The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments
Abstract
Simple Summary
Abstract
1. Introduction
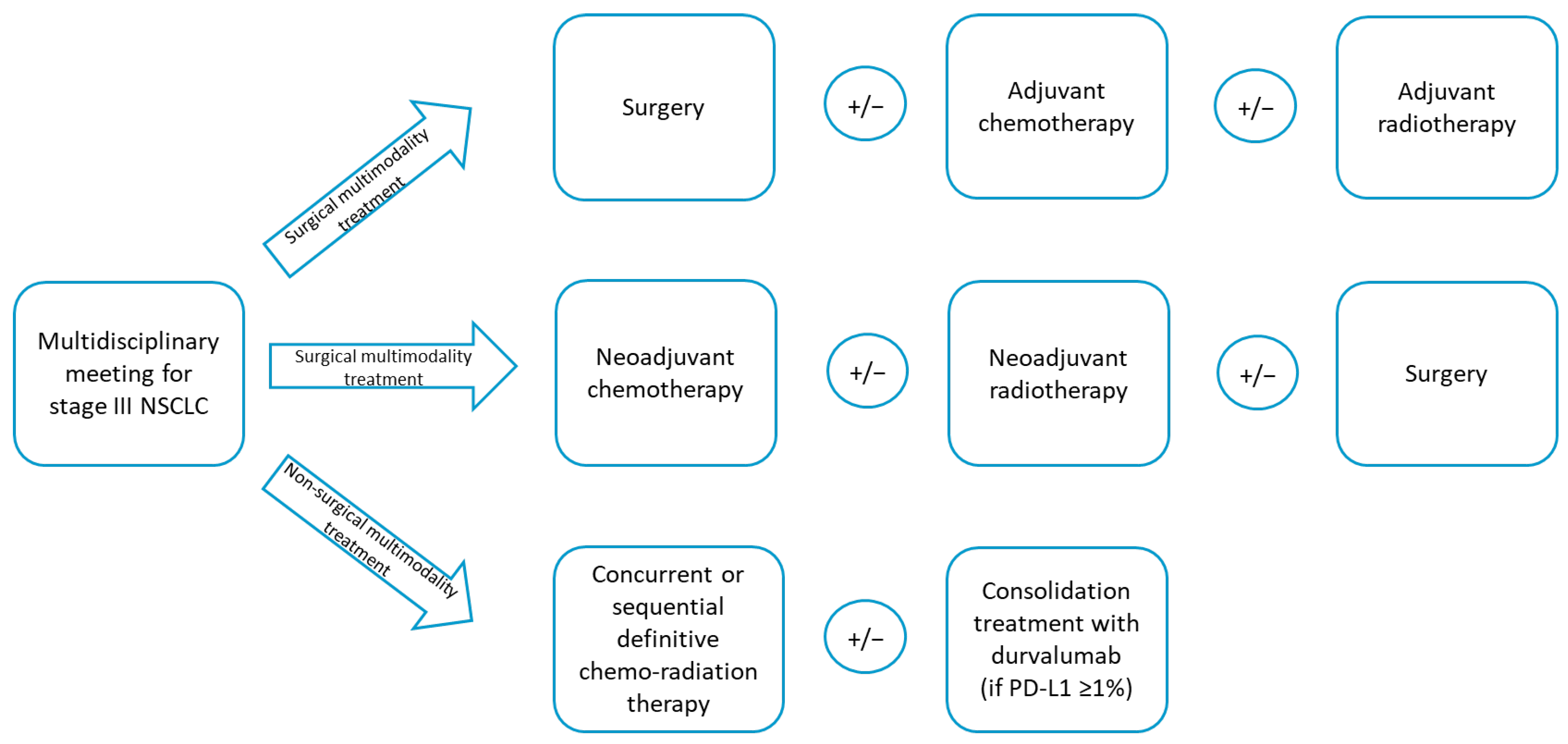
2. Materials and Methods
2.1. Immunohistochemistry for PD-L1, ALK and ROS-1
2.2. Molecular Investigation of EGFR Mutations
2.3. Statistical Analysis
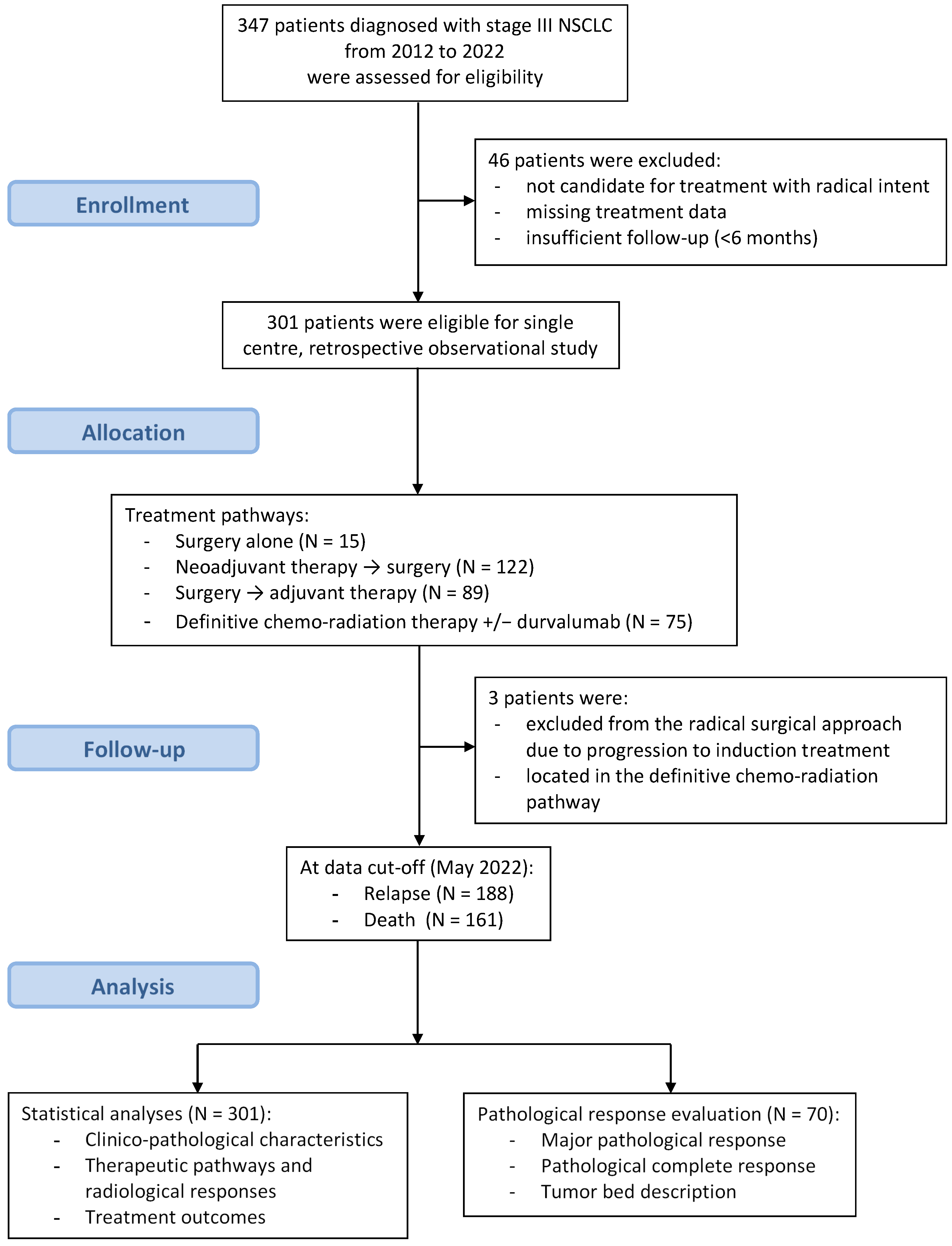
3. Results
3.1. Clinico-Pathological Characteristics
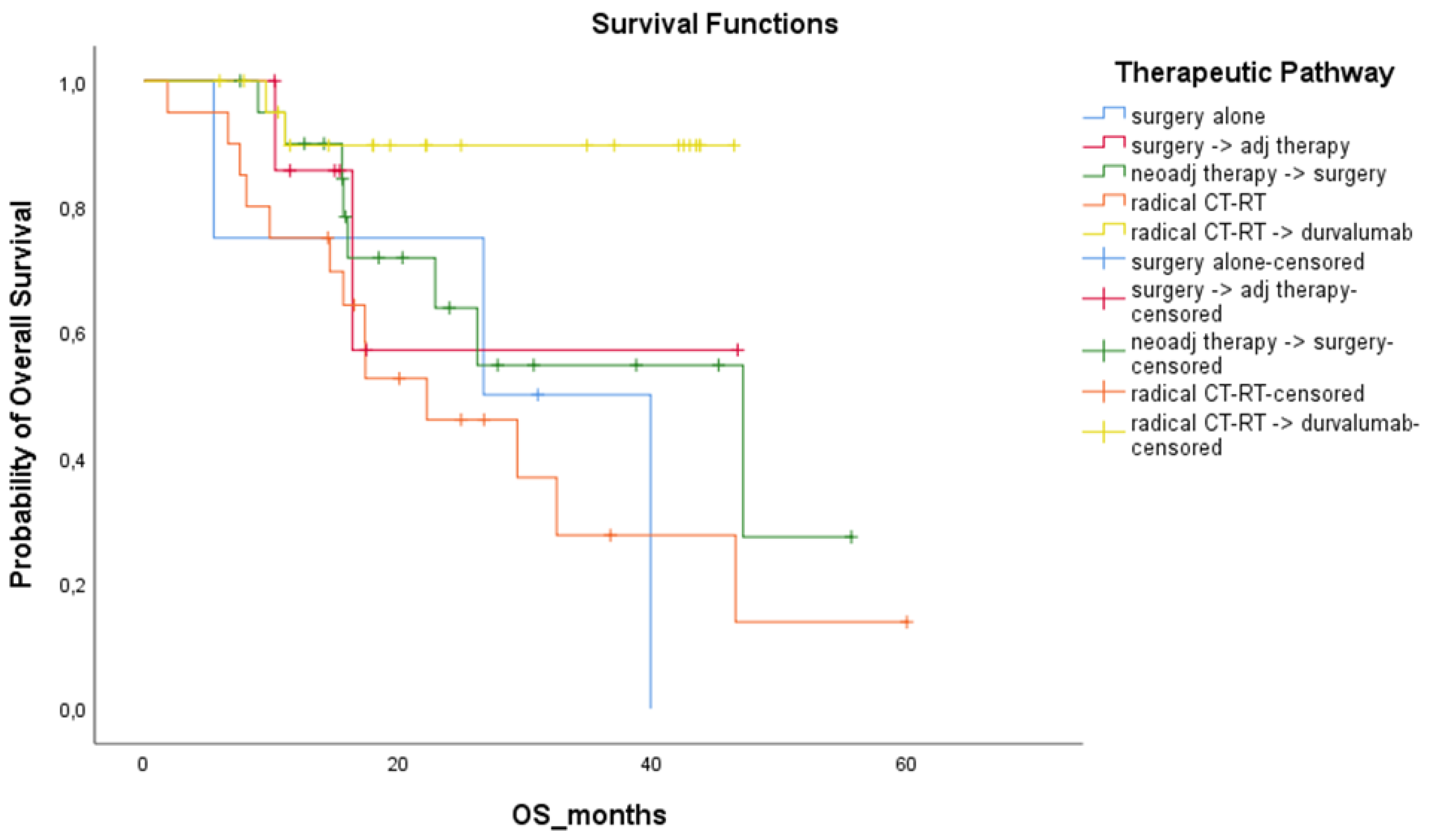
3.2. Therapeutic Pathways and Treatment Responses
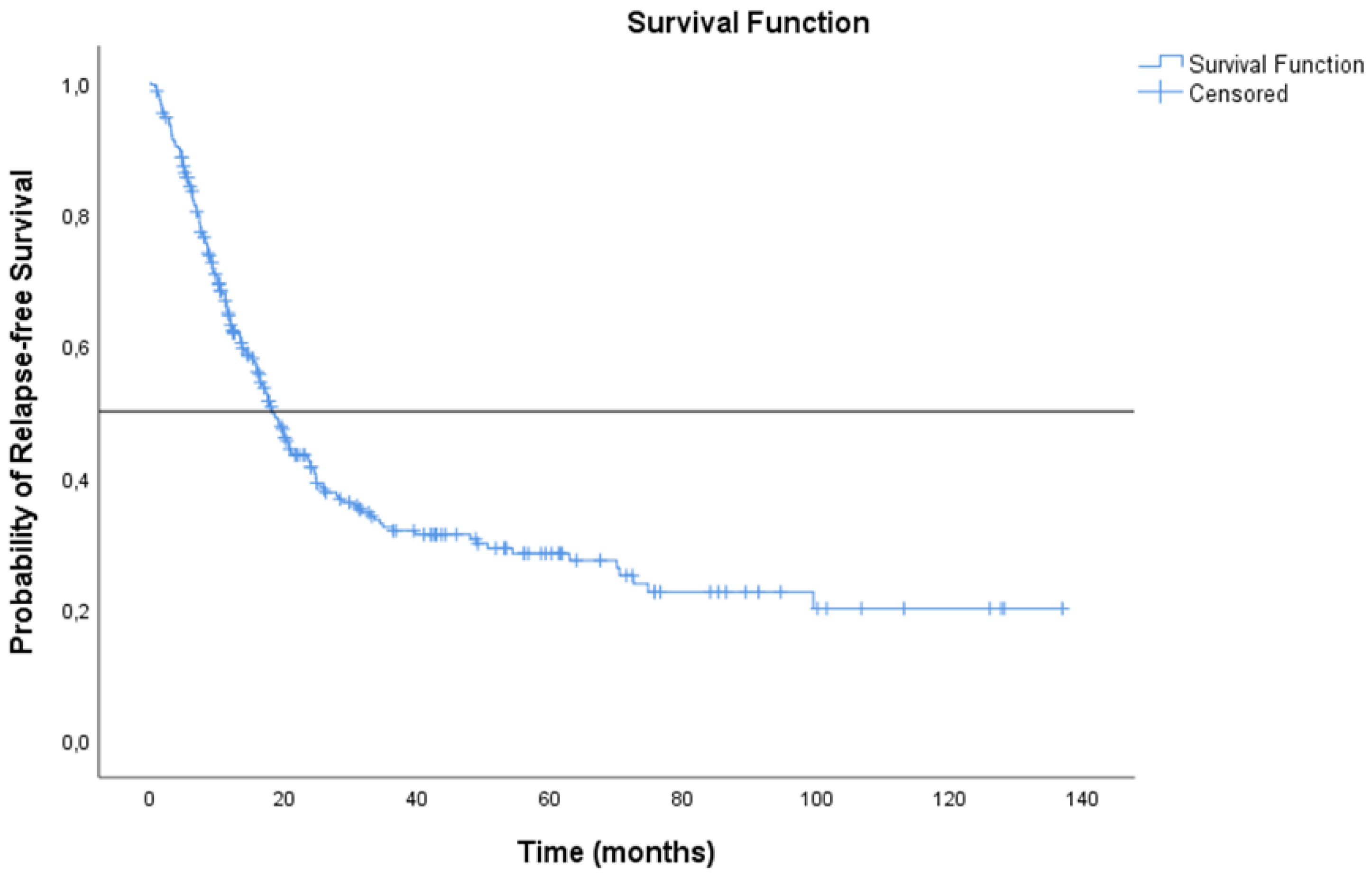
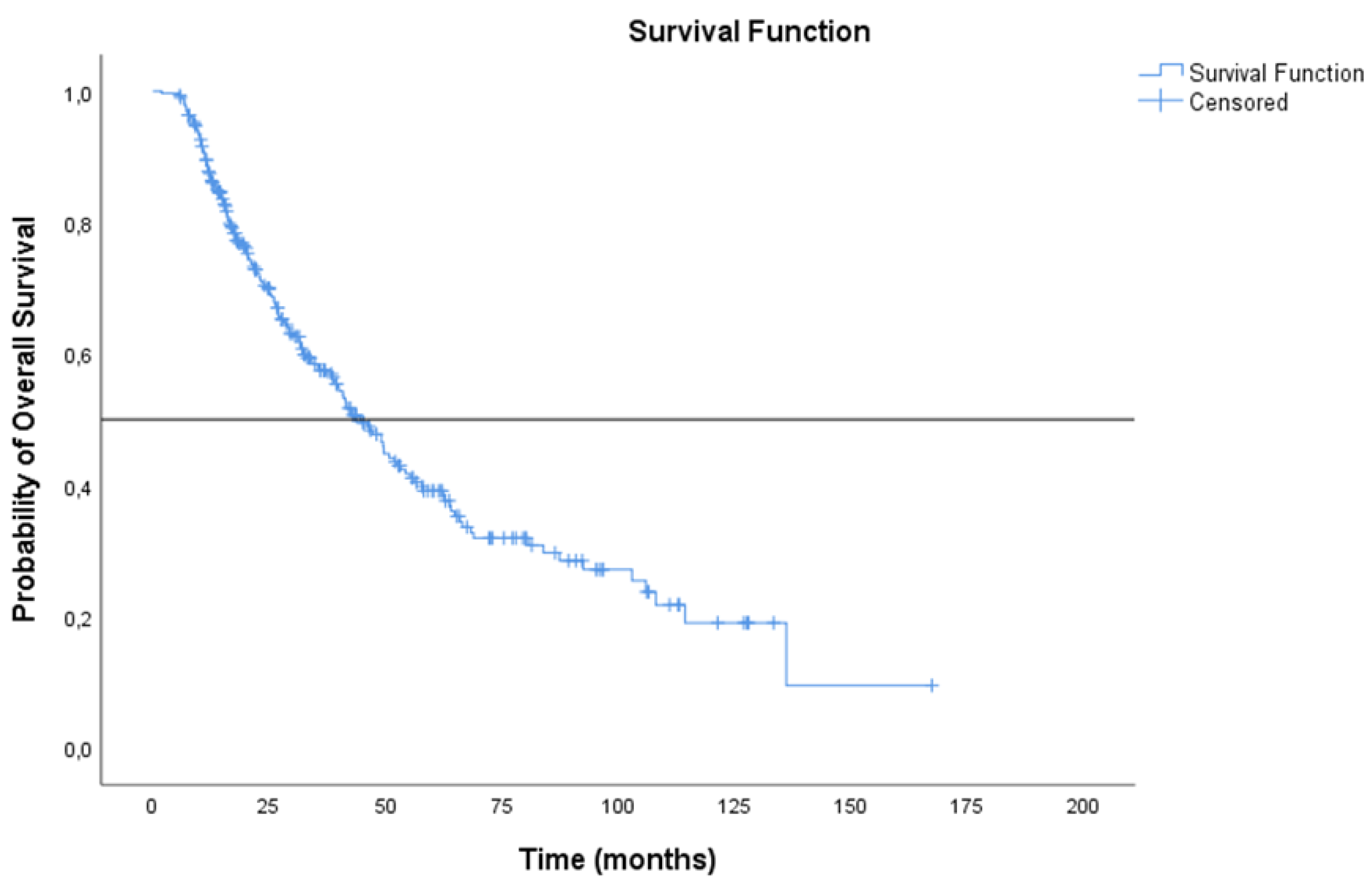
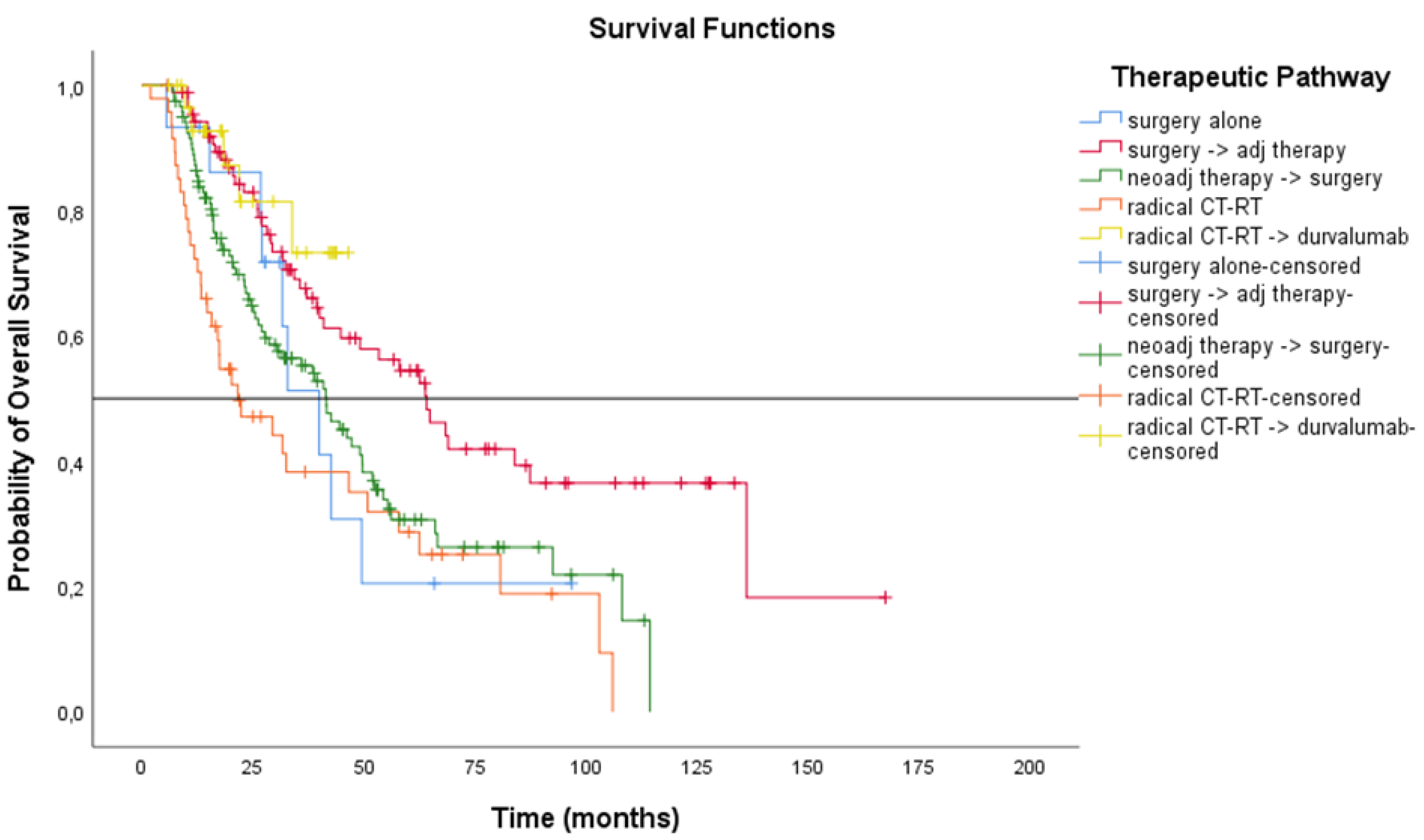
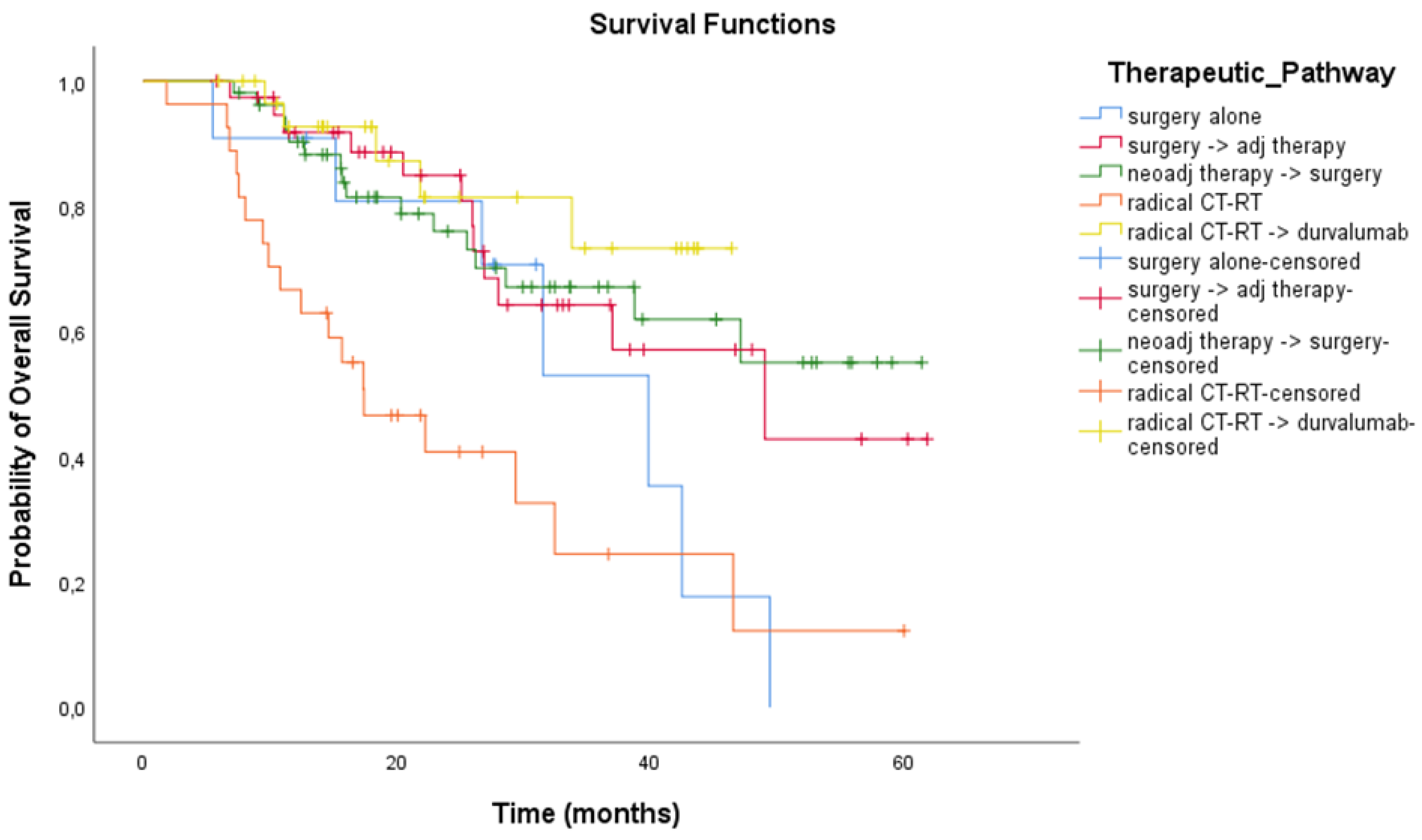
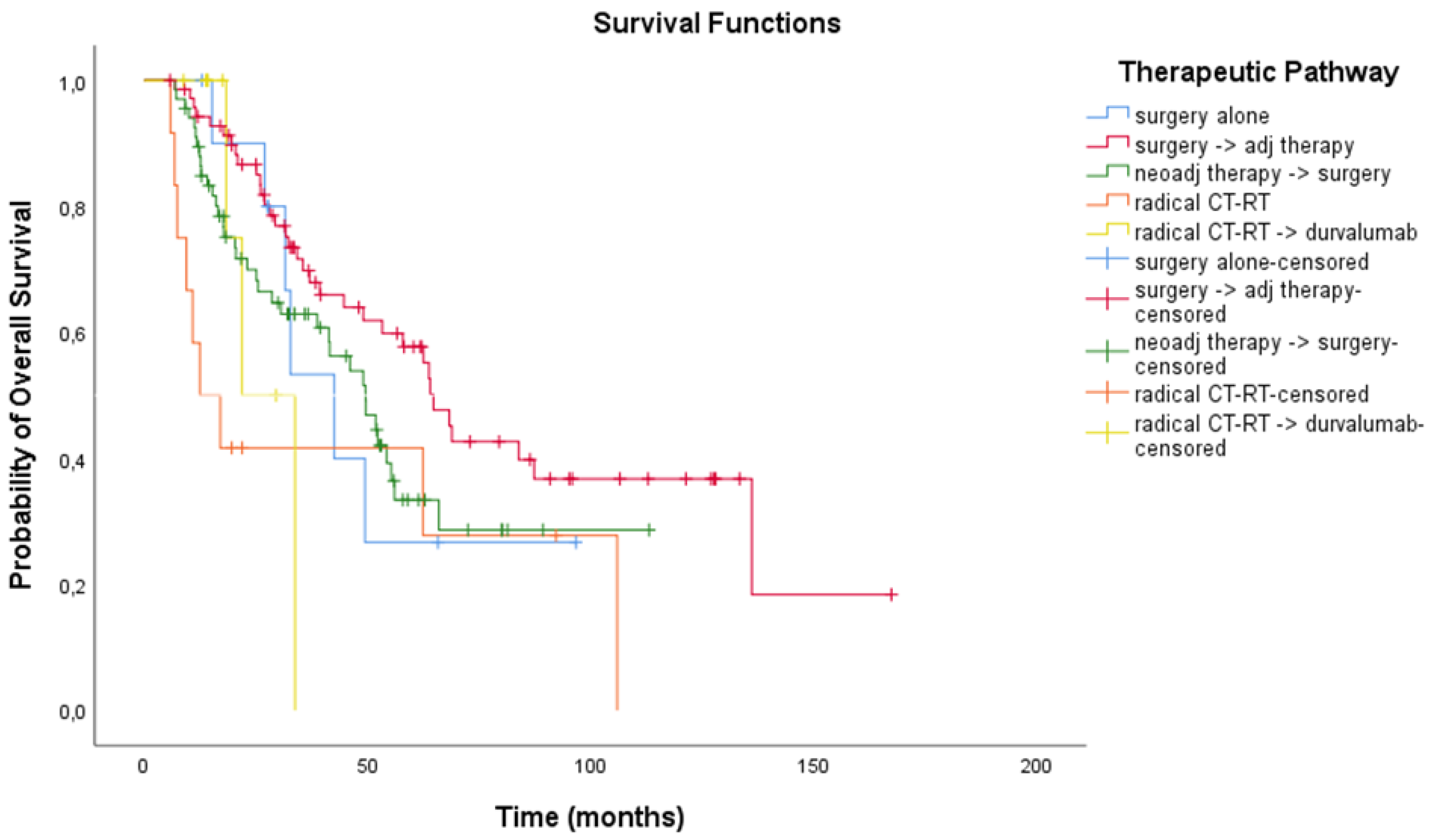
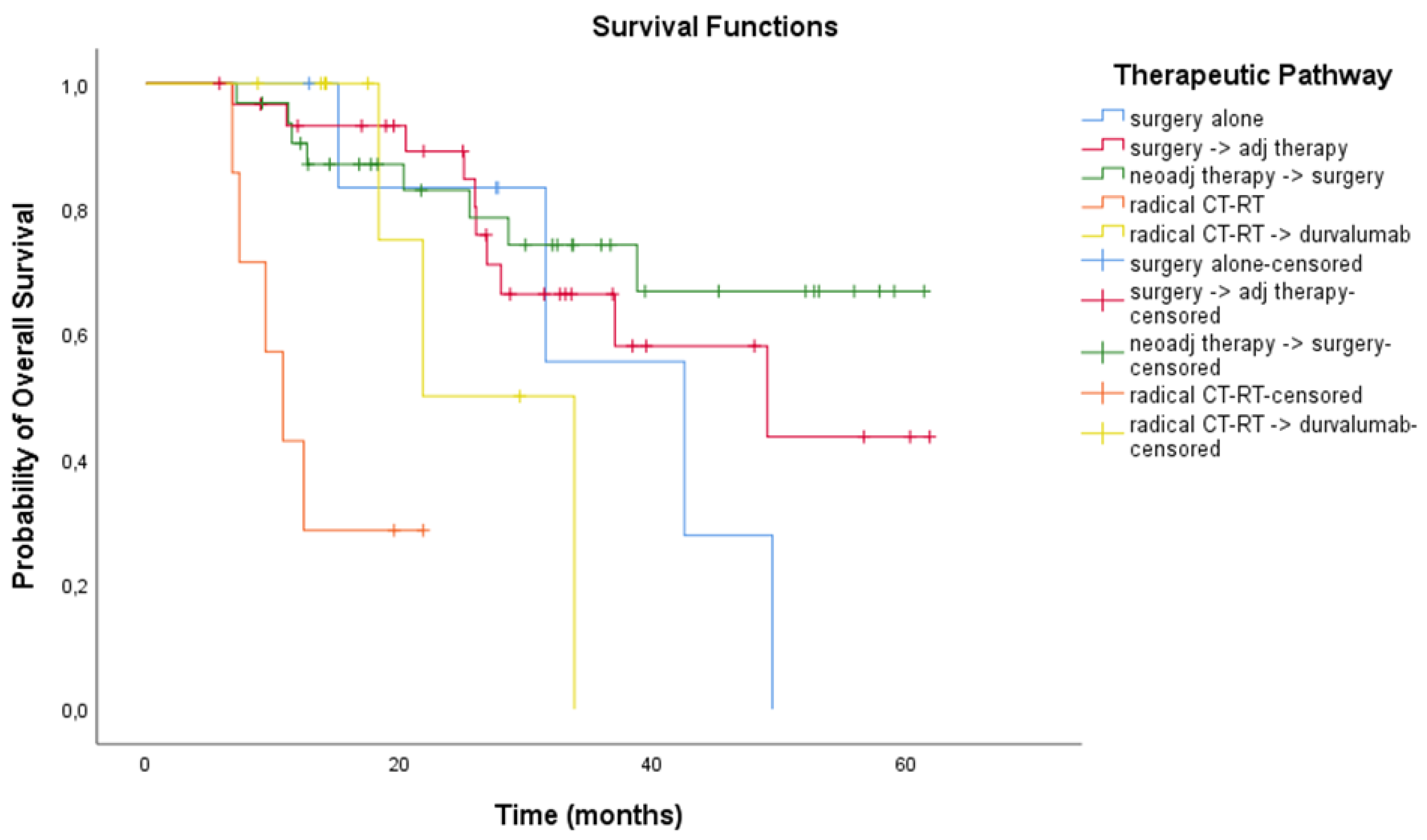
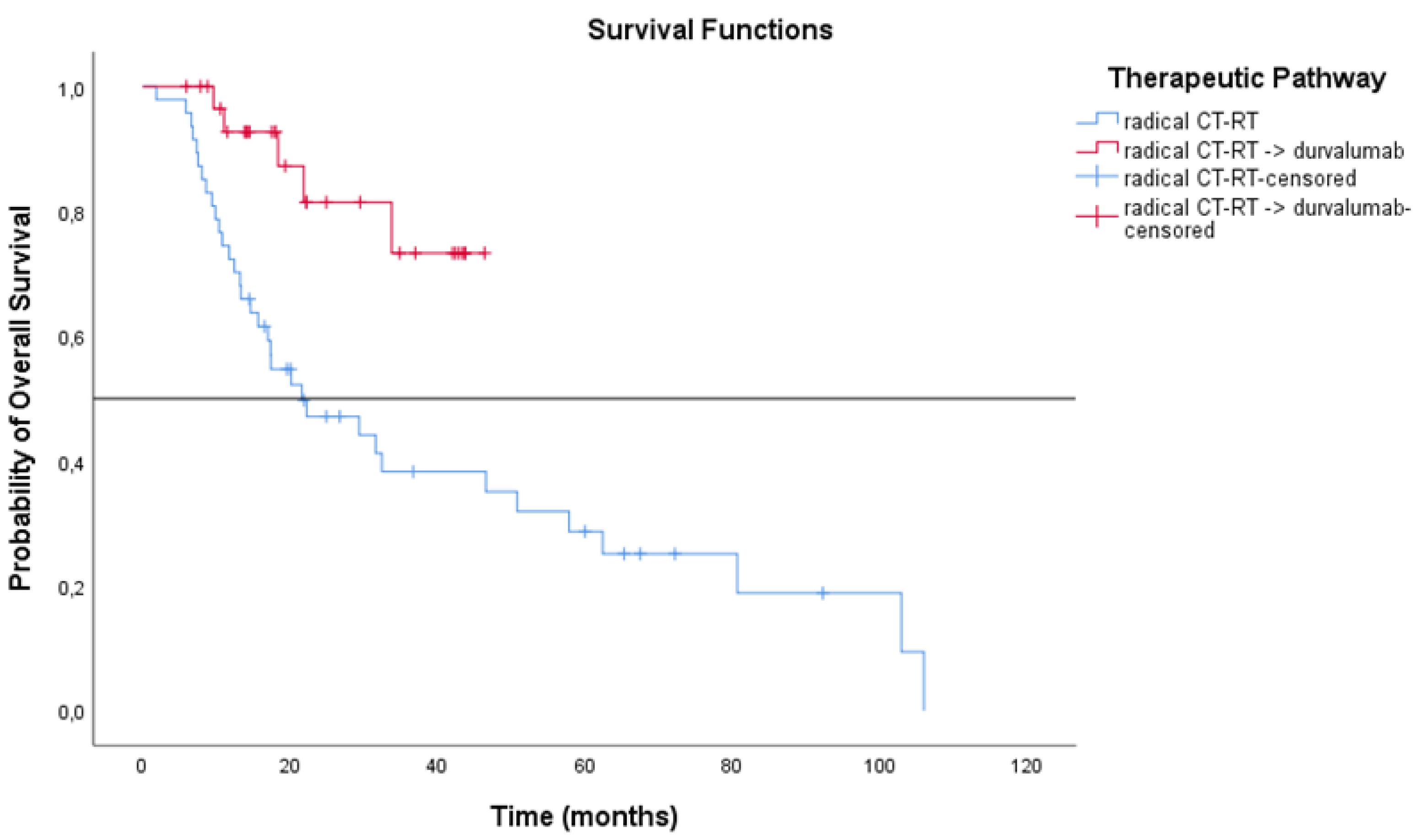
3.3. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lung and Bronchus Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 1 October 2022).
- Ko, E.C.; Raben, D.; Formenti, S.C. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non–Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5792–5806. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Salgia, R.; Boehmer, L.M.; Celestin, C.; Yu, H.; Spigel, D.R. Improving Care for Patients With Stage III or IV NSCLC: Learnings for Multidisciplinary Teams From the ACCC National Quality Survey. JCO Oncol. Pract. 2021, 17, e1120–e1130. [Google Scholar] [CrossRef]
- Kay, F.U.; Kandathil, A.; Batra, K.; Saboo, S.S.; Abbara, S.; Rajiah, P. Revisions to the Tumor, Node, Metastasis Staging of Lung Cancer (8th Edition): Rationale, Radiologic Findings and Clinical Implications. World J. Radiol. 2017, 9, 269. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 2.2021 Featured Updates to the NCCN Guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer: An Update of the ESMO Clinical Practice Guidelines Focusing on Diagnosis, Staging, Systemic and Local Therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Socinski, M.A.; Blackstock, A.W.; Bogart, J.A.; Wang, X.; Munley, M.; Rosenman, J.; Gu, L.; Masters, G.A.; Ungaro, P.; Sleeper, A.; et al. Randomized Phase II Trial of Induction Chemotherapy Followed by Concurrent Chemotherapy and Dose-Escalated Thoracic Conformal Radiotherapy (74 Gy) in Stage III Non-Small-Cell Lung Cancer: CALGB 30105. J. Clin. Oncol. 2008, 26, 2457–2463. [Google Scholar] [CrossRef]
