Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
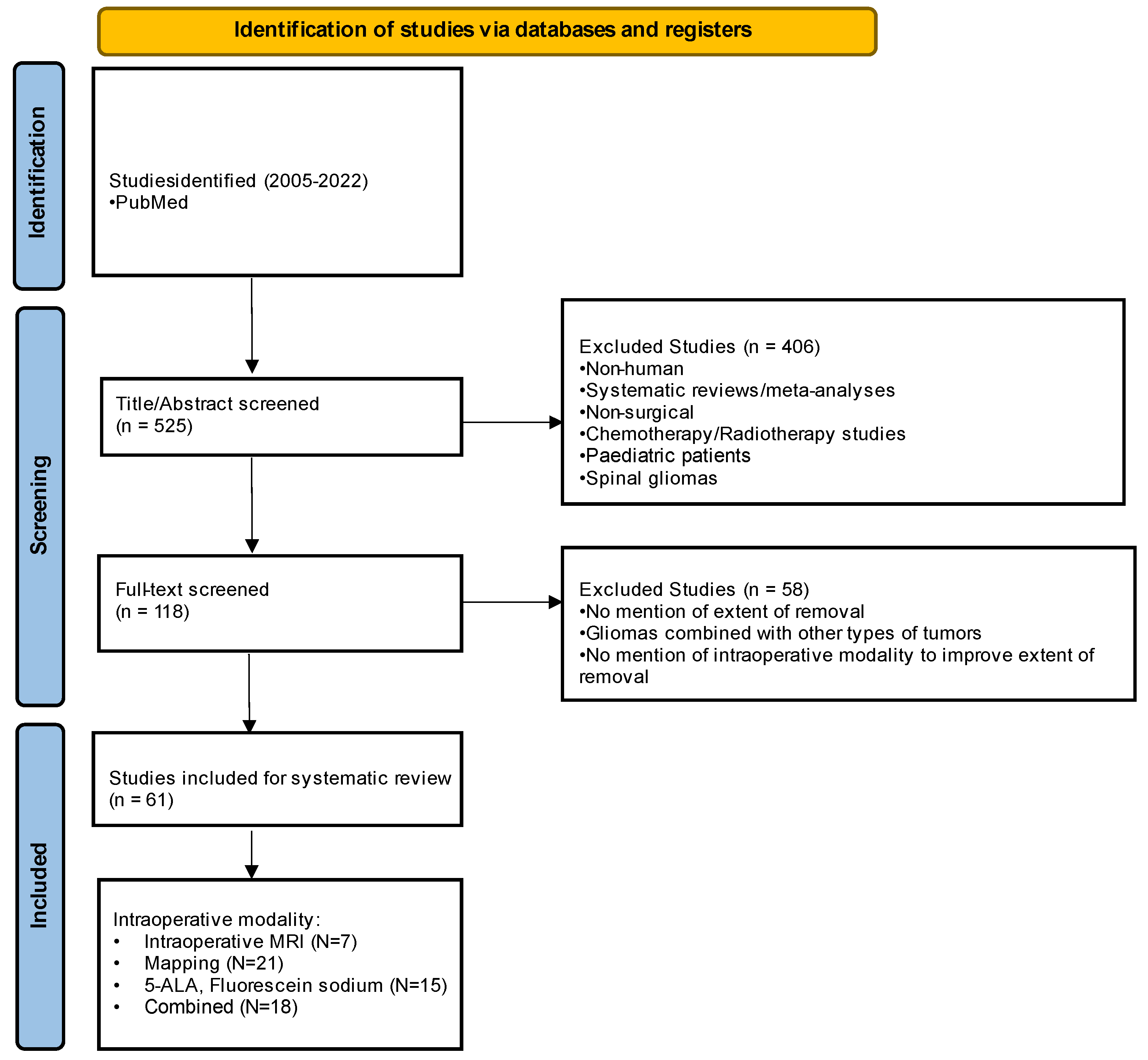
2. Materials and Methods
3. Results
3.1. Intraoperative Magnetic Resonance Imaging
3.2. Awake vs. Asleep Cortical and Subcortical Mapping
3.3. Fluorescence-Guided Imaging
3.4. Combined and Other Adjuvant Modalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larjavaara, S.; Mäntylä, R.; Salminen, T.; Haapasalo, H.; Raitanen, J.; Jääskeläinen, J.; Auvinen, A. Incidence of Gliomas by Anatomic Location. Neuro Oncol. 2007, 9, 319–325. [Google Scholar] [CrossRef] [PubMed]
- La Torre, D.; Maugeri, R.; Angileri, F.F.; Pezzino, G.; Conti, A.; Cardali, S.M.; Calisto, A.; Sciarrone, G.; Misefari, A.; Germanò, A.; et al. Human Leukocyte Antigen Frequency in Human High-Grade Gliomas: A Case-Control Study in Sicily. Neurosurgery 2009, 64, 1082–1088; discussion 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shubham, S.; Mandal, K.; Trivedi, V.; Chauhan, R.; Naseera, S. Survival and Prognostic Factors for Glioblastoma Multiforme: Retrospective Single-Institutional Study. Indian J. Cancer 2017, 54, 362–367. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A.R. Independent Association of Extent of Resection with Survival in Patients with Malignant Brain Astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef]
- Yong, R.L.; Lonser, R.R. Surgery for Glioblastoma Multiforme: Striking a Balance. World Neurosurg. 2011, 76, 528–530. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A Multivariate Analysis of 416 Patients with Glioblastoma Multiforme: Prognosis, Extent of Resection, and Survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing Percent Resection and Residual Volume Thresholds Affecting Survival and Recurrence for Patients with Newly Diagnosed Intracranial Glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef]
- De Benedictis, A.; Moritz-Gasser, S.; Duffau, H. Awake Mapping Optimizes the Extent of Resection for Low-Grade Gliomas in Eloquent Areas. Neurosurgery 2010, 66, 1074–1084; discussion 1084. [Google Scholar] [CrossRef]
- Morshed, R.A.; Young, J.S.; Lee, A.T.; Hervey-Jumper, S.L. Functional Mapping for Glioma Surgery, Part 2: Intraoperative Mapping Tools. Neurosurg. Clin. N. Am. 2021, 32, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Knauth, M.; Wirtz, C.R.; Tronnier, V.M.; Aras, N.; Kunze, S.; Sartor, K. Intraoperative MR Imaging Increases the Extent of Tumor Resection in Patients with High-Grade Gliomas. AJNR Am. J. Neuroradiol. 1999, 20, 1642–1646. [Google Scholar] [PubMed]
- Krivosheya, D.; Rao, G.; Tummala, S.; Kumar, V.; Suki, D.; Bastos, D.C.A.; Prabhu, S.S. Impact of Multi-Modality Monitoring Using Direct Electrical Stimulation to Determine Corticospinal Tract Shift and Integrity in Tumors Using the Intraoperative MRI. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 375–380. [Google Scholar] [CrossRef]
- Coburger, J.; Segovia von Riehm, J.; Ganslandt, O.; Wirtz, C.R.; Renovanz, M. Is There an Indication for Intraoperative MRI in Subtotal Resection of Glioblastoma? A Multicenter Retrospective Comparative Analysis. World Neurosurg. 2018, 110, e389–e397. [Google Scholar] [CrossRef]
- Scherer, M.; Jungk, C.; Younsi, A.; Kickingereder, P.; Müller, S.; Unterberg, A. Factors Triggering an Additional Resection and Determining Residual Tumor Volume on Intraoperative MRI: Analysis from a Prospective Single-Center Registry of Supratentorial Gliomas. Neurosurg. Focus 2016, 40, E4. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the Extent of Tumor Volume Resection and Patient Survival in Surgery of Glioblastoma Multiforme with High-Field Intraoperative MRI Guidance. Neuro Oncol. 2011, 13, 1339–1348. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI Guidance and Extent of Resection in Glioma Surgery: A Randomised, Controlled Trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Weinberg, J.S.; Suki, D.; Rao, G.; Prabhu, S.S.; Shah, K.; Jackson, E.; Sawaya, R. Impact of Intraoperative High-Field Magnetic Resonance Imaging Guidance on Glioma Surgery: A Prospective Volumetric Analysis. Neurosurgery 2009, 64, 1073–1081; discussion 1081. [Google Scholar] [CrossRef]
- Kubben, P.L.; Scholtes, F.; Schijns, O.E.M.G.; Ter Laak-Poort, M.P.; Teernstra, O.P.M.; Kessels, A.G.H.; van Overbeeke, J.J.; Martin, D.H.; van Santbrink, H. Intraoperative Magnetic Resonance Imaging versus Standard Neuronavigation for the Neurosurgical Treatment of Glioblastoma: A Randomized Controlled Trial. Surg. Neurol. Int. 2014, 5, 70. [Google Scholar] [CrossRef]
- Shah, A.S.; Sylvester, P.T.; Yahanda, A.T.; Vellimana, A.K.; Dunn, G.P.; Evans, J.; Rich, K.M.; Dowling, J.L.; Leuthardt, E.C.; Dacey, R.G.; et al. Intraoperative MRI for Newly Diagnosed Supratentorial Glioblastoma: A Multicenter-Registry Comparative Study to Conventional Surgery. J. Neurosurg. 2020, 135, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Sarkar, R.; Brandel, M.G.; Wali, A.R.; Rennert, R.C.; Lopez Ramos, C.; Padwal, J.; Steinberg, J.A.; Santiago-Dieppa, D.R.; Cheung, V.; et al. Cost-Effectiveness of Intraoperative MRI for Treatment of High-Grade Gliomas. Radiology 2019, 291, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Riva, M.; Fava, E.; Ferpozzi, V.; Castellano, A.; Raneri, F.; Pessina, F.; Bizzi, A.; Falini, A.; Cerri, G. Tailoring Neurophysiological Strategies with Clinical Context Enhances Resection and Safety and Expands Indications in Gliomas Involving Motor Pathways. Neuro Oncol. 2014, 16, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, A.M.; Worrell, G.A.; Tatum, W.O.; Mahato, D.; Brinkmann, B.H.; Rosenfeld, S.S.; ReFaey, K.; Bechtle, P.S.; Quinones-Hinojosa, A. High-Frequency Oscillations in Awake Patients Undergoing Brain Tumor-Related Epilepsy Surgery. Neurology 2018, 90, e1119–e1125. [Google Scholar] [CrossRef] [PubMed]
- Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Lee, Y.M.; Nangiana, J.; Vivas-Buitrago, T.; Quiñones-Hinojosa, A. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery 2017, 81, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Chalise, L.; Ohka, F.; Aoki, K.; Tanahashi, K.; Hirano, M.; Nishikawa, T.; Yamaguchi, J.; Shimizu, H.; Wakabayashi, T.; et al. Impact of the Extent of Resection on the Survival of Patients with Grade II and III Gliomas Using Awake Brain Mapping. J. Neurooncol. 2021, 153, 361–372. [Google Scholar] [CrossRef]
- Morsy, A.A.; Ismail, A.M.; Nasr, Y.M.; Waly, S.H.; Abdelhameed, E.A. Predictors of Stimulation-Induced Seizures during Perirolandic Glioma Resection Using Intraoperative Mapping Techniques. Surg. Neurol. Int. 2021, 12, 117. [Google Scholar] [CrossRef]
- Gogos, A.J.; Young, J.S.; Morshed, R.A.; Avalos, L.N.; Noss, R.S.; Villanueva-Meyer, J.E.; Hervey-Jumper, S.L.; Berger, M.S. Triple Motor Mapping: Transcranial, Bipolar, and Monopolar Mapping for Supratentorial Glioma Resection Adjacent to Motor Pathways. J. Neurosurg. 2020, 134, 1728–1737. [Google Scholar] [CrossRef]
- Burks, J.D.; Bonney, P.A.; Conner, A.K.; Glenn, C.A.; Briggs, R.G.; Battiste, J.D.; McCoy, T.; O’Donoghue, D.L.; Wu, D.H.; Sughrue, M.E. A Method for Safely Resecting Anterior Butterfly Gliomas: The Surgical Anatomy of the Default Mode Network and the Relevance of Its Preservation. J. Neurosurg. 2017, 126, 1795–1811. [Google Scholar] [CrossRef]
- Nossek, E.; Korn, A.; Shahar, T.; Kanner, A.A.; Yaffe, H.; Marcovici, D.; Ben-Harosh, C.; Ben Ami, H.; Weinstein, M.; Shapira-Lichter, I.; et al. Intraoperative Mapping and Monitoring of the Corticospinal Tracts with Neurophysiological Assessment and 3-Dimensional Ultrasonography-Based Navigation. Clinical Article. J. Neurosurg. 2011, 114, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Seidel, K.; Beck, J.; Murek, M.; Jilch, A.; Wiest, R.; Fung, C.; Raabe, A. Intraoperative Monopolar Mapping during 5-ALA-Guided Resections of Glioblastomas Adjacent to Motor Eloquent Areas: Evaluation of Resection Rates and Neurological Outcome. Neurosurg. Focus 2014, 37, E16. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Aubin, G.; Delion, M.; Lemée, J.-M.; Ter Minassian, A.; Menei, P. What Effects Does Awake Craniotomy Have on Functional and Survival Outcomes for Glioblastoma Patients? J. Neurooncol. 2021, 151, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Puglisi, G.; Conti Nibali, M.; Viganò, L.; Sciortino, T.; Gay, L.; Leonetti, A.; Zito, P.; Riva, M.; Bello, L. Asleep or Awake Motor Mapping for Resection of Perirolandic Glioma in the Nondominant Hemisphere? Development and Validation of a Multimodal Score to Tailor the Surgical Strategy. J. Neurosurg. 2022, 136, 16–29. [Google Scholar] [CrossRef]
- Giamouriadis, A.; Lavrador, J.P.; Bhangoo, R.; Ashkan, K.; Vergani, F. How Many Patients Require Brain Mapping in an Adult Neuro-Oncology Service? Neurosurg. Rev. 2020, 43, 729–738. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chiu, H.-Y.; Lin, Y.-J.; Chen, K.-T.; Hsu, P.-W.; Huang, Y.-C.; Chen, P.-Y.; Wei, K.-C. The Merits of Awake Craniotomy for Glioblastoma in the Left Hemispheric Eloquent Area: One Institution Experience. Clin. Neurol. Neurosurg. 2021, 200, 106343. [Google Scholar] [CrossRef]
- Alimohamadi, M.; Shirani, M.; Shariat Moharari, R.; Pour-Rashidi, A.; Ketabchi, M.; Khajavi, M.; Arami, M.; Amirjamshidi, A. Application of Awake Craniotomy and Intraoperative Brain Mapping for Surgical Resection of Insular Gliomas of the Dominant Hemisphere. World Neurosurg. 2016, 92, 151–158. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.-P.; Van Effenterre, R.; Capelle, L. Contribution of Intraoperative Electrical Stimulations in Surgery of Low Grade Gliomas: A Comparative Study between Two Series without (1985–96) and with (1996–2003) Functional Mapping in the Same Institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef]
- Martino, J.; Gomez, E.; Bilbao, J.L.; Dueñas, J.C.; Vázquez-Barquero, A. Cost-Utility of Maximal Safe Resection of WHO Grade II Gliomas within Eloquent Areas. Acta Neurochir. 2013, 155, 41–50. [Google Scholar] [CrossRef]
- Sacko, O.; Lauwers-Cances, V.; Brauge, D.; Sesay, M.; Brenner, A.; Roux, F.-E. Awake Craniotomy vs Surgery under General Anesthesia for Resection of Supratentorial Lesions. Neurosurgery 2011, 68, 1192–1198; discussion 1198–1199. [Google Scholar] [CrossRef]
- Skrap, M.; Mondani, M.; Tomasino, B.; Weis, L.; Budai, R.; Pauletto, G.; Eleopra, R.; Fadiga, L.; Ius, T. Surgery of Insular Nonenhancing Gliomas: Volumetric Analysis of Tumoral Resection, Clinical Outcome, and Survival in a Consecutive Series of 66 Cases. Neurosurgery 2012, 70, 1081–1093; discussion 1093–1094. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Ghareeb, F.; Duffau, H. Surgery for Low-Grade Glioma Infiltrating the Central Cerebral Region: Location as a Predictive Factor for Neurological Deficit, Epileptological Outcome, and Quality of Life. J. Neurosurg. 2013, 119, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Eseonu, C.I.; Rincon-Torroella, J.; Lee, Y.M.; ReFaey, K.; Tripathi, P.; Quinones-Hinojosa, A. Intraoperative Seizures in Awake Craniotomy for Perirolandic Glioma Resections That Undergo Cortical Mapping. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 239–246. [Google Scholar] [CrossRef]
