Brachytherapy in the Treatment of Non-Melanoma Skin Peri-Auricular Cancers—A Retrospective Analysis of a Single Institution Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
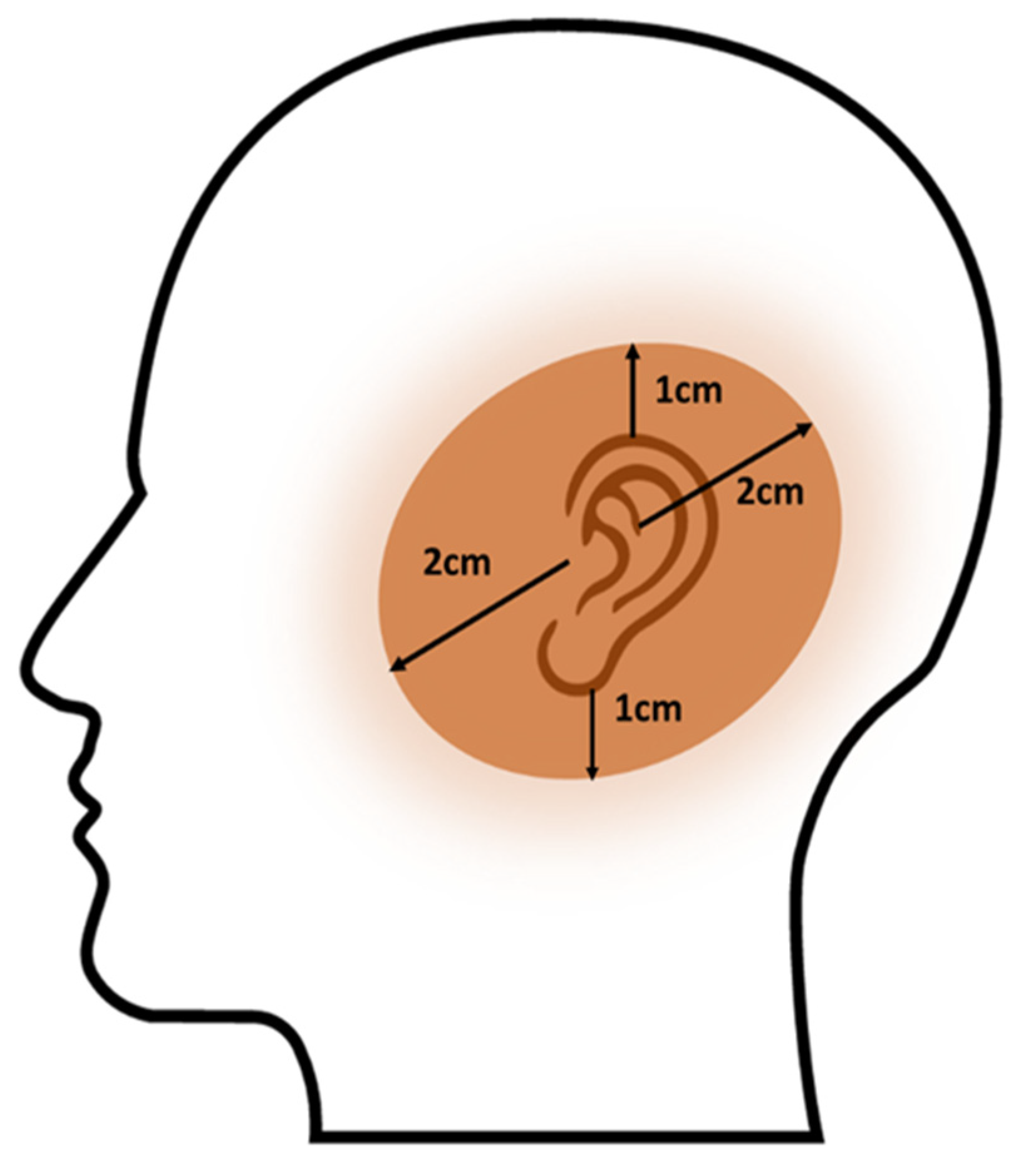
2.2. Procedure, Planning, and Treatment
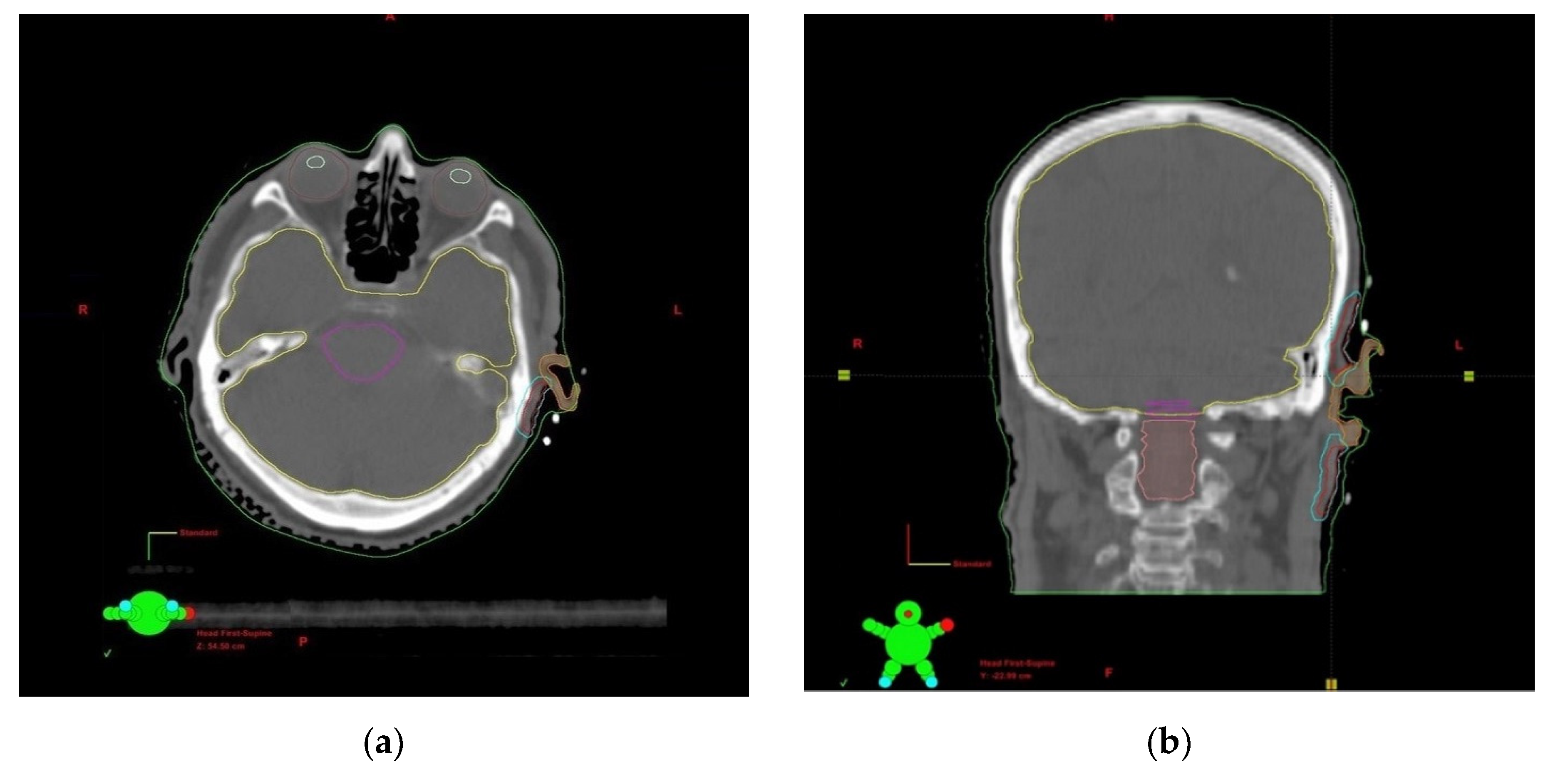
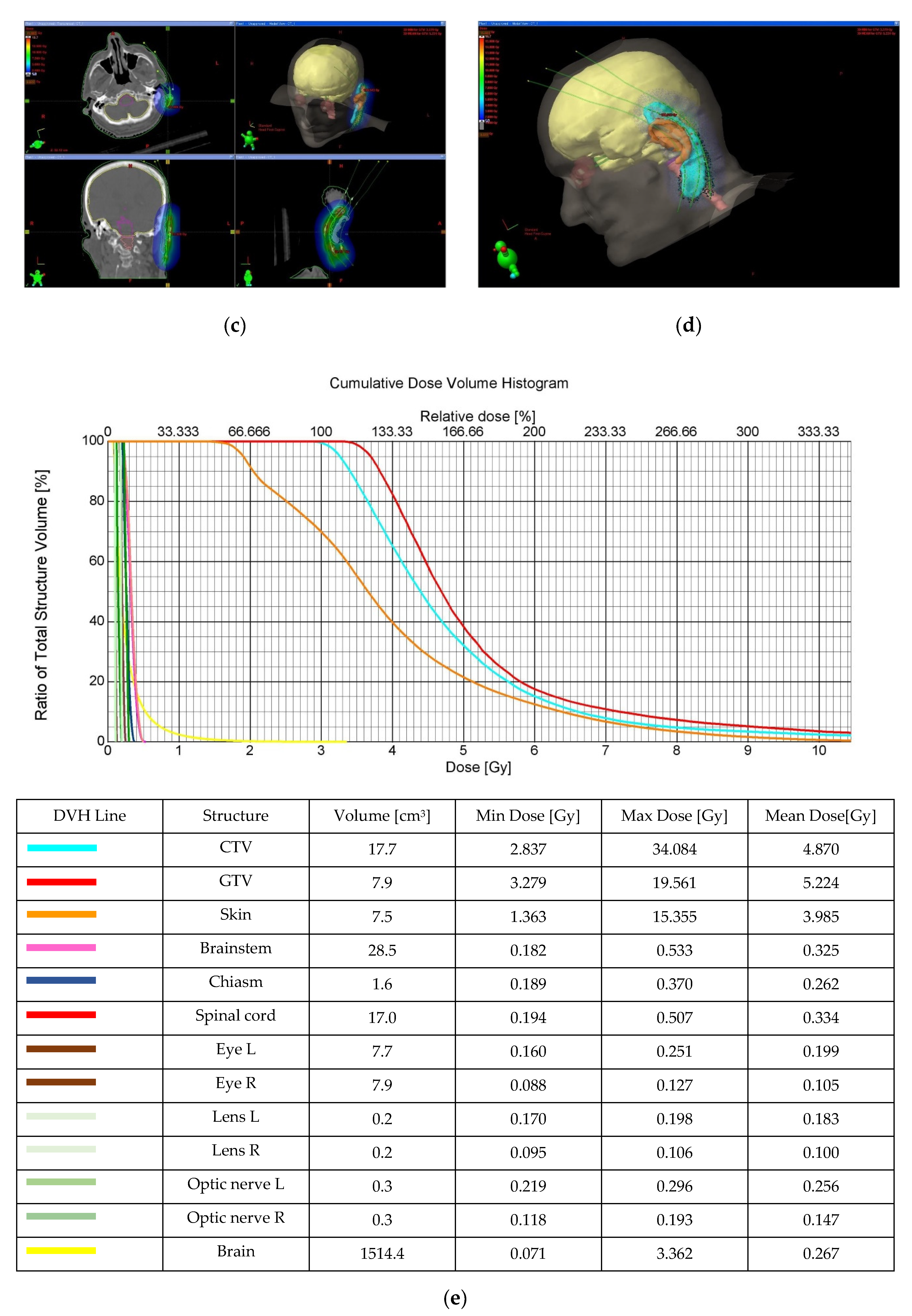
2.3. Treatment Planning and Dosimetry Analysis
2.4. Follow-Up after Treatment
2.5. Statistical Analysis
3. Results
Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/skin-cancer-statistics/ (accessed on 12 May 2022).
