Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ovarian Stimulation Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [PubMed]
- Guidelines for the Preservation of Fertility in Cancer Patients—Italian Association of Medical Oncology. 2020. Available online: https://www.aiom.it/2020 (accessed on 30 October 2020).
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Li, Z.; Guo, S.; Fu, J.; Sun, Y.; Sun, X. Oocyte Vitrification Temporarily Turns on Oxidation-Reduction Process Genes in Mouse Preimplantation Embryos. Reprod. Sci. 2021, 28, 1307–1315. [Google Scholar]
- de Moraes, C.C.; Marinho, V.F.; Campos, A.L.; de Souza Guedes, J.; de Sousa Xavier, É.B.; Caetano, J.P.; Marinho, R.M. Oocyte cryopreservation for future fertility: Comparison of ovarian response between cancer and non-cancer patients. JBRA Assist. Reprod. 2019, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.M.; Ramanathan, P. Fertility preservation in female oncology patients: The influence of the type of cancer on ovarian stimulation response. Hum. Reprod. 2018, 33, 2051–2059. [Google Scholar] [CrossRef]
- Quinn, M.M.; Cakmak, H.; Letourneau, J.M.; Cedars, M.I.; Rosen, M.P. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum. Reprod. 2017, 32, 568–574. [Google Scholar] [CrossRef][Green Version]
- Almog, B.; Azem, F.; Gordon, D.; Pauzner, D.; Amit, A.; Barkan, G.; Levin, I. Effects ofcancer on ovarian response in controlled ovarian stimulation for fertility preservation. Fertil. Steril. 2012, 98, 957–960. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Thomson, A.P.; Karmon, A.E.; Dickinson, K.A.; Wright, D.L.; Sabatini, M.E. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: A 17-year experience. J. Assist. Reprod. Genet. 2015, 32, 587–596. [Google Scholar] [CrossRef]
- Friedler, S.; Koc, O.; Gidoni, Y.; Raziel, A.; Ron-El, R. Ovarian response to stimulation for fertility preservation in women with malignant disease: A systematic review and meta-analysis. Fertil. Steril. 2012, 97, 125–133. [Google Scholar] [CrossRef]
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martinez, M.; Carmona, L.; Pellicer, A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil. Steril. 2013, 99, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Decanter, C.; Robin, G.; Mailliez, A.; Sigala, J.; Morschhauser, F.; Ramdane, N.; Devos, P.; Dewailly, D.; Leroy-Martin, B.; Keller, L. Prospective assessment of follicular growth and the oocyte cohort after ovarian stimulation for fertility preservation in 90 cancer patients versus 180 matched controls. Reprod. Biomed. Online 2018, 36, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Quintero, R.B.; Helmer, A.; Huang, J.Q.; Westphal, L.M. Ovarian stimulation for fertility preservation in patients with cancer. Fertil. Steril. 