- Motomura, K.; Natsume, A.; Iijima, K.; Kuramitsu, S.; Fujii, M.; Yamamoto, T.; Maesawa, S.; Sugiura, J.; Wakabayashi, T. Surgical Benefits of Combined Awake Craniotomy and Intraoperative Magnetic Resonance Imaging for Gliomas Associated with Eloquent Areas. J. Neurosurg. 2017, 127, 790–797. [Google Scholar] [CrossRef]
- Saito, T.; Muragaki, Y.; Tamura, M.; Maruyama, T.; Nitta, M.; Tsuzuki, S.; Fukuchi, S.; Ohashi, M.; Kawamata, T. Awake Craniotomy with Transcortical Motor Evoked Potential Monitoring for Resection of Gliomas in the Precentral Gyrus: Utility for Predicting Motor Function. J. Neurosurg. 2019, 132, 987–997. [Google Scholar] [CrossRef]
- Magill, S.T.; Han, S.J.; Li, J.; Berger, M.S. Resection of Primary Motor Cortex Tumors: Feasibility and Surgical Outcomes. J. Neurosurg. 2018, 129, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Shah, A.H.; Bregy, A.; Shah, N.H.; Thambuswamy, M.; Barbarite, E.; Fuhrman, T.; Komotar, R.J. Awake Craniotomy for Brain Tumor Resection: The Rule Rather than the Exception? J. Neurosurg. Anesth. 2013, 25, 240–247. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Viëtor, C.L.; Rizopoulos, D.; Schouten, J.W.; Klimek, M.; Dirven, C.M.F.; Vincent, A.J.-P.E. Awake Craniotomy versus Craniotomy under General Anesthesia without Surgery Adjuncts for Supratentorial Glioblastoma in Eloquent Areas: A Retrospective Matched Case-Control Study. Acta Neurochir. 2019, 161, 307–315. [Google Scholar] [CrossRef]
- Li, Y.; Rey-Dios, R.; Roberts, D.W.; Valdés, P.A.; Cohen-Gadol, A.A. Intraoperative Fluorescence-Guided Resection of High-Grade Gliomas: A Comparison of the Present Techniques and Evolution of Future Strategies. World Neurosurg. 2014, 82, 175–185. [Google Scholar] [CrossRef]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Greco Crasto, S.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M.; 5-ALA Recurrent Glioma Study Group. Five-Aminolevulinic Acid for Fluorescence-Guided Resection of Recurrent Malignant Gliomas: A Phase Ii Study. Neurosurgery 2009, 65, 1070–1076; discussion 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Díez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; García de Eulate, R.; Domínguez Echávarri, P.; Aristu Mendiroz, J. Surgery Guided by 5-Aminolevulinic Fluorescence in Glioblastoma: Volumetric Analysis of Extent of Resection in Single-Center Experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein Sodium-Guided Surgery in Glioblastoma Multiforme: A Prospective Evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive Resection at the Infiltrative Margins of Glioblastoma Facilitated by Intraoperative Fluorescein Guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic Acid Is a Promising Marker for Detection of Anaplastic Foci in Diffusely Infiltrating Gliomas with Nonsignificant Contrast Enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef]
- Chan, D.T.M.; Yi-Pin Sonia, H.; Poon, W.S. 5-Aminolevulinic Acid Fluorescence Guided Resection of Malignant Glioma: Hong Kong Experience. Asian J. Surg. 2018, 41, 467–472. [Google Scholar] [CrossRef]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross Total Resection of Glioma with the Intraoperative Fluorescence-Guidance of Fluorescein Sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the Biodistribution of Fluorescein in Glioma-Infiltrated Mouse Brain and Histopathological Correlation of Intraoperative Findings in High-Grade Gliomas Resected under Fluorescein Fluorescence Guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Catapano, G.; Sgulò, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Francaviglia, N.; Iacopino, D.G.; Costantino, G.; Villa, A.; Impallaria, P.; Meli, F.; Maugeri, R. Fluorescein for Resection of High-Grade Gliomas: A Safety Study Control in a Single Center and Review of the Literature. Surg. Neurol. Int. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Stummer, W.; Suero Molina, E.J.; Wölfer, J. Simultaneous Fluorescein Sodium and 5-ALA in Fluorescence-Guided Glioma Surgery. Acta Neurochir. 2015, 157, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Shi, C.; Liu, Y.; Teng, L.; Han, D.; et al. Intraoperative Fluorescence-Guided Resection of High-Grade Malignant Gliomas Using 5-Aminolevulinic Acid-Induced Porphyrins: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef]
- Yamada, S.; Muragaki, Y.; Maruyama, T.; Komori, T.; Okada, Y. Role of Neurochemical Navigation with 5-Aminolevulinic Acid during Intraoperative MRI-Guided Resection of Intracranial Malignant Gliomas. Clin. Neurol. Neurosurg. 2015, 130, 134–139. [Google Scholar] [CrossRef]
- Maesawa, S.; Fujii, M.; Nakahara, N.; Watanabe, T.; Wakabayashi, T.; Yoshida, J. Intraoperative Tractography and Motor Evoked Potential (MEP) Monitoring in Surgery for Gliomas around the Corticospinal Tract. World Neurosurg. 2010, 74, 153–161. [Google Scholar] [CrossRef]
- Maldaun, M.V.C.; Khawja, S.N.; Levine, N.B.; Rao, G.; Lang, F.F.; Weinberg, J.S.; Tummala, S.; Cowles, C.E.; Ferson, D.; Nguyen, A.-T.; et al. Awake Craniotomy for Gliomas in a High-Field Intraoperative Magnetic Resonance Imaging Suite: Analysis of 42 Cases. J. Neurosurg. 2014, 121, 810–817. [Google Scholar] [CrossRef]