- Vokes, E.E.; Herndon, J.E.; Crawford, J.; Leopold, K.A.; Perry, M.C.; Miller, A.A.; Green, M.R. Randomized Phase II Study of Cisplatin with Gemcitabine or Paclitaxel or Vinorelbine as Induction Chemotherapy Followed by Concomitant Chemoradiotherapy for Stage IIIB Non-Small-Cell Lung Cancer: Cancer and Leukemia Group B Study 9431. J. Clin. Oncol. 2002, 20, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Aguillo, M.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, Z.; Dang, J.; Li, G. Comparative Efficacy and Safety for Different Chemotherapy Regimens Used Concurrently with Thoracic Radiation for Locally Advanced Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis. Radiat. Oncol. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Kiura, K.; Takigawa, N.; Kamei, H.; Harita, S.; Hiraki, S.; Watanabe, Y.; Sugimoto, K.; Shibayama, T.; Yonei, T.; et al. Phase III Trial Comparing Docetaxel and Cisplatin Combination Chemotherapy with Mitomycin, Vindesine, and Cisplatin Combination Chemotherapy with Concurrent Thoracic Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: OLCSG 0007. J. Clin. Oncol. 2010, 28, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nakagawa, K.; Nishimura, Y.; Tsujino, K.; Satouchi, M.; Kudo, S.; Hida, T.; Kawahara, M.; Takeda, K.; Katakami, N.; et al. Phase III Study Comparing Second- and Third-Generation Regimens with Concurrent Thoracic Radiotherapy in Patients with Unresectable Stage III Non-Small-Cell Lung Cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 2010, 28, 3739–3745. [Google Scholar] [CrossRef]