2010, 93, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.; Guillén, V.; Ayllón, Y.; Martínez, M.; Muñoz, E.; Pellicer, A.; Garcia-Velasco, J.A. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil. Steril. 2012, 97, 930–934. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Inc.; Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Indian Med. Assoc. 2009, 107, 403–405. [Google Scholar]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Moravek, M.B.; Confino, R.; Lawson, A.K.; Smith, K.N.; Kazer, R.R.; Klock, S.C.; Gradishar, W.J.; Jeruss, J.S.; Pavone, M.E. Predictors and outcomes in breast cancer patients who did or did not pursue fertility preservation. Breast Cancer Res. Treat. 2021, 186, 429–437. [Google Scholar] [CrossRef]
- Buonomo, B.; Peccatori, F.A. Fertility preservation in endocrine responsive breast cancer: Data and prejudices. Ecancermedicalscience 2020, 14, 1157. [Google Scholar] [CrossRef]
- Paris, I.; Di Giorgio, D.; Carbognin, L.; Corrado, G.; Garganese, G.; Franceschini, G.; Sanchez, A.M.; De Vincenzo, R.P.; Accetta, C.; Terribile, D.A.; et al. Pregnancy-Associated Breast Cancer: A Multidisciplinary Approach. Clin. Breast Cancer 2021, 21, e120–e127. [Google Scholar] [CrossRef]
- Moragón, S.; Di Liello, R.; Bermejo, B.; Hernando, C.; Olcina, E.; Chirivella, I.; Lluch, A.; Cejalvo, J.M.; Martínez, M.T. Fertility and breast cancer: A literature review of counseling, preservation options and outcomes. Crit. Rev. Oncol. Hematol. 2021, 166, 103461. [Google Scholar] [CrossRef]
- Daniele, A.; Divella, R.; Pilato, B.; Tommasi, S.; Pasanisi, P.; Patruno, M.; Digennaro, M.; Minoia, C.; Dellino, M.; Pisconti, S.; et al. Can harmful lifestyle, obesity and weight changes increase the risk of breast cancer in BRCA 1 and BRCA 2 mutation carriers? A Mini review. Hered. Cancer Clin. Pract. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Bafunno, D.; Romito, F.; Lagattolla, F.; Delvino, V.A.; Minoia, C.; Loseto, G.; Dellino, M.; Guarini, A.; Catino, A.; Montrone, M.; et al. Psychological well-being in cancer outpatients during COVID-19. J. BUON 2021, 26, 1127–1134. [Google Scholar] [PubMed]
