Radiol. 2011, 34, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Matulonis, U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: A potential synergy? Gynecol. Oncol. 2019, 154, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Kim, S.; Harrison, L.B. Novel Opportunities to Use Radiation Therapy with Immune Checkpoint Inhibitors for Melanoma Management. Surg. Oncol. Clin. N. Am. 2017, 26, 515–529. [Google Scholar] [CrossRef]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, e29620. [Google Scholar] [CrossRef]
- den Brok, M.H.; Sutmuller, R.P.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Magraner, L.; Miles, J.; Baker, C.L.; Applebee, C.J.; Lee, D.J.; Elsheikh, S.; Lashin, S.; Withers, K.; Watts, A.G.; Parry, R.; et al. High PD-1/PD-L1 Checkpoint Interaction Infers Tumor Selection and Therapeutic Sensitivity to Anti-PD-1/PD-L1 Treatment. Cancer Res. 2020, 80, 4244–4257. [Google Scholar] [CrossRef]
- Larijani, B.; Miles, J.; Ward, S.G.; Parker, P.J. Quantification of biomarker functionality predicts patient outcomes. Br. J. Cancer 2021, 124, 1618–1620. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Pfannschmidt, J.; Hoffmann, H.; Dienemann, H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J. Thorac. Oncol. 2010, 5, S172–S178. [Google Scholar] [CrossRef] [PubMed]
- Sansom, D.M.; Walker, L.S.K. Dimers Aren’t Forever: CD80 Breaks up with PD-L1. Immunity 2019, 51, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Larijani, B.; Miles, J. Quantification of protein-protein interactions and activation dynamics: A new path to predictive biomarkers. Biophys. Chem. 2022, 283, 106768. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Nguyen, P.; Engle, E.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Green, B.; Soni, A.; Cuda, J.D.; Stein, J.E.; et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J. Immunother. Cancer 2018, 6, 99. [Google Scholar] [CrossRef]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. Immunomodulatory therapy for melanoma: Ipilimumab and beyond. Clin. Dermatol. 2013, 31, 191–199. [Google Scholar] [CrossRef]
- García-Mulero, S.; Alonso, M.H.; Pardo, J.; Santos, C.; Sanjuan, X.; Salazar, R.; Moreno, V.; Piulats, J.M.; Sanz-Pamplona, R. Lung metastases share common immune features regardless of primary tumor origin. J. ImmunoTher. Cancer 2020, 8, e000491. [Google Scholar] [CrossRef]
- Mlecnik, B.; Van den Eynde, M.; Bindea, G.; Church, S.E.; Vasaturo, A.; Fredriksen, T.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Debetancourt, D.; et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J. Natl. Cancer Inst. 2018, 110, 97–108. [Google Scholar] [CrossRef]
- Bortolomeazzi, M.; Keddar, M.R.; Montorsi, L.; Acha-Sagredo, A.; Benedetti, L.; Temelkovski, D.; Choi, S.; Petrov, N.; Todd, K.; Wai, P.; et al. Immunogenomics of Colorectal Cancer Response to Checkpoint Blockade: Analysis of the KEYNOTE 177 Trial and Validation Cohorts. Gastroenterology 2021, 161, 1179–1193. [Google Scholar] [CrossRef]
- Wang, C.; Fakih, M. Targeting MSS colorectal cancer with immunotherapy: Are we turning the corner? Expert Opin. Biol. Ther. 2021, 21, 1347–1357. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miles, J.; Soubeyran, I.; Marliot, F.; Pangon, N.; Italiano, A.; Bellera, C.; Ward, S.G.; Pagès, F.; Palussière, J.; Larijani, B. Determination of Interactive States of Immune Checkpoint Regulators in Lung Metastases after Radiofrequency Ablation. Cancers 2022, 14, 5738. https://doi.org/10.3390/cancers14235738
Miles J, Soubeyran I, Marliot F, Pangon N, Italiano A, Bellera C, Ward SG, Pagès F, Palussière J, Larijani B. Determination of Interactive States of Immune Checkpoint Regulators in Lung Metastases after Radiofrequency Ablation. Cancers. 2022; 14(23):5738. https://doi.org/10.3390/cancers14235738
Chicago/Turabian StyleMiles, James, Isabelle Soubeyran, Florence Marliot, Nicolas Pangon, Antoine Italiano, Carine Bellera, Stephen G. Ward, Franck Pagès, Jean Palussière, and Banafshé Larijani. 2022. "Determination of Interactive States of Immune Checkpoint Regulators in Lung Metastases after Radiofrequency Ablation" Cancers 14, no. 23: 5738. https://doi.org/10.3390/cancers14235738
APA StyleMiles, J., Soubeyran, I., Marliot, F., Pangon, N., Italiano, A., Bellera, C., Ward, S. G., Pagès, F., Palussière, J., & Larijani, B. (2022). Determination of Interactive States of Immune Checkpoint Regulators in Lung Metastases after Radiofrequency Ablation. Cancers, 14(23), 5738. https://doi.org/10.3390/cancers14235738