- Zhuang, D.-X.; Wu, J.-S.; Yao, C.-J.; Qiu, T.-M.; Lu, J.-F.; Zhu, F.-P.; Xu, G.; Zhu, W.; Zhou, L.-F. Intraoperative Multi-Information-Guided Resection of Dominant-Sided Insular Gliomas in a 3-T Intraoperative Magnetic Resonance Imaging Integrated Neurosurgical Suite. World Neurosurg. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Ghinda, D.; Zhang, N.; Lu, J.; Yao, C.-J.; Yuan, S.; Wu, J.-S. Contribution of Combined Intraoperative Electrophysiological Investigation with 3-T Intraoperative MRI for Awake Cerebral Glioma Surgery: Comprehensive Review of the Clinical Implications and Radiological Outcomes. Neurosurg. Focus 2016, 40, E14. [Google Scholar] [CrossRef] [PubMed]
- Leuthardt, E.C.; Lim, C.C.H.; Shah, M.N.; Evans, J.A.; Rich, K.M.; Dacey, R.G.; Tempelhoff, R.; Chicoine, M.R. Use of Movable High-Field-Strength Intraoperative Magnetic Resonance Imaging with Awake Craniotomies for Resection of Gliomas: Preliminary Experience. Neurosurgery 2011, 69, 194–205; discussion 205-206. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Yao, C.; Zhuang, D.; Qiu, T.; Hu, X.; Zhang, J.; Gong, X.; Liang, W.; Mao, Y.; et al. Awake Language Mapping and 3-Tesla Intraoperative MRI-Guided Volumetric Resection for Gliomas in Language Areas. J. Clin. Neurosci. 2013, 20, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Zavarella, S.; Jarchin, L.; Nardi, D.; Schaffer, S.; Schulder, M. Combined Brain Mapping and Compact Intraoperative MRI for Brain Tumor Resection. Stereotact. Funct. Neurosurg. 2018, 96, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Whiting, B.B.; Lee, B.S.; Mahadev, V.; Borghei-Razavi, H.; Ahuja, S.; Jia, X.; Mohammadi, A.M.; Barnett, G.H.; Angelov, L.; Rajan, S.; et al. Combined Use of Minimal Access Craniotomy, Intraoperative Magnetic Resonance Imaging, and Awake Functional Mapping for the Resection of Gliomas in 61 Patients. J. Neurosurg. 2019, 132, 159–167. [Google Scholar] [CrossRef]
- Peruzzi, P.; Bergese, S.D.; Viloria, A.; Puente, E.G.; Abdel-Rasoul, M.; Chiocca, E.A. A Retrospective Cohort-Matched Comparison of Conscious Sedation versus General Anesthesia for Supratentorial Glioma Resection. Clinical Article. J. Neurosurg. 2011, 114, 633–639. [Google Scholar] [CrossRef]
- Tuominen, J.; Yrjänä, S.; Ukkonen, A.; Koivukangas, J. Awake Craniotomy May Further Improve Neurological Outcome of Intraoperative MRI-Guided Brain Tumor Surgery. Acta Neurochir. 2013, 155, 1805–1812. [Google Scholar] [CrossRef]
- Roder, C.; Bisdas, S.; Ebner, F.H.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the Extent of Resection and Survival Benefit of Patients in Glioblastoma Surgery: High-Field IMRI versus Conventional and 5-ALA-Assisted Surgery. Eur. J. Surg. Oncol. 2014, 40, 297–304. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for Glioblastoma: Impact of the Combined Use of 5-Aminolevulinic Acid and Intraoperative MRI on Extent of Resection and Survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef]
- Schatlo, B.; Fandino, J.; Smoll, N.R.; Wetzel, O.; Remonda, L.; Marbacher, S.; Perrig, W.; Landolt, H.; Fathi, A.-R. Outcomes after Combined Use of Intraoperative MRI and 5-Aminolevulinic Acid in High-Grade Glioma Surgery. Neuro Oncol. 2015, 17, 1560–1567. [Google Scholar] [CrossRef]
- Feigl, G.C.; Ritz, R.; Moraes, M.; Klein, J.; Ramina, K.; Gharabaghi, A.; Krischek, B.; Danz, S.; Bornemann, A.; Liebsch, M.; et al. Resection of Malignant Brain Tumors in Eloquent Cortical Areas: A New Multimodal Approach Combining 5-Aminolevulinic Acid and Intraoperative Monitoring. J. Neurosurg. 2010, 113, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the Combination of 5-Aminolevulinic Acid-Induced Fluorescence with Intraoperative Magnetic Resonance Imaging-Guided Surgery for Glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; De Pellegrin, S.; d’Avella, E.; Gioffrè, G.; Rossetto, M.; Gerardi, A.; Lombardi, G.; Manara, R.; Munari, M.; Saladini, M.; et al. 5-Aminolevulinic Acid (5-ALA) Fluorescence Guided Surgery of High-Grade Gliomas in Eloquent Areas Assisted by Functional Mapping. Our Experience and Review of the Literature. Acta Neurochir. 2013, 155, 965–972; discussion 972. [Google Scholar] [CrossRef] [PubMed]
- Pichierri, A.; Bradley, M.; Iyer, V. Intraoperative Magnetic Resonance Imaging-Guided Glioma Resections in Awake or Asleep Settings and Feasibility in the Context of a Public Health System. World Neurosurg. X 2019, 3, 100022. [Google Scholar] [CrossRef]
- Morin, F.; Courtecuisse, H.; Reinertsen, I.; Le Lann, F.; Palombi, O.; Payan, Y.; Chabanas, M. Brain-Shift Compensation Using Intraoperative Ultrasound and Constraint-Based Biomechanical Simulation. Med. Image Anal. 2017, 40, 133–153. [Google Scholar] [CrossRef]
- Prada, F.; Bene, M.D.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of Residual Tumor with Intraoperative Contrast-Enhanced Ultrasound during Glioblastoma Resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Neidert, M.C.; Hostettler, I.C.; Burkhardt, J.-K.; Mohme, M.; Held, U.; Kofmehl, R.; Eisele, G.; Woernle, C.M.; Regli, L.; Bozinov, O. The Influence of Intraoperative Resection Control Modalities on Survival Following Gross Total Resection of Glioblastoma. Neurosurg. Rev. 2016, 39, 401–409. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Ji, M.; Orringer, D.A.; Freudiger, C.W.; Ramkissoon, S.; Liu, X.; Lau, D.; Golby, A.J.; Norton, I.; Hayashi, M.; Agar, N.Y.R.; et al. Rapid, Label-Free Detection of Brain Tumors with Stimulated Raman Scattering Microscopy. Sci. Transl. Med. 2013, 5, 201ra119. [Google Scholar] [CrossRef]
