Epidemiological Study of p16 Incidence in Head and Neck Squamous Cell Carcinoma 2005–2015 in a Representative Northern European Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
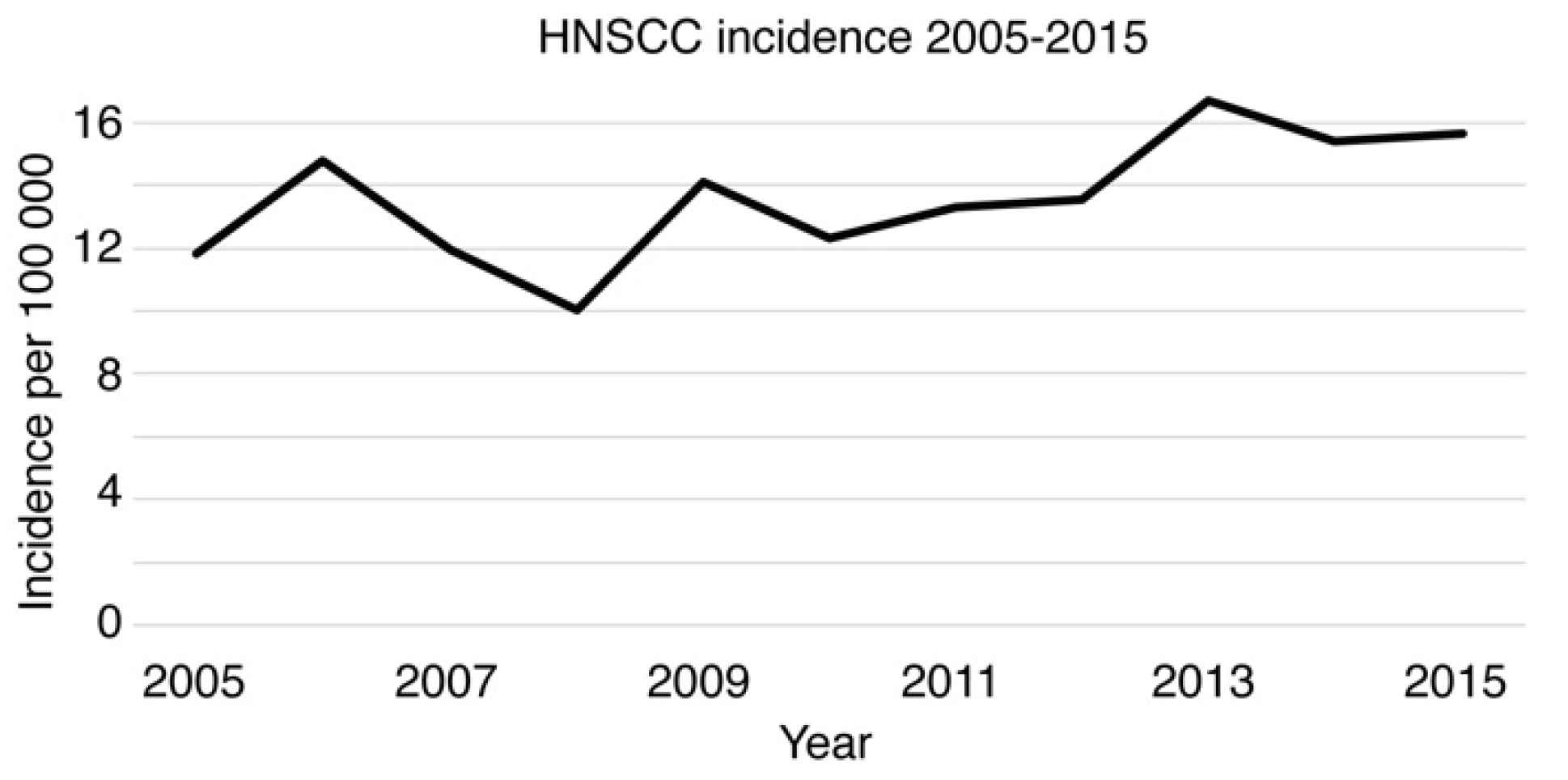
3.1. Epidemiology of HNSCC in Southwest Finland 2005–2015
3.2. Locoregional Distribution of HNSCC between 2005 and 2015
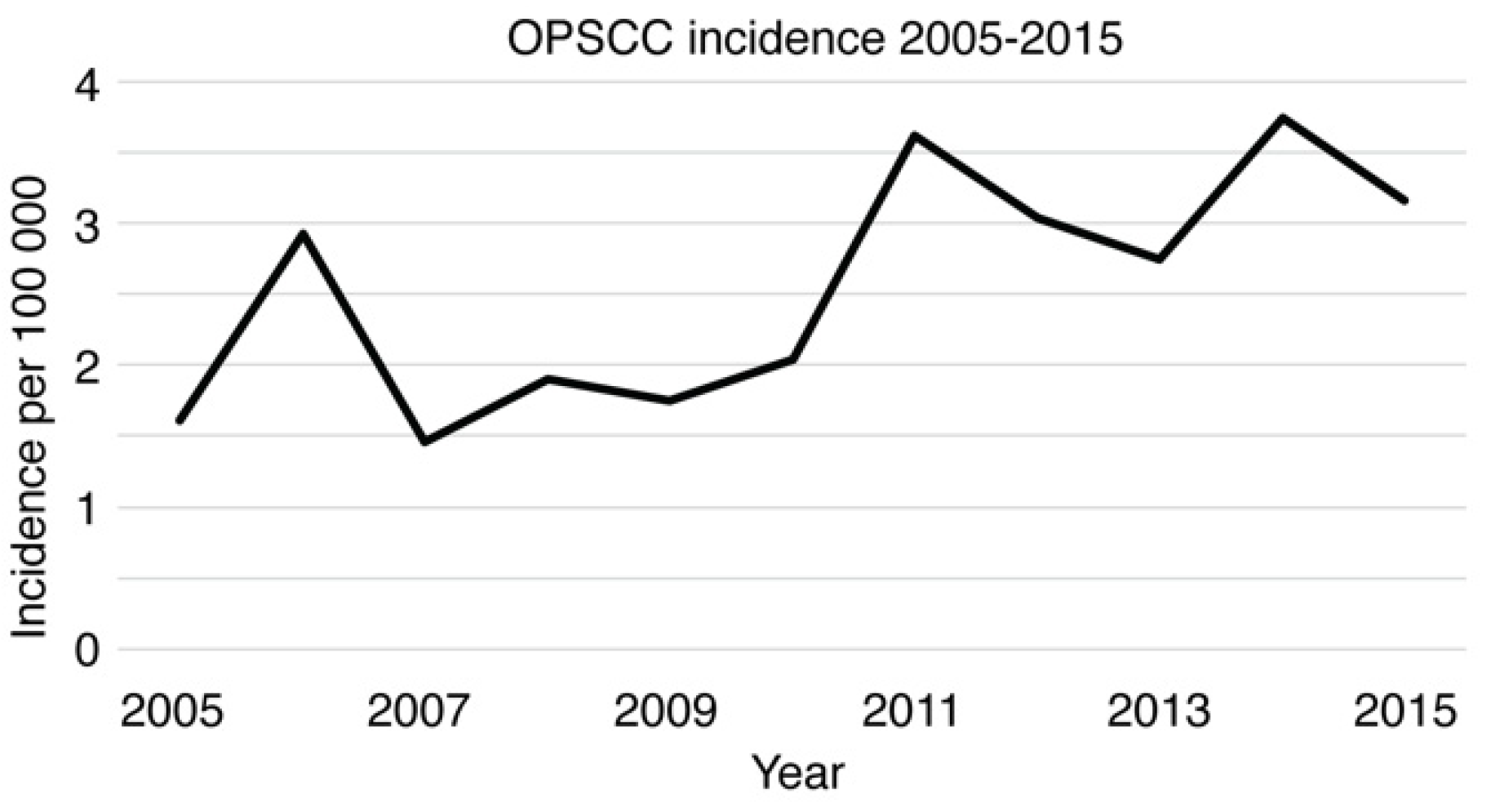
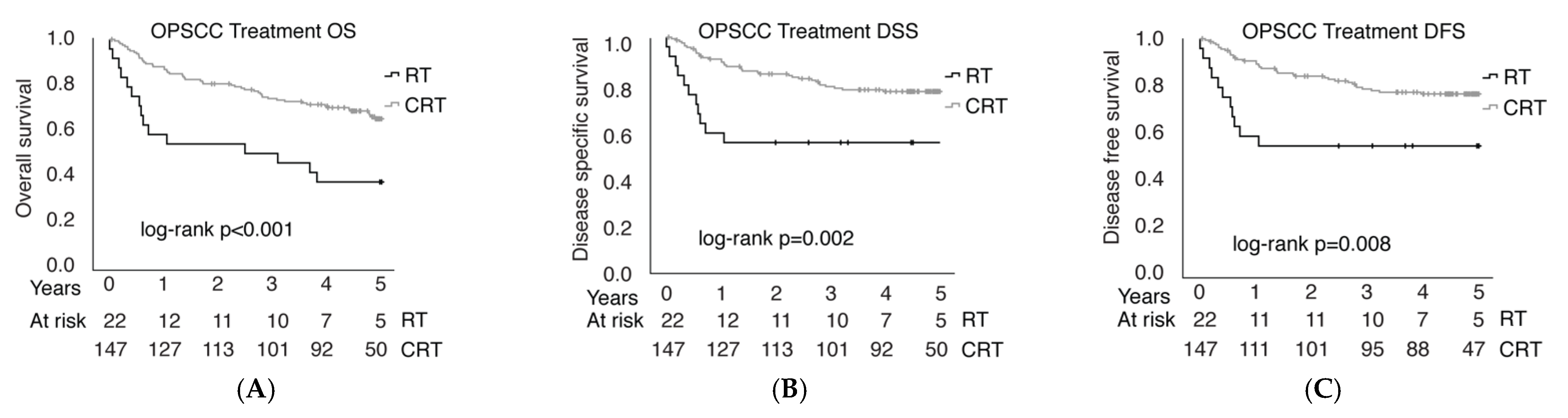
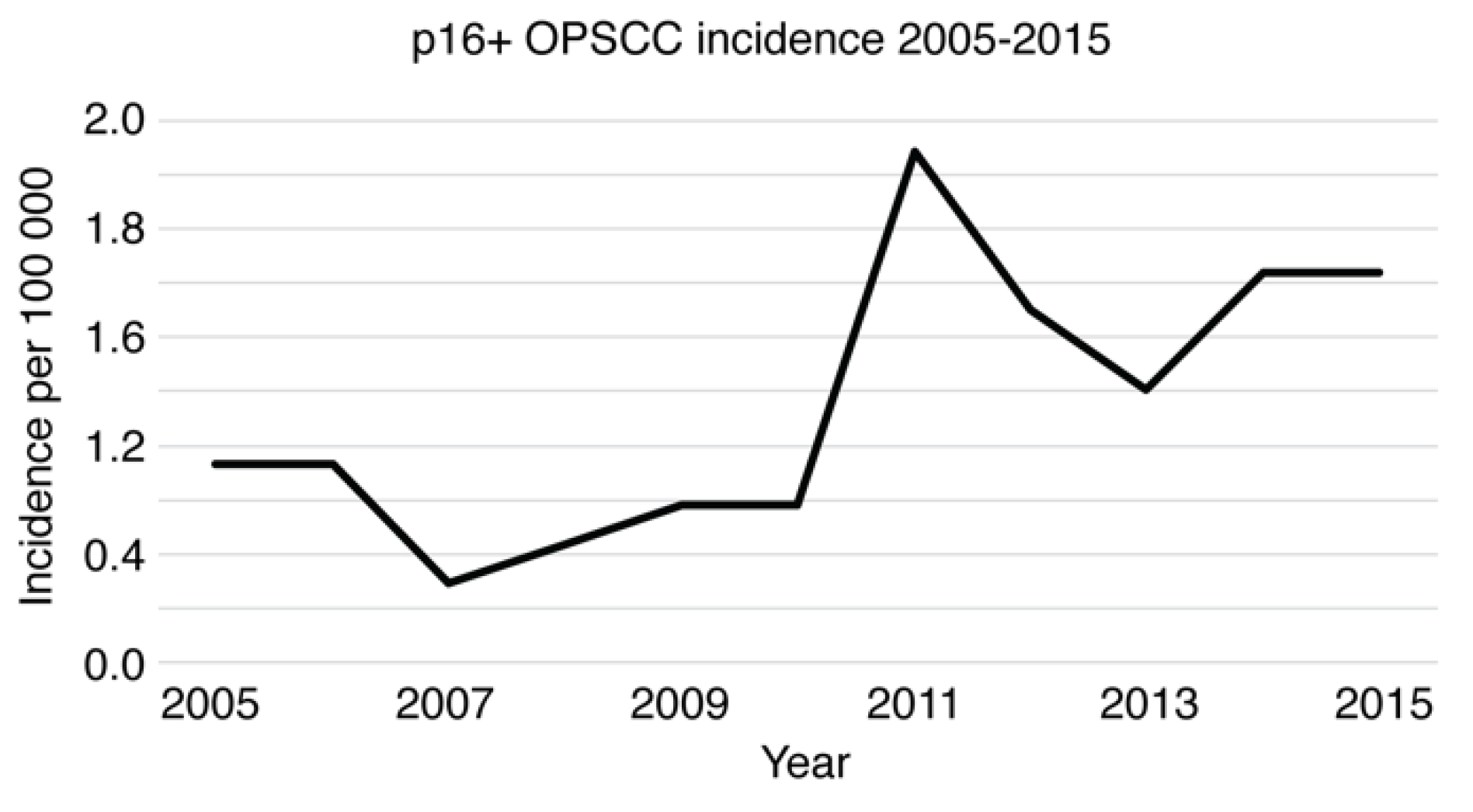
3.3. Epidemiology of Oropharyngeal Squamous Cell Carcinoma (OPSCC)
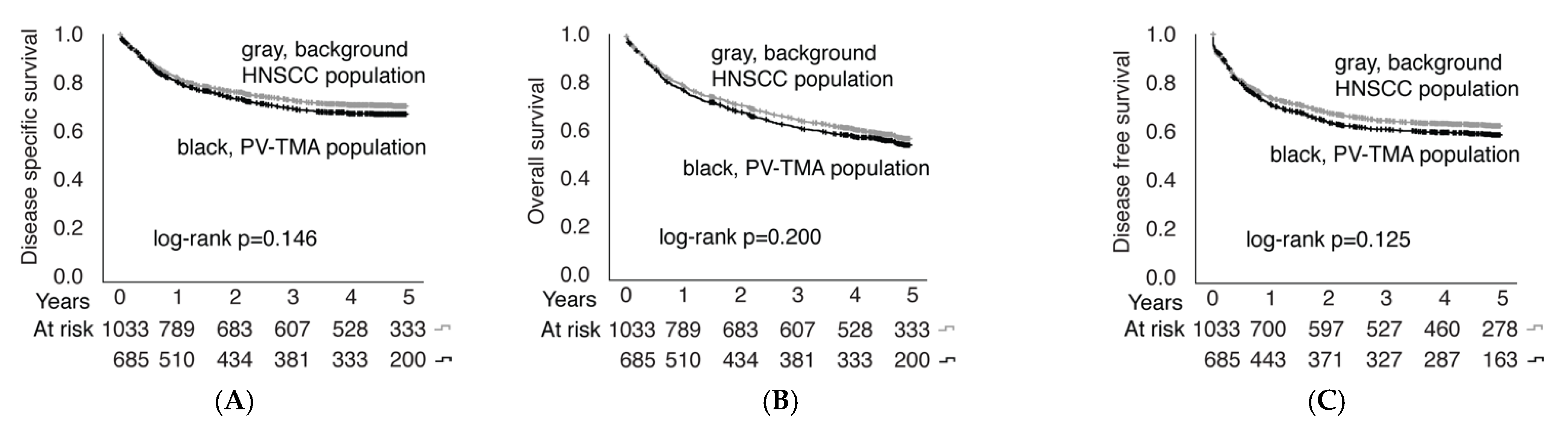
3.4. Establishment of a Population-Validated Tissue Microarray (PV-TMA) Corresponding to an Epidemiological HNSCC Background Population
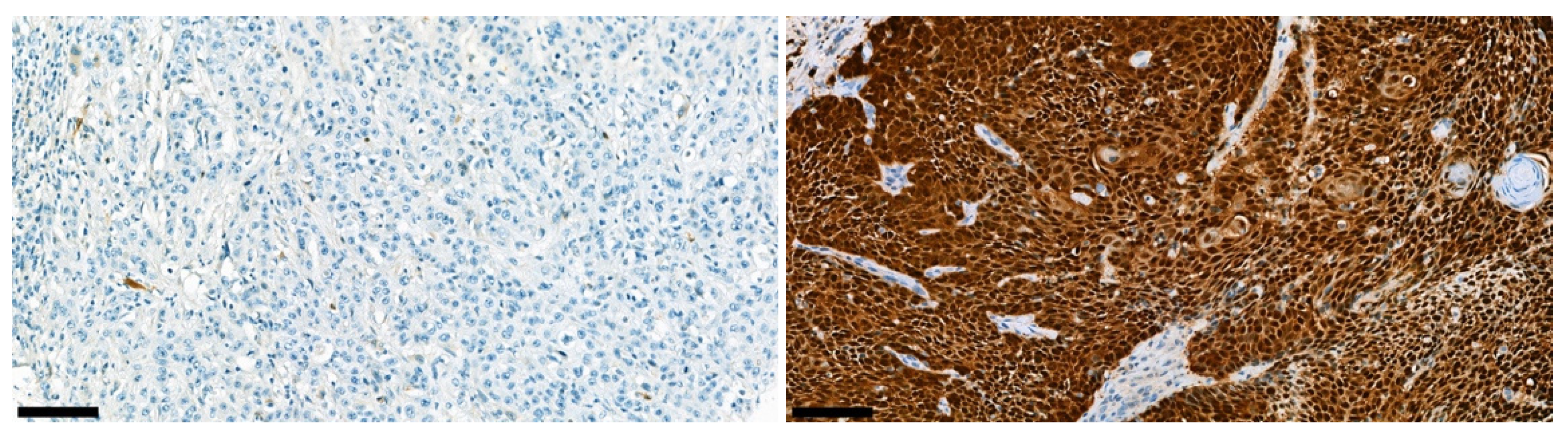
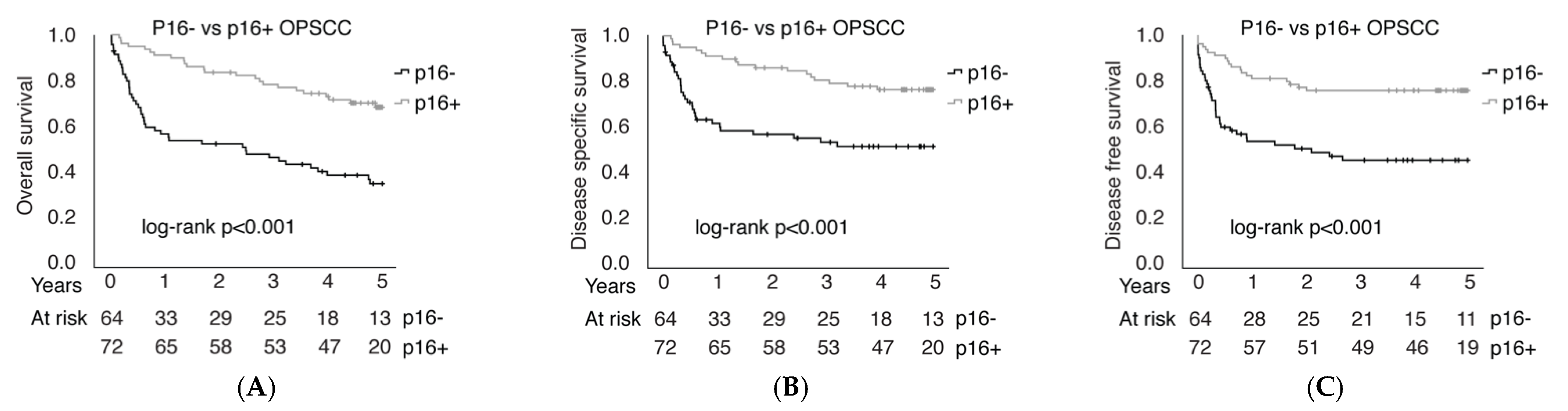
3.5. P16 Immunohistochemical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Dietz, A.; Gewelke, U.; Heller, W.D.; Weidauer, H. Tobacco and alcohol and the risk of head and neck cancer. Clin. Investig. 1992, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Economopoulou, P.; De Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Paver, E.C.; Currie, A.M.; Gupta, R.; Dahlstrom, J.E. Human papilloma virus related squamous cell carcinomas of the head and neck: Diagnosis, clinical implications and detection of HPV. Pathology 2019, 52, 179–191. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Westra, W.H. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: A guide for interpretative relevance and consistency. Head Neck 2011, 34, 459–461. [Google Scholar] [CrossRef]
- Lewis, J.S. p16 Immunohistochemistry As a Standalone Test for Risk Stratification in Oropharyngeal Squamous Cell Carcinoma. Head Neck Pathol. 2012, 6, 75–82. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takahashi, A.; Motoi, N.; Yoshimoto, S.; Tajima, T.; Yamakoshi, K.; Hirao, A.; Yanagi, S.; Fukami, K.; Ishikawa, Y.; et al. Intrinsic Cooperation between p16INK4a and p21Waf1/Cip1 in the Onset of Cellular Senescence and Tumor Suppression In vivo. Cancer Res. 2010, 70, 9381–9390. [Google Scholar] [CrossRef]
- Reimers, N.; Kasper, H.U.; Weissenborn, S.J.; Stützer, H.; Preuss, S.F.; Hoffmann, T.K.; Speel, E.J.M.; Dienes, H.P.; Pfister, H.J.; Guntinas-Lichius, O.; et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer 2007, 120, 1731–1738. [Google Scholar] [CrossRef]
- Singhi, A.D.; Westra, W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010, 116, 2166–2173. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Gültekin, E.; Weissenborn, S.J.; Wieland, U.; Dries, V.; Dienes, H.P.; Eckel, H.E.; Pfister, H.J.; Fuchs, P.G. Expression of p16 Protein Identifies a Distinct Entity of Tonsillar Carcinomas Associated with Human Papillomavirus. Am. J. Pathol. 2003, 162, 747–753. [Google Scholar] [CrossRef]