- Schmalbach, C.E.; Malloy, K.M. Head and Neck Cutaneous Cancer. Otolaryngol. Clin. N. Am. 2021, 54, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.-A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. The Burden of Skin Diseases [BOSD] in Europe: Preliminary results about skin cancers diagnosis and care pathway. In Proceedings of the Abstract no 353 Presented at EADV Spring Symposium 2022, Ljubljana, Slovenia, 12–14 May 2022. [Google Scholar]
- Newlands, C.; Currie, R.; Memon, A.; Whitaker, S.; Woolford, T. Non-melanoma skin cancer: United kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Van Lee, C.B.; Roorda, B.M.; Wakkee, M.; Voorham, Q.; Mooyaart, A.L.; de Vijlder, H.C.; Nijsten, T.; van den Bos, R.R. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: A retrospective cohort study. Br. J. Dermatol. 2019, 181, 338–343. [Google Scholar]
- Dalal, A.J.; Ingham, J.; Collard, B.; Merrick, G. Review of outcomes of 500 consecutive cases of non-melanoma skin cancer of the head and neck managed in an oral and maxillofacial surgical unit in a District General Hospital. Br. J. Oral. Maxillofac. Surg. 2018, 56, 805–809. [Google Scholar] [CrossRef]
- De Freitas, C.; Santos, A.N.; Bittner, G.C.; Sanabria, B.D.; Levenhagen, M.; Hans-Filho, G. Nonmelanoma Skin Cancer at Critical Facial Sites: Results and Strategies of the Surgical Treatment of 102 Patients. J. Skin Cancer 2019, 2019, 4798510. [Google Scholar] [CrossRef]
- Rieger, K.E.; Linos, E.; Egbert, B.M.; Swetter, S.M. Recurrence rates associated with incompletely excised low-risk nonmelanoma skin cancer. J Cutan Pathol. 2010, 37, 59–67. [Google Scholar] [CrossRef]
- Kumar, P.; Orton, C.I.; McWilliam, L.J.; Watson, S. Incidence of incomplete excision in surgically treated basal cell carcinoma: A retrospective clinical audit. Br. J. Plast Surg. 2000, 53, 563–566. [Google Scholar] [CrossRef]
- Lara, F.; Santamaría, J.R.; Garbers, L.E. Recurrence rate of basal cell carcinoma with positive histopathological margins and related risk factors. An. Bras. Dermatol. 2017, 92, 58–62. [Google Scholar] [CrossRef]
- Spigariolo, C.B.; Berti, E.; Brambilla, R.; Piccinno, R. Radiation therapy of non-melanoma skin cancer of the pinna: An Italian 35-year experience. Ital. J. Dermatol. Venerol. 2022, 157, 92–100. [Google Scholar] [CrossRef]