- Perez, C.A.; Stanley, K.; Rubin, P.; Kramer, S.; Brady, L.; Perez-Tamayo, R.; Brown, G.S.; Concannon, J.; Rotman, M.; Seydel, H.G. A Prospective Randomized Study of Various Irradiation Doses and Fractionation Schedules in the Treatment of Inoperable Non-oat-cell Carcinoma of the Lung. Preliminary Report by the Radiation Therapy Oncology Group. Cancer 1980, 45, 2744–2753. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant versus Sequential Radiochemotherapy in Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Finazzi, T.; Schneiders, F.L.; Senan, S. Developments in Radiation Techniques for Thoracic Malignancies. Eur. Respir. Rev. 2021, 30, 200224. [Google Scholar] [CrossRef]
- Haslett, K.; Bayman, N.; Franks, K.; Groom, N.; Harden, S.V.; Harris, C.; Hanna, G.; Harrow, S.; Hatton, M.; McCloskey, P.; et al. Isotoxic Intensity Modulated Radiation Therapy in Stage III Non-Small Cell Lung Cancer: A Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1341–1348. [Google Scholar] [CrossRef]
- Nestle, U.; De Ruysscher, D.; Ricardi, U.; Geets, X.; Belderbos, J.; Pöttgen, C.; Dziadiuszko, R.; Peeters, S.; Lievens, Y.; Hurkmans, C.; et al. ESTRO ACROP Guidelines for Target Volume Definition in the Treatment of Locally Advanced Non-Small Cell Lung Cancer. Radiother. Oncol. 2018, 127, 1–5. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-Dose versus High-Dose Conformal Radiotherapy with Concurrent and Consolidation Carboplatin plus Paclitaxel with or without Cetuximab for Patients with Stage IIIA or IIIB Non-Small-Cell Lung Cancer (RTOG 0617): A Randomised, Two-by-Two Factorial P. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Bradley, J.D.; Hu, C.; Komaki, R.R.; Masters, G.A.; Blumenschein, G.R.; Schild, S.E.; Bogart, J.A.; Forster, K.M.; Magliocco, A.M.; Kavadi, V.S.; et al. Long-Term Results of NRG Oncology RTOG 0617: Standard-versus High-Dose Chemoradiotherapy with or without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.C.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with Durvalumab by Tumour PD-L1 Expression in Unresectable, Stage III Non-Small-Cell Lung Cancer in the PACIFIC Trial. Ann. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef]
- Travis, W.D.; Dacic, S.; Wistuba, I.; Sholl, L.; Adusumilli, P.; Bubendorf, L.; Bunn, P.; Cascone, T.; Chaft, J.; Chen, G.; et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J. Thorac. Oncol. 2020, 15, 709–740. [Google Scholar] [CrossRef]