- Marklund, A.; Lundberg, F.E.; Eloranta, S.; Hedayati, E.; Pettersson, K.; Rodriguez-Wallberg, K.A. Reproductive Outcomes After Breast Cancer in Women With vs. Without Fertility Preservation. JAMA Oncol. 2021, 7, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Sermondade, N.; Calvo, J.; Benard, J.; Sifer, C.; Grynberg, M. Impact of letrozole supplementation during ovarian stimulation for fertility preservation in breast cancer patients. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100049. [Google Scholar] [CrossRef]
- Goldrat, O.; Van Den Steen, G.; Gonzalez-Merino, E.; Dechène, J.; Gervy, C.; Delbaere, A.; Devreker, F.; De Maertelaer, V.; Demeestere, I. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients versus conventional controlled ovarian hyperstimulation in infertile patients: Assessment of oocyte quality related biomarkers. Reprod. Biol. Endocrinol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, R.; Vicenti, R.; Macciocca, M.; Seracchioli, R.; Rossi, S.; Fabbri, R. High cytokine expression and reduced ovarian reserve in patients with Hodgkin lymphoma or non-Hodgkin lymphoma. Fertil. Steril. 2016, 106, 1176–1182. [Google Scholar] [CrossRef]
- Emori, M.; Drapkin, R. The hormonal composition of follicular fluid and its implications for ovarian cancer pathogenesis. Reprod. Biol. Endocrinol. 2014, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Romito, A.; Bove, S.; Romito, I.; Zace, D.; Raimondo, I.; Fragomeni, S.M.; Rinaldi, P.M.; Pagliara, D.; Lai, A.; Marazzi, F.; et al. Ovarian Reserve after Chemotherapy in Breast Cancer: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Ferrante, M.G.; Meneghini, C.; Licata, E.; Paciotti, G.; Gallo, M.; Schiavi, M.; Spina, V.; Guarino, A.; Caserta, D.; et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. Int. J. Gynaecol. Obstet. 2022, 156, 166–171. [Google Scholar] [CrossRef]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reproduction 2018, 156, F11–F27. [Google Scholar] [CrossRef]
- Merkison, J.; Malcom, C.; Decherney, A. Use of gonadotropin-releasing hormone (GnRH) agonist trigger in fertility preservation for patients with inherited genetic disorders. Front. Endocrinol. (Lausanne) 2022, 13, 826419. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, R.; Mangili, G.; Sarais, V.; Cervini, L.; Longo, V.; Bergamini, A.; Vanni, V.S.; Pagliardini, L.; Candiani, M.; Papaleo, E. Do stage and grade of malignancy impact fertility preservation in breast cancer patients? J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102215. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Marchetti, C.; Trozzi, R.; Scambia, G.; Fagotti, A. Fertility preservation in patients with BRCA mutations or Lynch syndrome. Int. J. Gynecol. Cancer 2021, 31, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Goldrat, O.; Ferreira, A.R.; Dechene, J.; Azim Jr, H.A.; Desir, J.; Delbaere, A.; de Roodenbeke, M.D.; De Azambuja, E.; Ignatiadis, M.; et al. Reproductive potential and performance of fertility preservation strategies inBRCA-mutated breast cancer patients. Ann. Oncol. 2018, 29, 237–243. [Google Scholar] [CrossRef]
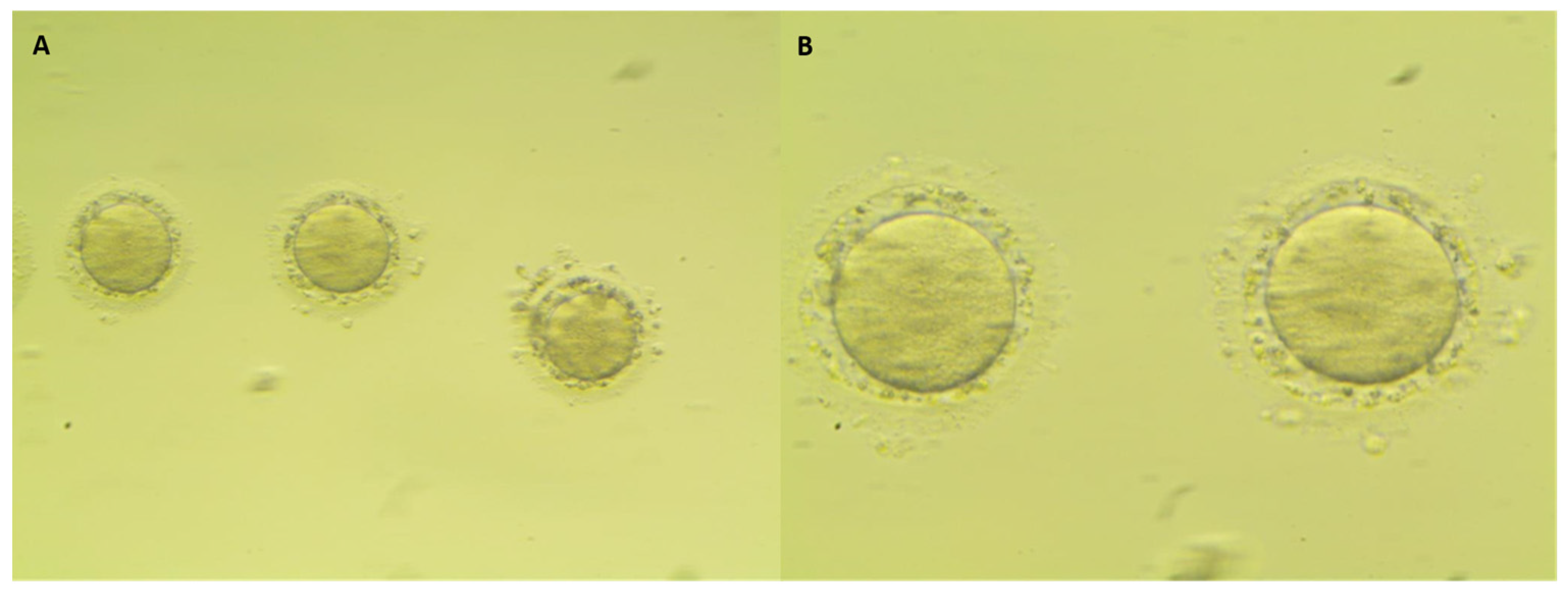
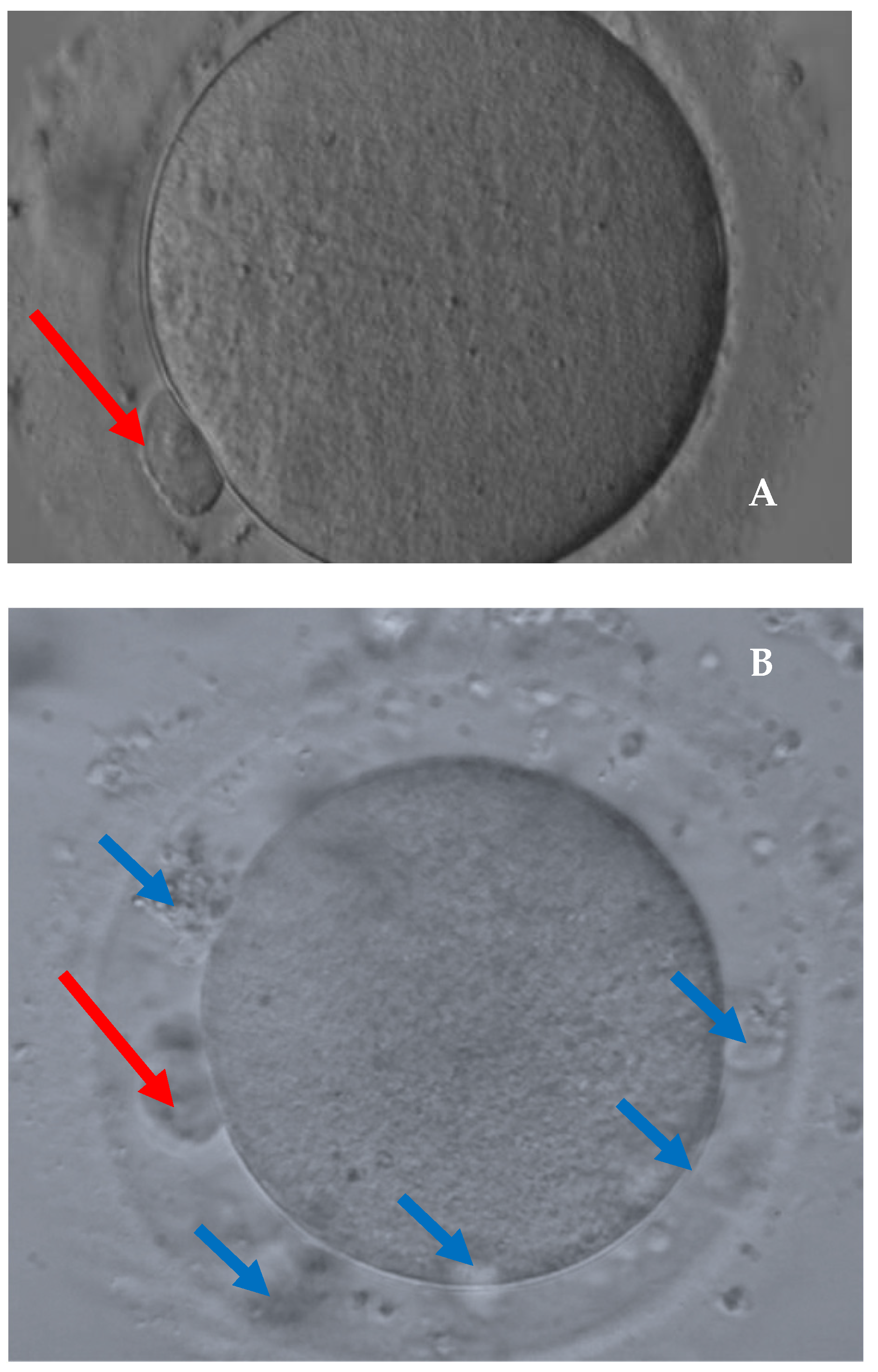
| Characteristic | All Cases n = 294 | Cancer Patients n = 105 | Control Group n = 189 | p-Value |
|---|---|---|---|---|
| Age, years | ||||
| Median (Q1–Q3) | 34 (32–36) | 33 (32–36) | 34 (32–37) | 0.059 |
| Mean ± SD | 33.6 ± 3.4 | 33.1 ± 3.6 | 33.9 ± 3.3 | |
| BMI, kg/m2 | ||||
| Median (Q1–Q3) | 21.8 (20.1–24.5) | 21.3 (19.8–23.9) | 21.9 (20.2–25) | 0.119 |
| Mean ± SD | 22.6 ± 3.5 | 22.2 ± 3.4 | 22.9 ± 3.6 | |
| AMH, ng/mL | ||||
| Median (Q1–Q3) | 2.6 (1.6–4.1) | 2.3 (1.3–3.7) | 2.8 (1.8–4.3) | 0.103 |
| Mean ± SD | 3.2 ± 2.4 | 3 ± 2.4 | 3.3 ± 2.3 | |
| E2 peak at triggering, pg/mL | ||||
| Median (Q1–Q3) | 705 (370.5–1350.3) | 317.5 (198.3–690) | 1043.5 (597.8–1495.8) | <0.0001 |
| Mean ± SD | 920.3 ± 697.6 | 522.1 ± 572.1 | 1140.5 ± 663.4 | |
| PR peak at triggering, ng/mL | ||||
| Median (Q1–Q3) | 1.2 (0.7–1.9) | 1.6 (1.1–2.5) | 0.9 (0.6–1.6) | <0.0001 |
| Mean ± SD | 1.8 ± 2.7 | 2.9 ± 4.2 | 1.2 ± 0.7 | |
| FSH cumulative dose, IU | ||||
| Median (Q1–Q3) | 1725 (1200–2400) | 2250 (1700–3000) | 1425 (1050–1950) | <0.0001 |
| Mean ± SD | 1832.9 ± 944.2 | 2377.3 ± 994.3 | 1527.1 ± 761.9 | |
| Duration of ovarian stimulation, days | ||||
| Median (Q1–Q3) | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.475 |
| Mean ± SD | 11.1 ± 1.9 | 11.1 ± 2.5 | 11.1 ± 1.6 |
| Characteristic | Breast Cancer Patients n = 105 |
|---|---|
| Stage | |
| I | 51/100 (51.0) |
| II | 32/100 (32.0) |
| III | 17/100 (17.0) |
| Histotype | |
| Duttal | 94/103 (91.3) |
| Lobular | 4/103 (3.9) |
| Other | 5/103 (4.9) |
| BRCA | |
| Wild-type | 74/95 (77.9) |
| Mutated | 19/95 (20.0) |
| VUS * | 2/95 (2.1) |
| Grade of differentiation | |
| 1 | 5/95 (5.3) |
| 2 | 39/95 (41.1) |
| 3 | 51/95 (53.7) |
| BRCA mutation type | |
| BRCA1 | 7/21 (33.3) |
| BRCA2 | 6/21 (28.6) |
| Unknown | 8/21 (38.1) |
| Hormone receptors | |
| ER+ and PR+ | 75/103 (72.8) |
| ER− and PR+ | 0/103 (0) |
| ER+ and PR− | 10/103 (9.7) |
| ER− and PR− | 18/103 (17.5) |