- Ji, M.; Lewis, S.; Camelo-Piragua, S.; Ramkissoon, S.H.; Snuderl, M.; Venneti, S.; Fisher-Hubbard, A.; Garrard, M.; Fu, D.; Wang, A.C.; et al. Detection of Human Brain Tumor Infiltration with Quantitative Stimulated Raman Scattering Microscopy. Sci. Transl. Med. 2015, 7, 309ra163. [Google Scholar] [CrossRef]
- Garzon-Muvdi, T.; Kut, C.; Li, X.; Chaichana, K.L. Intraoperative Imaging Techniques for Glioma Surgery. Future Oncol. 2017, 13, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, B.Y.; Keizer, M.E.; Vincent, A.J.P.E.; Schouten, J.W.; Stolker, R.J.; Klimek, M. Awake Craniotomy versus Craniotomy under General Anesthesia for the Surgical Treatment of Insular Glioma: Choices and Outcomes. Neurol. Res. 2018, 40, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Shiban, E.; Droese, D.; Gempt, J.; Buchmann, N.; Pape, H.; Ryang, Y.-M.; Meyer, B.; Ringel, F. Predictive Value and Safety of Intraoperative Neurophysiological Monitoring with Motor Evoked Potentials in Glioma Surgery. Neurosurgery 2012, 70, 1060–1070; discussion 1070-1071. [Google Scholar] [CrossRef] [PubMed]
- Przybylowski, C.J.; Hervey-Jumper, S.L.; Sanai, N. Surgical Strategy for Insular Glioma. J. Neurooncol. 2021, 151, 491–497. [Google Scholar] [CrossRef]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-Grade Gliomas and High-Grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411; discussion 411. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A Prospective Phase II Clinical Trial of 5-Aminolevulinic Acid to Assess the Correlation of Intraoperative Fluorescence Intensity and Degree of Histologic Cellularity during Resection of High-Grade Gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Yashin, K.; Bonsanto, M.M.; Achkasova, K.; Zolotova, A.; Wael, A.-M.; Kiseleva, E.; Moiseev, A.; Medyanik, I.; Kravets, L.; Huber, R.; et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics 2022, 12, 335. [Google Scholar] [CrossRef]
- Dasenbrock, H.H.; See, A.P.; Smalley, R.J.; Bi, W.L.; Dolati, P.; Frerichs, K.U.; Golby, A.J.; Chiocca, E.A.; Aziz-Sultan, M.A. Frameless Stereotactic Navigation during Insular Glioma Resection Using Fusion of Three-Dimensional Rotational Angiography and Magnetic Resonance Imaging. World Neurosurg. 2019, 126, 322–330. [Google Scholar] [CrossRef]
- Shalan, M.E.; Soliman, A.Y.; Nassar, I.A.; Alarabawy, R.A. Surgical Planning in Patients with Brain Glioma Using Diffusion Tensor MR Imaging and Tractography. Egypt. J. Radiol. Nucl. Med. 2021, 52, 110. [Google Scholar] [CrossRef]
| Study (Years) | Study Period | Study Design | Tumors | Histology | Location | Extent of Resection | Overall Survival | Complications |
|---|---|---|---|---|---|---|---|---|
| Coburger et al. (2017) [15] | 2008–2013 | Retrospective | 62 | GBM | Non-specific | EOR 78 ± 4.0% | - | 9 (27%) complications 2 (6%) CSF leak, 2 (6%) bleeding, 3 (9%) ischemia, 1 (3%) infection 1 (3%) hydrocephalus |
| Scherer et al. (2016) [16] | 2011–2014 | Prospective | 224 | 141 (62.9%) GBM, 83 (37.1%) lower-grade gliomas | Frontal, temporal, parietal, occipital, intraventricular | 70% underwent additional resection after iMRI | - | 15 (6.7%) permanent neurological deficits |
| Kuhnt et al. (2011) [17] | 2002–2008 | Retrospective | 135 | GBM | Supratentorial | Residual tumor in 88 patients. Further resection was performed in 19 patients. GTR for 9 patients increased from 47 (34.8%) to 56 (41.5%) patients. | Mean OS in patients with GTR (EOR > 98%) = 14 months compared to 9 months in EOR < 98%) | 1 (0.7%) permanent language deficit |
| Senft et al. (2011) [18] | 2007–2010 | Prospective | 49 | 46 (93.9%) GBM, 3 (6.1%) lower-grade gliomas | Non-specific | GTR 23/24 (96%) in the iMRI group, and 17/25 (68%) in the control group. | Median PFS: 226 days (95% CI 0–454) in the iMRI group and 154 days (60–248) in the conventional group | 5 (10.2%) permanent neurological deficits |
| Hatiboglu et al. (2009) [19] | 2006–2007 | Prospective | 46 | 29 (63.0%) GBM, 17 (37.0%) lower-grade gliomas | Non-specific | EOR increased from 76% (range, 35%-97%) to 96% (range, 48%-100%) 29 patients had GTR, 15 (52%) due to iMRI | - | 4/46 (9%) permanent neurological deficits (3 hemiparesis and 1 visual deficit) |
| Kubben et al. (2014) [20] | 2010–2012 | Prospective | 14 | GBM | Supratentorial | Residual tumor volume: control group median IQR (6.5%, 2.5–14.75%), iMRI group (13%, 3.75–27.75%) | iMRI: median (IQR) = 396 (191–599) days, control: 472 (244–619) days | - |
| Shah et al. (2020) [21] | 1996–2008 | Retrospective | 640 | GBM | Non-specific | Additional resection was performed in 104/122 with STR after iMRI, | Median OS was 17.0 months for patients with and without iMRI. iMRI was not associated with increased OS | Use of iMRI was not associated with increased rate of new permanent neurological deficit |
| Study (Years) | Study Period | Study Design | Tumors | Histology | Location | Mapping | Extent of Resection | Overall Survival | Complications |
|---|---|---|---|---|---|---|---|---|---|
| (A): General Anesthesia | |||||||||
| Gogos et al. (2020) [19] | 2018–2019 | Prospective | 58 | 37 (62.7%) GBM, 21 (37.3%) lower-grade gliomas | Frontal, temporal, parietal, insula | GA | Median EOR 98.0% | - | 2 (3.4%) permanent neurological deficits |