- Junker, K.; Langner, K.; Klinke, F.; Bosse, U.; Thomas, M. Grading of Tumor Regression in Non-Small Cell Lung Cancer: Morphology and Prognosis. Chest 2001, 120, 1584–1591. [Google Scholar] [CrossRef]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer after Neoadjuvant Chemotherapy. J. Thorac. Oncol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Chaft, J.E.; William, W.N.; Rusch, V.; Pisters, K.M.W.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological Response after Neoadjuvant Chemotherapy in Resectable Non-Small-Cell Lung Cancers: Proposal for the Use of Major Pathological Response as a Surrogate Endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Emoto, K.; Eguchi, T.; Aly, R.G.; Zheng, H.; Chaft, J.E.; Tan, K.S.; Jones, D.R.; Kris, M.G.; Adusumilli, P.S.; et al. Pathologic Assessment After Neoadjuvant Chemotherapy for NSCLC: Importance and Implications of Distinguishing Adenocarcinoma From Squamous Cell Carcinoma. J. Thorac. Oncol. 2019, 14, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Bar, J.; Garrido, P.; Garassino, M.C.; McDonald, F.; Mornex, F.; Filippi, A.R.; Smit, H.J.M.; Peters, S.; Field, J.K.; et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients with Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings from the PACIFIC-R Study. J. Thorac. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Woodford, K.L.; Koo, K.; Reynolds, J.; Stirling, R.G.; Harden, S.V.; Brand, M.; Senthi, S. OA01.04 Persisting Gaps in Optimal Care of Stage III Non-Small Cell Lung Cancer: An Australian Patterns of Care Analysis. J. Thorac. Oncol. 2022, 17, S4–S5. [Google Scholar] [CrossRef]
- Benet, J.; Toffart, A.C.; Brichon, P.Y.; Chollier, T.; Ruckly, S.; Villa, J.; Emprou, C.; Pierret, T.; Dumas, I.; Ferretti, G.; et al. Survival of Clinical Stage III NSCLC According to Therapeutic Strategy: Relevance of the Tumor Board Decision in the Era of Immunotherapy. Cancer Treat. Res. Commun. 2022, 30, 100508. [Google Scholar] [CrossRef]