- Foley, H.; Hopley, S.; Brown, E.; Bernard, A.; Foote, M. Conformal orbit sparing radiation therapy: A treatment option for advanced skin cancer of the parotid and ear region. J. Med. Radiat. Sci. 2016, 63, 186–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cisek, P.; Kieszko, D.; Bilski, M.; Dębicki, R.; Grywalska, E.; Hrynkiewicz, R.; Bębnowska, D.; Kordzińska-Cisek, I.; Rolińska, A.; Niedźwiedzka-Rystwej, P.; et al. Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye. Cancers 2021, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Tanous, A.; Tighe, D.; Bartley, J.; Gottschalk, G.; Gilmour, T.; Lotz, N.; Fogarty, G.B. Lesion-based radiotherapy of the ears, lips and eyelids for skin cancer. Int. J. Radiol. Radiat. Ther. 2021, 8, 32–42. [Google Scholar] [CrossRef]
- Kim, J.W.; Yun, B.M.; Shin, M.S.; Kang, J.K.; Kim, J.; Kim, Y.S. Effectiveness of radiotherapy for head and neck skin cancers: A single-institution study. Radiat. Oncol. J. 2019, 37, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Giarrizzo, I.; Fionda, B.; Rigante, M.; Pagliara, M.M.; Casà, C.; Parrilla, C.; Lancellotta, V.; Placidi, E.; Salvati, A.; et al. ORIFICE (Interventional Radiotherapy for Face Aesthetic Preservation) Study: Results of Interdisciplinary Assessment of Interstitial Interventional Radiotherapy (Brachytherapy) for Periorificial Face Cancer. J. Pers. Med. 2022, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Guinot, J.L.; Rembielak, A.; Perez-Calatayud, J.; Rodríguez-Villalba, S.; Skowronek, J.; Tagliaferri, L.; Guix, B.; Gonzalez-Perez, V.; Valentini, V.; Kovacs, G.; et al. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018, 126, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Landier, W. Ototoxicity and cancer therapy. Cancer 2016, 122, 1647–1658. [Google Scholar] [CrossRef]
- Nader, M.E.; Gidley, P.W. Challenges of hearing rehabilitation after radiation and chemotherapy. J. Neurol. Surg. B Skull. Base 2019, 80, 214–224. [Google Scholar] [CrossRef]
- Mazeron, J.-J.; Ghalie, R.; Zeller, J.; Marinello, G.; Marin, L.; Raynal, M.; Le Bourgeois, J.-P.; Pierquin, B. Radiation therapy for carcinoma of the pinna using iridium 192 wires: A series of 70 patients. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1757–1763. [Google Scholar] [CrossRef]