| Schucht et al. (2014) [32] | 2010–2014 | Retrospective | 67 | GBM | Proximity to corticospinal tract | GA | Complete resection in 49/67 (73%) | - | 3 (4%) persisting postoperative motor deficits |
| Duffau et al. (2005) [38] | 1985–1996 and 1996–2003 | Retrospective | 222 | Lower-grade gliomas | Eloquent areas | 122 (54.9%) GA | Total resection: GA monitoring 31 (25.4%), no GA monitoring 6 (6%) | Survival: in GA monitoring: 11 (9.0%), non-GA monitoring: 43 (43%) | - |
| (B): Awake Craniotomy | |||||||||
| Motomura et al. (2021) [27] | 2012–2020 | Retrospective | 126 | Lower-grade gliomas | Frontal, insular, temporal, parietal, occipital | AC | Median EOR 93.1% >100% (=supratotal resection) 15 (11.9%) 100% (=gross total resection) 32 (25.4%) ≥90%, <100% (=subtotal resection) 27 (21.4%) <90% (=partial resection) 52 (41.3%) | - | 5 (4.0%) permanent speech disturbance 9 (7.1%) permanent motor disturbance |
| Burks et al. (2017) [30] | 2012–2015 | Retrospective | 15 | 11 (73%) GBM, 4 (26%) lower-grade gliomas | Non-specific | AC | 100% (GTR) 12 (80%) 90%–99% (NTR)1 (7%) 70%–89% (STR) 2 (13%) | 4 died (26.6%) | 1 (7%) abulia 1 (7%) hemorrhage 1 (7%) Infection |
| Clavreul et al. (2021) [33] | 2004–2019 | Retrospective | 46 | GBM | Non-specific | AC | Complete resection in 28 (61%) | Median PFS was 6.8 months (CI 6.1; 9.7) and median OS was 17.6 months (CI 14.8; 34.1). | 3 (6.5%) new motor deficits |
| Alimohamadi et al. (2016) [37] | 2015–2016 | Retrospective | 10 | 3 (30%) GBM, 7 (70%) lower-grade gliomas | Non-specific | AC | 73–100% EOR | - | 1 (10%) deteriorated aphasia |
| Martino et al. (2013) [39] | 2009–2011 | Retrospective | 22 | Lower-grade gliomas | SMA, premotor, posterior temporal | 11 (50%) AC | With monitoring: EOR (%) 91.7%, GTR 5 (45.5%), NTR 4 (36.4%), STR 2 (18.2%) without monitoring: EOR (%) 48.7%, GTR 0, NTR 4 (36.4%), STR 7 (63.6%), | 5.3 years (3.5–7) monitoring 3.7 years (3.5–4) no monitoring | 1 (9.1%) in AC (mild dysphasia), and 5 (45.5%) in no monitoring group (4 dysphasia, 3 hemiparesis). |
| Schucht et al. (2013) [42] | 2007–2010 | Retrospective | 64 | Lower-grade gliomas | Central, frontal | AC | Median EOR 92% (range 80–97%) | - | 3 (9.1%) in central and 2 (6.5%) in frontal |
| Saito et al. (2019) [45] | 2000–2018 | Retrospective | 30 | 3 (10.0%) GBM, 27 (90%) low-grade gliomas | Precentral gyrus | AC | EOR, Mean 93% (range = 75–100) | - | 8 (26.6%) motor decline |
| Motomura et al. (2017) [44] | 2012–2014 | Retrospective | 33 | 4 (12.1%) GBM, 29 (87.9%) lower-grade gliomas | Frontal, insular, parietal, temporal, occipital | AC | EOR ≥ 90% 15 (45.5%), <90% 18 (54.5%) increased rate of EOR due to iMRI in 16 patients by mean (SD) 15.8 (12.2) | - | 3 (9.0%) permanent neurological deficits |
| Eseonu et al. (2018) [43] | 2015–2016 | Retrospective | 57 | 17 (29.8) GBM, 40 (70.2%) low-grade gliomas | Peri-Rolandic motor area | AC (33 positive mapping (PM), 24 negative mapping (NM)) | EOR 87.8% (7.1%) in positive monitoring, 92.4 (9.4%) in NM HG, positive monitoring: 85.7 (9.4%), HG, negative monitoring: 90.7 (10.2%). LG, positive monitoring: 90.8 (10.4%), LG, negative monitoring: 97.0 (5.2%) | - | 3 (9.2%) in PM |
| (C): General Anesthesia and Awake Craniotomy | |||||||||
| Morsy et al. (2021) [28] | 2014–2019 | Retrospective | 64 | 26 (40.6%) GBM, 48 (59.4%) lower-grade gliomas | Peri-Rolandic | 40 (62.5%) AC, 24 (37.5%) GA | AC: EOR mean (SD) 92.03 (3.1) GTR > 98% 18 (45%) NTR > 90–98 14 (35%) STR 50–90% 8 (20%) GA: EOR, mean (SD) 90.05 (3.9) GTR > 98% 7 (29.2%) NTR > 90–98 8 (33.3%) STR 50–90% 9 (37.5%) | - | AC: 2 (5%), GA: 2 (8.3%) permanent neuro deficit |
| Nossek et al. (2011) [31] | 2007–2009 | Retrospective | 55 | 22 (40%) GBM, 33 (60%) lower-grade gliomas | Frontal, parietal, temporal | 35 (63.7%) AC, 20 (36.3%) GA | GTR 39 (71%) STR 16 (29%) | - | 7 (12.7%) patients had varying degrees of permanent motor deficits |
| Rossi et al. (2021) [34] | 2018–2019 | Retrospective | 135 | High-grade gliomas 73 (35%), low-grade gliomas 48 (54%) | Peri-Rolandic | 66 (49%) AC, 69 (51%) GA | 95% mean EOR (94% vs. 96% AC) | - | - |
| 2018–2019 | Prospective | 52 | High-grade gliomas 16 (31%), low-grade gliomas 34 (65%) | Peri-Rolandic | 35 (67%) AC, 17 (33%) GA | 97% mean EOR in both AC and sleep | - | - | |
| Giamouriadis et al. (2020) [35] | 2017 | Prospective | 48 | 26 (56.5%) GBM, 32 (43.5%) lower-grade gliomas | Frontal, insular, temporal, parietal, occipital. | 16 (33.3%) AC, 32 (66.6%) GA | GA: GTR 27 (84.3%) STR 1 (3.1%) near GTR 3 (9.3%) AC: STR 0 near GTR 4 (25%) GTR 12 (75%) | - | GA: 1 (3.1%) permanent deficit AC: 2 (12.5%) permanent deficits |
| Eseonu et al. (2017) [47] | 2005–2015 | Retrospective | 58 | 11 (18.9%) GBM, 47 (81.1%) lower-grade gliomas | Peri-Rolandic | 27 (46.5%) AC, 31 (53.4%) GA | AC: mean (SD) EOR 86.3% (20.5%), GA: mean (SD) EOR 79.6% (23.1%) | - | - |
| Li et al. (2021) [48] | 2008–2019 | Retrospective | 109 | GBM | Primary motor cortex, primary sensory cortex, premotor cortex, language cortex | 48 (44.0) AC, 61 (55.9%) GA | GA: mean (SD) EOR: 90.2% (7.44) Gross total (>95%), 28 (45.9) Subtotal (85–95%), 18 (29.5) AC: EOR: mean (SD) EOR: 94.9% (5.73) Gross total (>95%), 40 (83.3) Subtotal (85–95%), 6 (12.5) Partial (< 85%), 2 (4.2) Partial (< 85%), 15 (24.6) | AC: mean PFS 23.2 months mean OS 28.1 months GA: mean PFS was 18.9 months mean OS 23.4 months | Permanent motor 3 (9.7%) in AC vs. 3 (11.1%) in GA language deficit: 2 (6.5%) in AC vs. 4 (14.8%) in GA |