- Ronden, M.I.; Bahce, I.; Claessens, N.J.M.; Barlo, N.; Dahele, M.R.; Daniels, J.M.A.; Tissing-Tan, C.; Hekma, E.; Hashemi, S.M.S.; van der Wel, A.; et al. The Impact of the Availability of Immunotherapy on Patterns of Care in Stage III NSCLC: A Dutch Multicenter Analysis. JTO Clin. Res. Rep. 2021, 2, 100195. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Lerouge, D.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; Dansin, E.; Paumier, A.; et al. Postoperative Radiotherapy versus No Postoperative Radiotherapy in Patients with Completely Resected Non-Small-Cell Lung Cancer and Proven Mediastinal N2 Involvement (Lung ART): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 104–114. [Google Scholar] [CrossRef]
- Hui, Z.; Men, Y.; Hu, C.; Kang, J.; Sun, X.; Bi, N.; Zhou, Z.; Liang, J.; Lv, J.; Feng, Q.; et al. Effect of Postoperative Radiotherapy for Patients with PIIIA-N2 Non-Small Cell Lung Cancer after Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1178–1185. [Google Scholar] [CrossRef]
- Zens, P.; Bello, C.; Scherz, A.; Koenigsdorf, J.; Pöllinger, A.; Schmid, R.A.; Ochsenbein, A.; Neppl, C.; Langer, R.; Berezowska, S. A Prognostic Score for Non-Small Cell Lung Cancer Resected after Neoadjuvant Therapy in Comparison with the Tumor-Node-Metastases Classification and Major Pathological Response. Mod. Pathol. 2021, 34, 1333–1344. [Google Scholar] [CrossRef]
- Stein, J.E.; Lipson, E.J.; Cottrell, T.R.; Forde, P.M.; Anders, R.A.; Cimino-Mathews, A.; Thompson, E.D.; Allaf, M.E.; Yarchoan, M.; Feliciano, J.; et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020, 26, 545–551. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant Atezolizumab and Chemotherapy in Patients with Resectable Non-Small-Cell Lung Cancer: An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant Chemotherapy and Nivolumab in Resectable Non-Small-Cell Lung Cancer (NADIM): An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Cortiula, F.; Reymen, B.; Peters, S.; Van Mol, P.; Wauters, E.; Vansteenkiste, J.; De Ruysscher, D.; Hendriks, L.E.L. Immunotherapy in Unresectable Stage III Non-Small-Cell Lung Cancer: State of the Art and Novel Therapeutic Approaches. Ann. Oncol. 