- Lewis, A.; Kang, R.; Levine, A.; Maghami, E. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology 2015, 29, 616. [Google Scholar] [PubMed]
- Prigge, E.-S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16INK4aimmunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Gordis, T.M.; Cagle, J.L.; Nguyen, S.A.; Newman, J.G. Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis of Clinical Trial Demographics. Cancers 2022, 14, 4061. [Google Scholar] [CrossRef]
- Ragin, C.C.R.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef]
- Huang, S.H.; Xu, W.; Waldron, J.; Siu, L.; Shen, X.; Tong, L.; Ringash, J.; Bayley, A.; Kim, J.; Hope, A.; et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM Stage and Prognostic Groups for Human Papillomavirus–Related Oropharyngeal Carcinomas. J. Clin. Oncol. 2015, 33, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Corry, J.; Peters, L.J.; Rischin, D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol. 2010, 11, 287–291. [Google Scholar] [CrossRef]
- Bhatia, A.; Burtness, B. Human Papillomavirus–Associated Oropharyngeal Cancer: Defining Risk Groups and Clinical Trials. J. Clin. Oncol. 2015, 33, 3243–3250. [Google Scholar] [CrossRef]
- Mirghani, H.; Amen, F.; Blanchard, P.; Moreau, F.; Guigay, J.; Hartl, D.; Guily, J.L.S. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int. J. Cancer 2014, 136, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Rosenthal, B.T.; Clark, D.P.; Gillison, M.L. Associations between Oral HPV16 Infection and Cytopathology: Evaluation of an Oropharyngeal “Pap-Test Equivalent” in High-Risk Populations. Cancer Prev. Res. 2011, 4, 1378–1384. [Google Scholar] [CrossRef]
- Kim, K.Y.; McShane, L.M.; Conley, B.A. Designing biomarker studies for head and neck cancer. Head Neck 2013, 36, 1069–1075. [Google Scholar] [CrossRef]
- Mirghani, H.; Amen, F.; Moreau, F.; Guigay, J.; Ferchiou, M.E.; Melkane, A.; Hartl, D.M.; Guily, J.L.S. Human papilloma virus testing in oropharyngeal squamous cell carcinoma: What the clinician should know. Oral Oncol. 2014, 50, 1–9. [Google Scholar] [CrossRef]
- Lothaire, P.; de Azambuja, E.; Dequanter, D.; Lalami, Y.; Sotiriou, C.; Andry, G.; Castro, G.; Awada, A. Molecular markers of head and neck squamous cell carcinoma: Promising signs in need of prospective evaluation. Head Neck 2006, 28, 256–269. [Google Scholar] [CrossRef]
- Routila, J.; Leivo, I.; Minn, H.; Westermarck, J.; Ventelä, S. Evaluation of prognostic biomarkers in a population-validated Finnish HNSCC patient cohort. Eur. Arch Otorhinolaryngol. 2021, 278, 4575–4585. [Google Scholar] [CrossRef]