| Sacko et al. (2011) [40] | 2002–2007 | Prospective | 575 | 120 GBM, 455 lower-grade gliomas | Frontal, temporal, parietal, occipital | 214 (37.2%) AC, 316 (62.8%) GA | AC: 37% total, 45% subtotal. GA 52% total, 34% subtotal | - | Permanent neurological deficit: AC 20 (9.3%), and GA: 26 (8.2%) |
| Skrap et al. (2012) [41] | 2000–2010 | Retrospective | 66 | Lower-grade gliomas | Insular | 46 (69.9%) AC, 23 (30.1%) GA | Median EOR 80% EOR 90%, n = 22 (33.3%) EOR 70%-90%, n = 30 (45.4%) EOR 70%, n = 14 (21.2%) | - | 4 (6%) permanent deficits |
| Magill et al. (2018) [46] | 1998–2016 | Retrospective | 49 | 15 (28.3) GBM, 34 low-grade gliomas | Primary motor cortex | 34 (64.2%) AC, 19 (35.8%) GA | GTR 27 (50.9%) STR 26 (49.1%) Mean EOR 91% (range = 41–100) | - | 20 (37.7%) permanent deficits |
| Study (Years) | Study Period | Study Design | Tumors | Histology | Location | Modality | Extent of Resection | Overall Survival | Complications |
|---|---|---|---|---|---|---|---|---|---|
| (A): Fluorescein sodium | |||||||||
| Koc et al. (2008) [54] | 2003–2006 | Prospective | 80 | GBM | Non-specific | 47 (58.7%) fluorescein sodium | GTR: 39 (83%) fluorescein sodium vs. 18 (55%) in control group. | No difference in median survival | - |
| Neira et al. (2017) [55] | 2013–2014 | Retrospective | 32 | High-grade gliomas (3–4) | Non-specific | Fluorescein sodium | 27 (84%) GTR | - | - |
| Acerbi et al. (2018) [56] | 2011 | Prospective | 46 | High-grade gliomas | Non-specific | Fluorescein sodium | 38 (82.6%) complete resections | PFS-6 and PFS-12 were 56.6% and 15.2%. Median survival was 12 months | - |
| Chen et al. (2012) [60] | 2010–2011 | Prospective | 10 | 3 (30%) GBM, 7 (70%) lower-grade gliomas | Non-specific | Fluorescein sodium | 8/10 (80%) GTR | 7.2 PFS months vs. 5.4 in the control group | 1/12 (8.3%) permanent hemiplegia in the control group |
| Diaz et al. (2015) [61] | - | Prospective | 12 | GBM | Non-specific | Fluorescein sodium | 12/12 (100%) GTR | - | - |
| Catapano et al. (2017) [62] | 2016–2017 | Retrospective | 48 | GBM | Non-specific | 23 (47.9%) fluorescein sodium | 19/23 (82.6%) GTR vs. 9/25 (36%) in the control group | - | - |
| Francaviglia et al. (2017) [63] | 2015–2016 | Retrospective | 47 | 33 (70.2%) GBM, 14 (29.8%) lower-grade gliomas | Non-specific | Fluorescein sodium | 39/47 (83%) | - | 6 (12.7%) hemorrhage with permanent hemiparesis 4 (8.5%) Seizures 1 (2.1%) Hydrocephalus 1 (2.1%) Sepsis |
| (B): 5-ALA | |||||||||
| Stummer et al. (2006) [53] | 1999–2004 | Prospective | 322 | 237 (73.6%) GBM, 85 (26.4%) lower-grade gliomas | Non-specific | 139 (43.1%) 5-ALA | GTR: 50 (36%) in 5-ALA group, 49 (27%) in the control group. | 6-month PFS: 5-ALA: 57 (41.0%) vs. 39 (21.1%) in controls. | - |
| Nabavi et al. (2009) [51] | 2003–2005 | Prospective | 36 | 21 (58.3%) GBM, 15 (41.7%) lower-grade gliomas | Non-specific | 5-ALA | 7/36 (19.4%) GTR | - | - |
| Diez Valle et al. (2011) [52] | 2007–2009 | Prospective | 36 | GBM | Non-specific | 5-ALA | 30 (83.3%) complete resections | PFS 6.5 months (95% CI 3.8–9.2) for newly diagnosed GBM, and 5.3 months (95% CI 4.4–6.2) for recurrent cases | - |
| Widhalm et al. (2010) [58] | 2008–2009 | Prospective | 17 | Lower-grade gliomas | Frontal, central, temporal, occipital, parietal, insular, thalamus | 5-ALA | 14/17 (82%) GTR | - | - |
| Widhalm et al. (2013) [57] | 2008–2012 | Prospective | 59 | Lower-grade gliomas | Frontal, central, temporal, occipital, parietal, insular, thalamus | 5-ALA | 38/59 (64%) GTR | - | - |
| Chan et al. (2018) [59] | 2011–2016 | Retrospective | 16 | 10 (62.5%) GBM, 6 (37.5%) lower-grade gliomas | Non-specific | 5-ALA | 9/16 (56.2%) GTR | - | - |
| Teixidor et al. (2016) [64] | 2010–2014 | Prospective | 77 | 66 (85.7%) GBM, 11 (14.3%) lower-grade gliomas | Non-specific | 5-ALA | 42 (54%) complete resections | Six-month PFS in 45 (58%) and median overall survival was 14.2 months | No serious adverse events were reported |
| (C): Fluorescein Sodium and 5-ALA | |||||||||
| Zeppa et al. (2022) [50] | 2018–2021 | Retrospective | 99 | GBM | Precentral, postcentral, temporo-insular | 40 (40.4%) 5-ALA, 44 (44.4%) fluorescein sodium, 15 (15.2%) both | Total resection: 18/40 (45%%) 5-ALA, 21/44 (47.7%) in fluorescein sodium, and 6/15 (40%) in both | Mean (SD) OS 14.9 (9.91) months. mean OS in 5-ALA: 20 (16), SF: 12.3 (5.7), both 18.1 (11.9) months | - |
| Study (Years) | Study Period | Study Design | Tumors | Histology | Location | Modality | Extent of Resection | Overall Survival | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Maldaun et al. (2014) [69] | 2010–2011 | Retrospective | 41 | 9 (21.4%) GBM, 33 (78.6%) lower-grade gliomas | Frontal, temporal, parietal, insular | iMRI + AC | Median EOR overall was 90%, and gross total resection (EOR ≥ 95%) 17 (40.5%). After viewing the first MR images after initial resection, further resection was performed in 17 cases (40.5%); the mean EOR in these cases increased from 56% to 67% after further resection | - | Neurologic deficits 11 (26.2%) |
| Maesawa et al. (2010) [68] | 2007–2008 | Retrospective | 28 | Lower-grade gliomas | Proximity to corticospinal tract | iMRI, intraoperative tractography mapping | 24 (85.7%) STR | - | 1 (3.5%) permanent motor deficit |