2022, 33, 893–908. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Remon, J.; Hendriks, L.E.L. Who Benefits from Consolidation Durvalumab in Stage III Non-Small Cell Lung Cancer? Eur. J. Cancer 2022, 167, 149–151. [Google Scholar] [CrossRef]











| Trial | Regimen | Median OS (Months) | 2 y OS | 5 y OS |
|---|---|---|---|---|
| Meta-Analysis 2006 | ||||
| RT Alone | - | 12 months | 21% | 6% |
| Concurrent CT-RT | Carboplatin–etoposide | 14 months | 25% | 8% |
| Meta-Analysis 2010 | ||||
| Sequential | Cisplatin + vinca or etoposide | 14 months | 30% | 11% |
| Concurrent | 18 months | 36% | 15% | |
| RTOG 0617 standard arm | Carboplatin–paclitaxel | 29 months | 58% | 31% |
| PROCLAIM standard arm | Cisplatin–etoposide | 25 months | 52% | NA |
| PACIFIC | CT-RT followed by Durvalumab | 47.5 months | 66% | 43% |
| Variable | n (%) | Variable | n (%) |
|---|---|---|---|
| Overall population | 301 (100) | ||
| Sex | Histology at diagnosis | ||
| Female | 102 (33.9) | Adenocarcinoma | 198 (65.8) |
| Male | 199 (66.1) | Squamous cell carcinoma | 85 (28.2) |
| Median age at diagnosis | Adenosquamous cell carcinoma | 2 (0.7) | |
| years (IQR) | 67 (61–72) | Sarcomatoid carcinoma | 2 (0.7) |
| ECOG at diagnosis | Not otherwise specified | 14 (4.6) | |
| 0 | 133 (44.2) | PD-L1 % | |
| 1 | 162 (53.8) | <1% | 60 (19.9) |
| >=2 | 6 (2) | ≥1% | 74 (24.6) |
| Smoking habit | Unknown | 167 (55.5) | |
| Never-smoker | 47 (15.6) | Driver mutation (EGFR, ALK, ROS1) | |
| Former-smoker | 129 (42.9) | Positive | 24 (8) |
| Current-smoker | 125 (41.5) | Negative or unknown | 277 (92) |
| Chronic obstructive pulmonary disease | Stage at diagnosis | ||
| No | 189 (62.8) | IIIA | 170 (56.5) |
| Yes | 112 (37.2) | IIIB | 119 (39.5) |
| Weight Loss | IIIC | 12 (4) | |
| No | 256 (85) | Clinical T stage | |
| Yes | 45 (15) | TX | 4 (1.3) |
| Diagnostic techniques | T1 | 39 (13) | |
| Bronchoscopy | 152 (50.5) | T2 | 86 (28.6) |
| Image-guided transthoracic biopsy | 92 (30.6) | T3 | 85 (28.2) |