- Denissoff, A.; Huusko, T.; Ventelä, S.; Niemelä, S.; Routila, J. Exposure to alcohol and overall survival in head and neck cancer: A regional cohort study. Head Neck 2022, 44, 2109–2117. [Google Scholar] [CrossRef]
- Statistic Finland. Available online: https://stat.fi/tup/tilastotietokannat/index_en.html (accessed on 6 October 2022).
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Warpenius, K.; Mäkelä, P. The Finnish Drinking Habits Survey: Implications for alcohol policy and prevention. Nord. Stud. Alcohol Drugs 2020, 37, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Statistical Information on Welfare and Health in Finland. Available online: https://sotkanet.fi/sotkanet/en/taulukko/?indicator=szY0CQQA®ion=s07MBAA=&year=sy4rAwA=&gender=t&abs=f&color=f&buildVersion=3.0-SNAPSHOT&buildTimestamp=202109301228 (accessed on 18 September 2022).
- Smeets, S.J.; Hesselink, A.T.; Speel, E.-J.M.; Haesevoets, A.; Snijders, P.J.; Pawlita, M.; Meijer, C.J.; Braakhuis, B.J.; Leemans, C.R.; Brakenhoff, R.H. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer 2007, 121, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Routila, J.; Suvila, K.; Grénman, R.; Leivo, I.; Westermarck, J.; Ventelä, S. Cancer cell line microarray as a novel screening method for identification of radioresistance biomarkers in head and neck squamous cell carcinoma. BMC Cancer 2021, 21, 868. [Google Scholar] [CrossRef] [PubMed]