| HER-2 expression | |
| Absent | 83/100 (83.0) |
| Present | 17/100 (17.0) |
| Triple negative | 13/99 (13.1) |
| Recurrence | 2 (1.9) |
| Pregnancy | |
| No | 102/104 (98.1) |
| Spontaneous | 2/104 (1.9) |
| Assisted fecundation | 0/104 (0) |
| Characteristics | All Cases n = 294 | Breast Cancer Patients n = 105 | Control Group n = 189 | p-Value |
|---|---|---|---|---|
| Retrieved oocytes (mean ± SD) | ||||
| Median (Q1–Q3) | 8 (5–13) | 13 (8–17) | 7 (5–10) | <0.0001 |
| Mean ± SD | 9.9 ± 6.4 | 13.3 ± 7.3 | 7.9 ± 4.8 | |
| MII mature oocytes | ||||
| Presence | 290 (98.6) | 103 (98.1) | 187 (98.9) | 0.548 |
| Median (Q1–Q3) | 6 (4–10) | 8 (5–13) | 6 (3–7) | <0.0001 |
| Mean ± SD | 7.2 ± 4.7 | 8.9 ± 5.2 | 6.3 ± 4.1 | |
| Immature oocytes (MI + germinal vesicle) | ||||
| Presence | 167 (56.8) | 76 (72.4) | 91 (48.1) | <0.0001 |
| Median (Q1–Q3) | 1 (0–2) | 2 (0–4) | 0 (0–2) | <0.0001 |
| Mean ± SD | 1.7 ± 2.6 | 2.9 ± 3.6 | 1.1 ± 1.6 | |
| Dysmorphic oocytes | ||||
| Presence | 119 (40.5) | 58 (55.2) | 61 (32.3) | <0.0001 |
| <25% | 86/119 (72.3) | 40/58 (69.0) | 46/61 (75.4) | 0.433 |
| ≥25% | 33/119 (27.7) | 18/58 (31.0) | 15/61 (24.6) | |
| Median (Q1–Q3) | 0 (0–1) | 1 (0–2) | 0 (0–1) | <0.0001 |
| Mean ± SD | 0.9 ± 1.8 | 1.5 ± 2.3 | 0.6 ± 1.3 | |
| Percentage * | ||||
| Median (Q1–Q3) | 0 (0–14.3) | 5.3 (0–20.8) | 0 (0–11.1) | 0.0003 |
| Mean ± SD | 8.7 ± 14.8 | 11.5 ± 14.7 | 7.2 ± 14.7 |
| Characteristic | Patients at Risk | N° of Events | Univariable Analysis | Multivariable Analysis * | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |||
| Breast cancer | 294 | 119 | 2.59 (1.58–4.23) | < 0.0001 | 3.92 (1.84–8.35) | < 0.0001 |
| Age | ||||||
| Continuous | 294 | 119 | 0.94 (0.88–1.01) | 0.094 | 0.97 (0.89–1.05) | 0.425 |
| ≤34 years | 162 | 76 | 1 (Ref) | NI | - | |
| >34 years | 132 | 43 | 0.55 (0.34–0.88) | 0.013 | ||
| BMI | 270 | 114 | 1.00 (0.93–1.07) | 0.990 | 1.00 (0.92–1.08) | 0.995 |
| AMH | 248 | 100 | 1.06 (0.95–1.18) | 0.264 | 1.05 (0.93–1.19) | 0.409 |
| Duration of stimulation | 292 | 117 | 1.03 (0.91–1.16) | 0.632 | 1.05 (0.90–1.24) | 0.514 |
| E2 level at triggering day | 292 | 118 | 1.00 (0.99–1.01) | 0.553 | 1.00 (1.00–1.01) | 0.033 |
| Total FSH cumulative dose | 292 | 118 | 1.00 (0.99–1.01) | 0.254 | 1.00 (0.99–1.00) | 0.641 |
| Characteristic | Univariable Logistic Regression | |||
|---|---|---|---|---|
| Patients at Risk | N° of Events | Odds Ratio (95% CI) | p-Value | |
| Age | ||||
| Continuous | 105 | 58 | 0.95 (0.85–1.06) | 0.339 |