- Guix, B.; Finestres, F.; Tello, J.-I.; Palma, C.; Martinez, A.; Guix, J.-R.; Guix, R. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 95–102. [Google Scholar] [CrossRef]
- Gauden, R.; Pracy, M.; Avery, A.-M.; Hodgetts, I.; Gauden, S. HDR brachytherapy for superficial non-melanoma skin cancers. J. Med. Imaging Radiat. Oncol. 2013, 57, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Pons-Llanas, O.; Ballester-Sánchez, R.; Celada-Álvarez, F.J.; Candela-Juan, C.; García-Martínez, T.; Llavador-Ros, M.; Botella-Estrada, R.; Barker, C.; Ballesta, A.; Tormo-Micó, A.; et al. Clinical implementation of a new electronic brachytherapy system for skin brachytherapy. J. Contemp. Brachytherapy 2015, 6, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Tormo, A.; Celada, F.; Rodriguez, S.; Botella, R.; Ballesta, A.; Kasper, M.; Ouhib, Z.; Santos, M.; Perez-Calatayud, J. Non-melanoma skin cancer treated with HDR Valencia applicator: Clinical outcomes. J.Contemp. Brachytherapy 2014, 6, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kuncman, Ł.; Kozłowski, S.; Pietraszek, A.; Pietrzykowska-Kuncman, M.; Danielska, J.; Sobotkowski, J.; Łuniewska-Bury, J.; Fijuth, J. Highly conformal CT based surface mould brachytherapy for non-melanoma skin cancers of earlobe and nose. J. Contemp. Brachytherapy 2016, 8, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Bandyopadhyay, A.; Pal, J.; Ghosh, A.; Deb, A. A dosimetric study of electron beam therapy vs. high-dose-rate mould brachytherapy in adjuvant treatment of non-melanoma skin carcinomas of the head and neck region. J. Contemp. Brachytherapy 2019, 11, 547–555. [Google Scholar]
- Maroñas, M.; Guinot, J.L.; Arribas, L.; Carrascosa, M.; Tortajada, M.I.; Carmona, R.; Estornell, M.; Muelas, R. Treatment of facial cutaneous carcinoma with high-dose rate contact brachytherapy with customized molds. Brachytherapy 2011, 10, 221–227. [Google Scholar] [CrossRef]
- Arguis, M.; Murcia-Mejía, M.; Henríquez, I.; Gómez, D.; Lafuerza, A.; Esteban, C.; León, R.; Polo, Y.; Grau, I.; Arenas, M. The role of brachytherapy in non-melanoma skin cancer treatment. Radiother. Oncol. 2012, 103, S477. [Google Scholar] [CrossRef]