- Routila, J.; Qiao, X.; Weltner, J.; Rantala, J.K.; Carpén, T.; Hagström, J.; Mäkitie, A.; Leivo, I.; Ruuskanen, M.; Söderlund, J.; et al. Cisplatin overcomes radiotherapy resistance in OCT4-expressing head and neck squamous cell carcinoma. Oral Oncol. 2022, 127, 105772. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid Clearance Profile of Plasma Circulating Tumor HPV Type 16 DNA during Chemoradiotherapy Correlates with Disease Control in HPV-Associated Oropharyngeal Cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef]
- Kokko, L.-L.; Hurme, S.; Maula, S.-M.; Alanen, K.; Grénman, R.; Kinnunen, I.; Ventelä, S. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral Oncol. 2011, 47, 510–516. [Google Scholar] [CrossRef]
- Kang, H.; Kiess, A.; Chung, C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PLoS ONE 2013, 8, e68329. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections among Young Adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Hirth, J.M.; Chang, M.; Resto, V.A.; Guo, F.; Berenson, A.B. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30 years old). Vaccine 2017, 35, 3446–3451. [Google Scholar] [CrossRef]
- HPV, or Human Papillomavirus Vaccine. Available online: https://thl.fi/en/web/infectious-diseases-and-vaccinations/vaccines-a-to-z/hpv-or-human-papillomavirus-vaccine (accessed on 3 September 2022).