| ≤34 years | 66 | 41 | 1 (Ref) | |
| >34 years | 39 | 17 | 0.47 (0.21–1.05) | 0.067 |
| BMI | 105 | 58 | 0.98 (0.88–1.10) | 0.734 |
| AMH | 91 | 50 | 1.18 (0.98–1.44) | 0.087 |
| Duration of stimulation | 105 | 58 | 0.9 (0.76–1.05) | 0.172 |
| E2 level at triggering day | 104 | 57 | 0.99 (0.99–1.01) | 0.229 |
| Total FSH cumulative dose | 105 | 58 | 0.99 (0.99–1.01) | 0.615 |
| Stage | 100 | 55 | ||
| I | 51 | 29 | 1 (Ref) | |
| II | 32 | 15 | 0.67 (0.28–1.63) | 0.376 |
| III | 17 | 11 | 1.39 (0.45–4.34) | 0.570 |
| Histotype | 103 | 56 | ||
| Duttal | 94 | 49 | 1 (Ref) | |
| Lobular and Other | 9 | 7 | 3.21 (0.63–16.29) | 0.158 |
| Grade of differentiation | 95 | 53 | ||
| 1 | 5 | 4 | 1 (Ref) | |
| 2 | 39 | 18 | 0.21 (0.02–2.09) | 0.185 |
| 3 | 51 | 31 | 0.39 (0.04–3.72) | 0.411 |
| BRCA | ||||
| Wild-type versus mutated/VUS | 95 | 50 | 2.11 (0.76–5.83) | 0.149 |
| BRCA1 versus wild-type/BRCA2 | 87 | 44 | 1.33 (0.28–6.34) | 0.718 |
| Hormone receptors | 103 | 57 | ||
| ER+ and PR+ | 75 | 39 | 1 (Ref) | |
| ER + and PR - | 10 | 7 | 2.15 (0.52–8.97) | 0.292 |
| ER- and PR - | 18 | 11 | 1.45 (0.51–4.15) | 0.488 |
| HER-2 expression | 100 | 56 | ||
| Absent | 83 | 47 | 1 (Ref) | |
| Present | 17 | 9 | 0.86 (0.3–2.45) | 0.780 |
| Triple negative | 99 | 56 | ||
| No | 87 | 49 | 1 (Ref) | |
| Yes | 13 | 7 | 0.88 (0.27–2.84) | 0.832 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiani, C.; Guarino, A.; Meneghini, C.; Licata, E.; Paciotti, G.; Miriello, D.; Schiavi, M.C.; Spina, V.; Corno, R.; Gallo, M.; et al. Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation. Cancers 2022, 14, 5718. https://doi.org/10.3390/cancers14225718
Fabiani C, Guarino A, Meneghini C, Licata E, Paciotti G, Miriello D, Schiavi MC, Spina V, Corno R, Gallo M, et al. Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation. Cancers. 2022; 14(22):5718. https://doi.org/10.3390/cancers14225718
Chicago/Turabian StyleFabiani, Cristina, Antonella Guarino, Caterina Meneghini, Emanuele Licata, Gemma Paciotti, Donatella Miriello, Michele Carlo Schiavi, Vincenzo Spina, Roberta Corno, Mariagrazia Gallo, and et al. 2022. "Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation" Cancers 14, no. 22: 5718. https://doi.org/10.3390/cancers14225718
APA StyleFabiani, C., Guarino, A., Meneghini, C., Licata, E., Paciotti, G., Miriello, D., Schiavi, M. C., Spina, V., Corno, R., Gallo, M., & Rago, R. (2022). Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation. Cancers, 14(22), 5718. https://doi.org/10.3390/cancers14225718