| Mediastinoscopy | 14 (4.6) | T4 | 87 (28.9) |
| Surgery | 43 (14.3) | Clinical N stage | |
| N0 | 21 (7) | ||
| N1 | 38 (12.6) | ||
| N2 | 213 (70.8) | ||
| N3 | 29 (9.6) |
| Variable | n (%) | Variable | n (%) |
|---|---|---|---|
| Overall population | 301 (100) | ||
| Therapeutic pathway | Adjuvant systemic therapy | 85 (28.2) | |
| Surgery alone | 15 (5) | Platinum-based doublet | 78 (91.8) |
| Neoadjuvant therapy → surgery +/− adjuvant radiotherapy | 119 (39.5) | Platinum-based doublet +/− immunotherapy | 5 (5.9) |
| Surgery → adjuvant therapy | 89 (29.6) | Immunotherapy | 2 (2.3) |
| Definitive chemo-radiation therapy (CT-RT) | 47 (15.6) | Adjuvant radiotherapy | 101 (33.6) |
| Definitive CT-RT → durvalumab | 31 (10.3) | Adjuvant radiotherapy dose, Gy (range) | 54 (6–66) |
| Therapeutic pathway: resectable vs unresectable | Definitive CT-RT +/− durvalumab | 78 (25.9) | |
| Multimodality treatment with surgery | 223 (74.1) | Platinum-based doublet | 78 (100) |
| Multimodality treatment without surgery | 78 (25.9) | Durvalumab | 31 (39.7) |
| Neoadjuvant systemic therapy | 122 (40.5) | Radiotherapy | |
| Platinum-based doublet | 115 (94.3) | Sequential | 33 (42.3) |
| Platinum-based doublet +/− immunotherapy | 5 (4.1) | Concurrent | 45 (57.7) |
| Immunotherapy | 1 (0.8) | Definitive radiotherapy dose, Gy (range) | 60 (12–66) |
| Tyrosine kinase inhibitor | 1 (0.8) | ||
| Neoadjuvant radiotherapy | 8 (2.7) | ||
| Neoadjuvant radiotherapy dose, Gy (range) | 52.2 (50–60) |
| Treatment Modality | Entire Population | ||
|---|---|---|---|
| No. of Patients (n = 301) | mRFS (Months) 95% CI | mOS (Months) 95% CI | |
| Surgery alone | 15 | 16.4 (2.57 to 30.22) | 39.8 (27.88 to 51.82) |
| Neoadjuvant therapy + surgery | 119 | 15.9 (11.27 to 20.53) | 41.5 (32.67 to 50.38) |
| Surgery + Adjuvant therapy | 89 | 30.5 (20.42 to 40.58) | 64.1 (48.78 to 79.35) |
| Chemo-radiation therapy | 47 | 11.4 (7.91 to 14.89) | 21.6 (7.89 to 35.28) |
| Chemo-radiation therapy + durvalumab | 31 | 24.8 (12.12 to 37.48) | NR (NR to NR) |