| Characteristic | n | % | Survival Effect HR (95% CI) | p Value |
|---|---|---|---|---|
| Gender | Not included | |||
| Male | 679 | 65.7 | ||
| Female | 354 | 34.3 | ||
| Age | 1.03 (1.02–1.05)/year | <0.001 | ||
| <65 | 487 | 47.1 | ||
| >65 | 546 | 52.9 | ||
| Tumor site | Not included | |||
| Oral cavity | 505 | 48.9 | ||
| Oropharynx | 193 | 18.7 | ||
| Larynx | 184 | 17.8 | ||
| Hypopharynx | 40 | 3.9 | ||
| Other | 111 | 10.8 | ||
| T-class | ||||
| T1-2 | 676 | 65.4 | 1 | - |
| T3-4 | 357 | 34.6 | 2.45 (1.84–3.24) | <0.001 |
| N-class | ||||
| N0 | 638 | 61.8 | 1 | - |
| N+ | 395 | 38.2 | 1.79 (1.33–2.40) | <0.001 |
| Stage | Not included | |||
| 0–II | 481 | 46.6 | ||
| III–IV | 552 | 53.4 | ||
| Gradus | Not included | |||
| G1 | 321 | 32.9 | ||
| G2 | 435 | 44.6 | ||
| G3 | 219 | 22.5 | ||
| Treatment | Not included | |||
| Surgery only | 362 | 35.0 | ||
| Definitive RT | 85 | 8.2 | ||
| Definitive CRT | 159 | 15.4 | ||
| Surgery + CRT/RT | 372 | 36.0 | ||
| Palliative treatment | 55 | 5.3 | ||
| Tobacco use | ||||
| No | 570 | 55.2 | 1 | - |
| Yes | 463 | 44.8 | 1.46 (1.07–1.99) | 0.017 |
| Alcohol use | ||||
| No | 784 | 75.9 | 1 | - |
| Yes | 249 | 24.1 | 1.36 (1.00–1.85) | 0.047 |
| Smoking ≥20 PY (%) | Alcohol Current Use (%) | Gender Male (%) | Age ≥65 years (%) | |
|---|---|---|---|---|
| Oral cavity (OSCC) | 40.2 | 18.0 | 52.5 | 61.8 |
| Oropharynx (OPSCC) | 63.2 | 32.1 | 77.8 | 31.6 |
| Larynx (LSCC) | 83.2 | 32.1 | 86.4 | 48.9 |
| Hypopharynx (HPSCC) | 72.5 | 37.5 | 65.0 | 60.0 |
| Other | 38.7 | 19.8 | 71.2 | 53.2 |
| Total | TMA | TMA Inclusion | TMA Inclusion | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR (95% CI) | p | OR (95% CI) | p | |
| Gender | ||||||||
| Male | 679 | 66 | 438 | 64 | 0.79 (0.60–1.04) | 0.089 | 0.77 (0.56–1.05) | 0.103 |
| Female | 354 | 34 | 247 | 36 | 1 | - | 1 | - |
| Age | ||||||||
| <65 | 487 | 47 | 334 | 49 | 1.21 (0.94–1.57) | 0.145 | 1.02 (0.75–1.38) | 0.904 |
| ≥65 | 546 | 53 | 351 | 51 | 1 | - | 1 | - |
| Smoker | ||||||||
| <20 pack years | 483 | 47 | 311 | 45 | 0.85 (0.66–1.10) | 0.221 | 0.86 (0.62–1.18) | 0.340 |
| ≥20 pack years | 550 | 53 | 374 | 55 | 1 | - | 1 | - |
| Alcohol | ||||||||
| No | 784 | 76 | 513 | 75 | 0.85 (0.62–1.15) | 0.290 | 0.97 (0.68–1.39) | 0.877 |
| Yes | 249 | 24 | 172 | 25 | 1 | - | 1 | - |
| Tumor site | ||||||||
| Oral cavity | 505 | 49 | 352 | 51 | 1 | - | 1 | - |
| Oropharynx | 193 | 19 | 146 | 21 | 1.35 (0.92–1.97) | 0.121 | 0.94 (0.60–1.49) | 0.795 |
| Larynx | 184 | 18 | 109 | 16 | 0.63 (0.45–0.90) | 0.010 | 0.69 (0.44–1.07) | 0.097 |