| Zhuang et al. (2016) [70] | 2011–2013 | Retrospective | 30 | 6 (20%) GBM, 24 (80%) lower-grade gliomas | Dominant insular lobe | iMRI, + AC or GA mapping | iMRI increased resection from 90 to 93% in all cases, and 88% to 92% in low-grade gliomas. The use of iMRI also resulted in an increase in the percentage of gross and near-total resection from 53% to 77% | - | 3 (11%) permanent language, 2 (7.1%) motor deficits |
| Ghinda et al. (2016) [71] | 2011–2015 | Retrospective | 106 | 25 (23.6%) GBM, 81 (76.4%) lower-grade gliomas | Frontal, parietal, temporal, insular | iMRI, + AC | Mean EOR 92%, complete resection was achieved in 64 (60.4%). 30 (28.3%) patients underwent further resection after initial iMRI scanning, with 10.1% increase in mean EOR | - | - |
| Leuthardt et al. (2011) [72] | 2008 | Retrospective | 12 | 3 (25%) GBM, 9 (75%) lower-grade gliomas | Eloquent areas | iMRI, + AC | 5 (41.6%) GTR, 2 (16.6%) NTR, and 5 (41.6%) STR | - | 1 (8.3%) with worse outcome |
| Lu et al. (2013) [73] | 2011 | Retrospective | 30 | 11 (36.6%) high-grade gliomas, 19 (63.3%) low-grade gliomas | Eloquent areas | iMRI, + GA mapping | Median EOR significantly increased from 92.5% (range, 75.1–97.0%) to 100% (range, 92.6–100%) 11 (36.6%) additional tumor removal | - | 1 (3.3%) permanent deficit |
| White (2018) [74] | 2001–2016 | Retrospective | 36 | 17 (47.2%) GBM, 19 (52.7%) lower-grade gliomas | Left hemisphere | iMRI, + AC | 20 (55.6%) GTR, 19 (53%) further resections under iMRI | - | 3 (8.3%) permanent deficits |
| Whiting et al. (2019) [75] | 2010–2017 | Retrospective | 62 | 18 (29.0%) GBM, 43 (69.3%) lower-grade gliomas | Frontal, temporal, parietal, insular | iMRI, + AC | 41 (85.4%) had additional resection due to MRI. Median EOR 98.5% | - | 2 (3.2%) residual speech difficulty, and 2 (3.2%) permanent weakness postoperatively |
| Peruzzi et al. (2011) [76] | 2006–2008 | Retrospective | 44 | 28 (63.6%) GBM, 16 (36.3%) lower-grade gliomas | Frontal, temporal, parietal, occipital | iMRI, + AC/GA mapping | GTR in all patients | - | - |
| Tuominen et al. (2013) [77] | - | Retrospective | 40 | 10 (25%) GBM, 30 (75%) lower-grade gliomas | Frontal, temporal, parietal | 20 (50%) iMRI + AC, 20 (50%) iMRI | GTR: 10 (50%) iMRI + AC 11 (55%) iMRI | - | 1 (5%) permanent neurological deficit iMRI + AC 4 (20%) permanent neurological deficit iMRI |
| Roder et al. (2014) [78] | 2010–2012 | Retrospective | 117 | GBM | Non-specific | 66 (56.4%) 5-ALA, 27 (23.0%) iMRI, 19 (16.2%) iMRI and 5-ALA | iMRI EOR 53%, 5 ALA combined with iMRI increased EOR | - | 11 (9.4%) permanent severe deficits |
| Coburger et al. (2015) [79] | 2012–2014 | Prospective | 116 | GBM | Non-specific | 59 (50.8%) iMRI (Group 1), 57 (49.2%) 5-ALA and MRI (Group 2) | mean EOR 97.4% (87–100) iMRI, 99.7% (97–100) iMRI + 5-ALA GTR: 27 (82%) iMRI, 33 (100%) iMRI + 5-ALA | Median PFS (CI95%)Group 1: 6 months (2.4–9.6), Group 2: 6 months (4.6–7.4) OS (CI95%) Group 1: 17 months (7.6–26.4) Group 2: 18 (15.2–20.8) | 7 (21%) iMRI, 11 (27%) iMRI+ 5-ALA |
| Schatlo et al. (2015) [80] | 2003–2011 | Retrospective | 200 | 166 (83%) GBM, 44 (17%) lower-grade gliomas | Non-specific | 58 (29%) 5-ALA only, 55 (27.5%) iMRI + 5-ALA, 87 (43.5%) neither. | 5-ALA enhanced the achievement of gross total resection. GTR 25 (45%) with iMRI vs. 43 (30%) without iMRi | Median overall survival 13.8 months in the non-iMRI group and 17.9 months in the iMRI group, with no effect on PFS | - |
| Feigl et al. (2010) [81] | 2007–2009 | Prospective | 36 | 15 (41.6%) GBM, 21 (58.3%) lower-grade gliomas | Frontal, temporal, parietal, insular, cerebellar | 5-ALA, GA mapping | 16/25 (64%) GTR | - | 2 (1%) hemiparesis, and 1 (0.5%) homonymous hemianopia |
| Tsugu et al. (2011) [82] | 2005–2009 | Retrospective | 33 | 20 (60.6%) GBM, 13 (39.4%) lower-grade gliomas | Non-specific | 23 (69.6%) 5-ALA, 10 (30.4%) 5-ALA + MRI | GTR: 6/11 (54.5%) 5-ALA only 4/10 (40%) 5-ALA + iMRI | - | - |
| Della Puppa et al. (2013) [83] | 2011–2012 | Prospective | 31 | 25 (80.6%) GBM, 6 (19.4%) lower-grade gliomas | Insular, frontal, temporal, language area | 5-ALA with GA (N = 25, 80.6%) or AC (N = 6, 19.4%) mapping | GTR 23/31 (74%) | - | 1 (3%) severe morbidity |
| Yamada et al. (2015) [67] | 2004–2007 | Prospective | 99 | 67 (67.6%) GBM, 32 (32.4%) lower-grade gliomas | Non-specific | 5-ALA + iMRI | GTR 51/99 (52%) | - | - |
| Pichierri et al. (2019) [84] | 2014–2018 | Retrospective | 92 | 28 (30.5%) GBM, 64 (69.5%) lower-grade gliomas | Frontal, temporal, occipital, parietal, insular, cerebellar | 26 (28.3%) iMRI (G1), 20 (21.7%) iMRI + AC (G2), 46 (50%) control (G3) | Group 1: Grade 2 GTR 46%, Grade 3 GTR 57%, Grade 4 GTR 63%. Group 2: Grade 2 GTR 55%, Grade 3 GTR 66%, Grade 4 GTR 41%, Group 3: Grade 2 GTR 41%, Grade 3 GTR 30%, Grade 4 GTR 36% | - | Memory/cognition 1(4%) G1, 1(4%) G2, 5(10.8%) G3. Parietal syndrome 1 (5%) G2, Hemiparesis 2 (4.3%) G3, Dysphasia 2 (4.3%) G3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanbour, H.; Chotai, S. Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes. Cancers 2022, 14, 5705. https://doi.org/10.3390/cancers14225705
Chanbour H, Chotai S. Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes. Cancers. 2022; 14(22):5705. https://doi.org/10.3390/cancers14225705
Chicago/Turabian StyleChanbour, Hani, and Silky Chotai. 2022. "Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes" Cancers 14, no. 22: 5705. https://doi.org/10.3390/cancers14225705
APA StyleChanbour, H., & Chotai, S. (2022). Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes. Cancers, 14(22), 5705. https://doi.org/10.3390/cancers14225705