| Treatment Modality | Patients Treated After 2018 | ||
|---|---|---|---|
| No. of Patients (n = 161) | mRFS (Months) 95% CI | mOS (Months) 95% CI | |
| Surgery alone | 11 | 16.4 (NR to 35.58) | 39.9 (25.96 to 53.74) |
| Neoadjuvant therapy + surgery | 53 | 18 (13.27 to 22.73) | NR (NR to NR) |
| Surgery + Adjuvant therapy | 39 | 23.9 (22.54 to 25.26) | 49.05 (24.71 to 73.39) |
| Chemo-radiation therapy | 27 | 8.3 (4.93 to 11.68) | 17.4 (8.54 to 26.29) |
| Chemo-radiation therapy + durvalumab | 31 | 24.8 (12.12 to 37.48) | NR (NR to NR) |
| Treatment Modality | Unresectable Patients | ||
|---|---|---|---|
| No. of Patients (n = 78) | mRFS (Months) 95% CI | mOS (Months) 95% CI | |
| Chemo-radiation therapy | 47 | 11.4 (7.91 to 14.89) | 21.6 (7.89 to 35.28) |
| Chemo-radiation therapy + durvalumab | 31 | 24.8 (12.11 to 37.48) | NR (NR to NR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, A.; Sepulcri, M.; Schiavon, M.; Scagliori, E.; Mancin, E.; Lunardi, F.; Gennaro, G.; Frega, S.; Dal Maso, A.; Bonanno, L.; et al. The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments. Cancers 2022, 14, 5700. https://doi.org/10.3390/cancers14225700
Ferro A, Sepulcri M, Schiavon M, Scagliori E, Mancin E, Lunardi F, Gennaro G, Frega S, Dal Maso A, Bonanno L, et al. The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments. Cancers. 2022; 14(22):5700. https://doi.org/10.3390/cancers14225700
Chicago/Turabian StyleFerro, Alessandra, Matteo Sepulcri, Marco Schiavon, Elena Scagliori, Edoardo Mancin, Francesca Lunardi, Gisella Gennaro, Stefano Frega, Alessandro Dal Maso, Laura Bonanno, and et al. 2022. "The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments" Cancers 14, no. 22: 5700. https://doi.org/10.3390/cancers14225700
APA StyleFerro, A., Sepulcri, M., Schiavon, M., Scagliori, E., Mancin, E., Lunardi, F., Gennaro, G., Frega, S., Dal Maso, A., Bonanno, L., Paronetto, C., Caumo, F., Calabrese, F., Rea, F., Guarneri, V., & Pasello, G. (2022). The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments. Cancers, 14(22), 5700. https://doi.org/10.3390/cancers14225700