| Hypopharynx | 40 | 4 | 30 | 4 | 1.30 (0.62–2.73) | 0.482 | 0.92 (0.41–2.06) | 0.836 |
| Other | 111 | 10 | 48 | 7 | 0.33 (0.22–0.50) | <0.001 | 0.23 (0.14–0.38) | <0.001 |
| T-class | ||||||||
| T1–2 | 676 | 64 | 428 | 62 | 0.67 (0.51–0.89) | 0.005 | 0.85 (0.51–1.39) | 0.510 |
| T3–4 | 357 | 36 | 257 | 38 | 1 | - | 1 | - |
| N-class | ||||||||
| N0 | 638 | 62 | 387 | 57 | 0.50 (0.38–0.66) | <0.001 | 0.49 (0.30–0.81) | 0.005 |
| N+ | 395 | 38 | 298 | 43 | 1 | - | 1 | - |
| Stage | ||||||||
| I–II | 481 | 47 | 287 | 42 | 0.57 (0.44–0.74) | <0.001 | 0.94 (0.48–1.82) | 0.853 |
| III–IV | 552 | 53 | 398 | 58 | 1 | - | 1 | - |
| Treatment | ||||||||
| Surgery only | 362 | 35 | 220 | 32 | 1 | - | 1 | - |
| RT | 85 | 8 | 45 | 7 | 0.73 (0.45–1.17) | 0.187 | 0.66 (0.38–1.16) | 0.146 |
| CRT | 159 | 15 | 108 | 16 | 1.37 (0.92–2.03) | 0.120 | 1.25 (0.74–2.13) | 0.408 |
| RT + surgery | 85 | 8 | 66 | 10 | 2.24 (1.29–3.90) | 0.004 | 2.10 (1.17–3.78) | 0.013 |
| CRT + surgery | 287 | 28 | 209 | 30 | 1.73 (1.24–2.42) | 0.001 | 1.15 (0.73–1.38) | 0.559 |
| Palliative | 55 | 5 | 37 | 5 | 1.33 (0.73–2.42) | 0.357 | 0.98 (0.48–1.99) | 0.951 |
| Site | p16+ | p16− | ||
|---|---|---|---|---|
| n | % | n | % | |
| OSCC | 14 | 5 | 278 | 95 |
| OPSCC | 72 | 53 | 64 | 47 |
| LSCC | 5 | 5 | 89 | 95 |
| HPSCC | 1 | 4 | 24 | 96 |
| Other | 8 | 17 | 38 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylly, M.; Nissi, L.; Huusko, T.; Routila, J.; Vaittinen, S.; Irjala, H.; Leivo, I.; Ventelä, S. Epidemiological Study of p16 Incidence in Head and Neck Squamous Cell Carcinoma 2005–2015 in a Representative Northern European Population. Cancers 2022, 14, 5717. https://doi.org/10.3390/cancers14225717
Mylly M, Nissi L, Huusko T, Routila J, Vaittinen S, Irjala H, Leivo I, Ventelä S. Epidemiological Study of p16 Incidence in Head and Neck Squamous Cell Carcinoma 2005–2015 in a Representative Northern European Population. Cancers. 2022; 14(22):5717. https://doi.org/10.3390/cancers14225717
Chicago/Turabian StyleMylly, Mari, Linda Nissi, Teemu Huusko, Johannes Routila, Samuli Vaittinen, Heikki Irjala, Ilmo Leivo, and Sami Ventelä. 2022. "Epidemiological Study of p16 Incidence in Head and Neck Squamous Cell Carcinoma 2005–2015 in a Representative Northern European Population" Cancers 14, no. 22: 5717. https://doi.org/10.3390/cancers14225717
APA StyleMylly, M., Nissi, L., Huusko, T., Routila, J., Vaittinen, S., Irjala, H., Leivo, I., & Ventelä, S. (2022). Epidemiological Study of p16 Incidence in Head and Neck Squamous Cell Carcinoma 2005–2015 in a Representative Northern European Population. Cancers, 14(22), 5717. https://doi.org/10.3390/cancers14225717