| Clinical and Histopathological Factor | Number of Patients (Percentage) | Mean Value | Median Value (Range) |
|---|---|---|---|
| Age (years) | 33 | 80.79 +/− 9.76 | 83 (63–98) |
| Gender | |||
| Men | 14 (42%) | - | - |
| Women | 19 (58%) | - | - |
| Histopathological type | |||
| Squamous cell carcinoma | 20 (57%) | - | - |
| Basal cell carcinoma | 9 (26%) | - | - |
| Undifferentiated carcinoma | 4 (11%) | - | - |
| Stage | |||
| T1N0M0 | 7 (20%) | - | - |
| T2N0M0 | 15 (43%) | - | - |
| T3N0M0 | 11 (31%) | - | - |
| Largest lesion size (cm) | - | 3.46 +/− 1.79 | 3 (0–7.5) |
| Definitive brachytherapy | 15 (43%) | - | - |
| Brachytherapy after surgery | 18 (57%) | - | - |
| Application of brachytherapy | |||
| Contact brachytherapy | 21 (66%) | - | - |
| Interstitial brachytherapy | 11 (33%) | - | - |
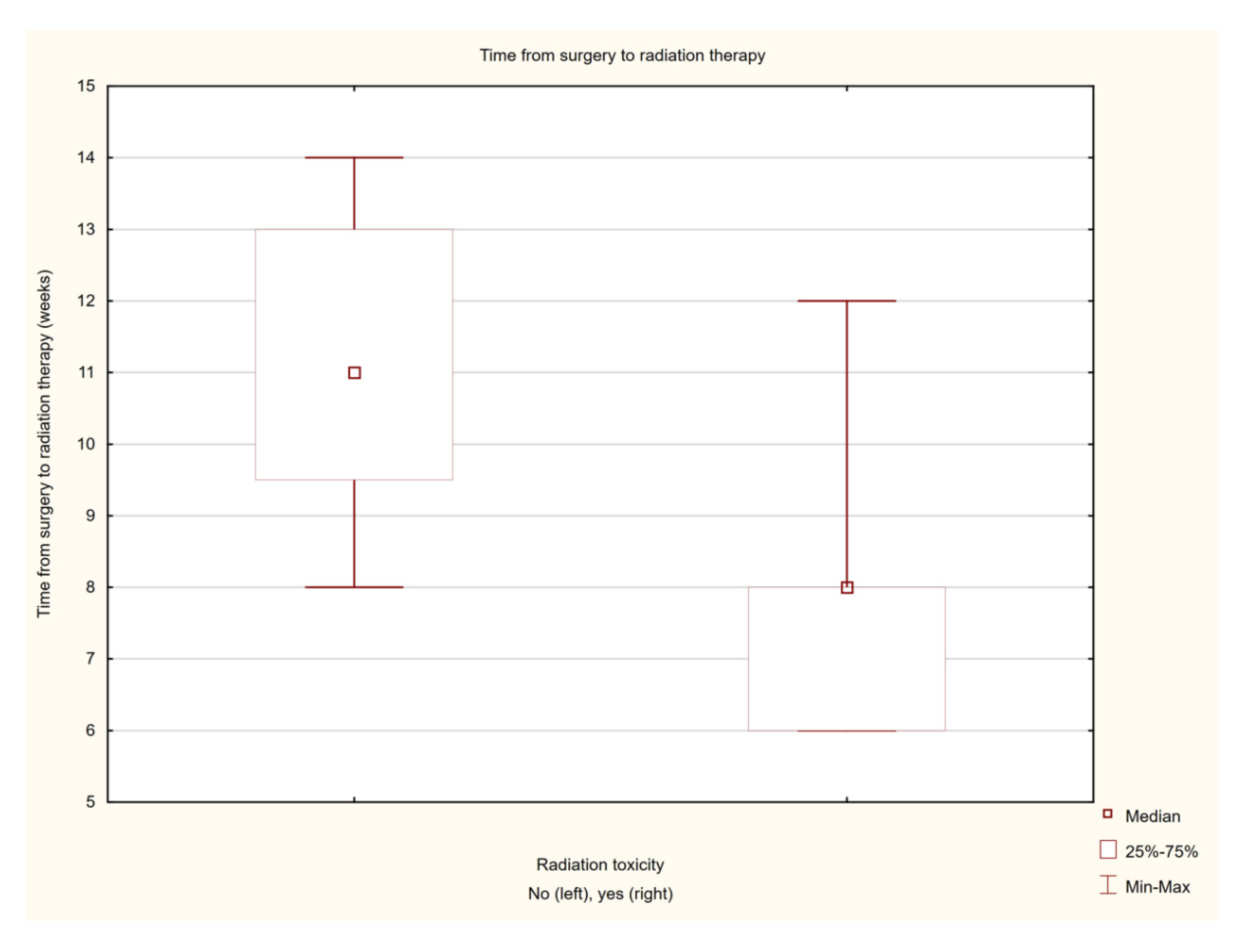
| Time from surgery to the start of brachytherapy treatment (weeks) | 19 | 9.16 +/− 2.54 | 8 (6–14) |
| Number of applicators | - | 6.42 +/− 2.61 | 6 (3–15) |
| Dose Fractionation Schedules | |||
|---|---|---|---|
| Total Number of Fractions | Number of Fractions per Day | Dose per Fraction | Total Dose |
| 15 | 1 | 3 Gy | 45 Gy |
| 9 | 2 (interval between fractions of minimum 6 h) | 5 Gy | 45 Gy |
| 14 | 2 (interval between fractions of minimum 6 h) | 3 Gy | 42 Gy |
| 5 | 1 | 4 Gy | 20 Gy |
| 3 | 1 every 7 days | 7 Gy | 21 Gy |
| Variable | Mean Value | Median Value (Range) | |
|---|---|---|---|
| General prescription | Total dose (Gy) | 37.73 ± 12.31 | 45 (7–49) |
| Fraction dose (Gy) | 3.65 ± 0.88 | 3.5 (3–7) | |
| Number of fractions | 11.18 ± 4.63 | 14 (1–15) | |
| Number of irradiation days | 16.15 ± 10.4 | 19 (1–42) | |
| Target dose | CTV D0.10cc [Gy] | 18.14 ± 18.44 | 12.39 (5.78–88.01) |
| CTV D0.10cc [Gy] × number of fractions | 164.02 ± 113.61 | 147 (28.9–645.21) | |
| CTV D90 [Gy] | 4.09 ± 0.87 | 3.8 (3.1–6.2) | |
| CTV D90 [Gy] × number of fractions | 43.67 ± 17.34 | 49.5 (6.2–92.55) | |
| CTV D90 [Gy] × number of fractions BED 10 | 61.42 ± 25.96 | 68.78 (10.04–149.65) | |
| CTV D100[Gy] | 2.75 ± 0.56 | 2.58 (2.04–4.44) | |
| CTV D100 [Gy] × number of fractions | 29.72 ± 12.5 | 34.35 (3.74–66.6) | |
| CTV D100 [Gy] × number of fractions | 37.93 ± 16.92 | 42.22 (5.14–96.17) | |
| CTV V100 [% of volume] | 96.76 ± 2.72 | 97.71 (90.05–99.8) | |
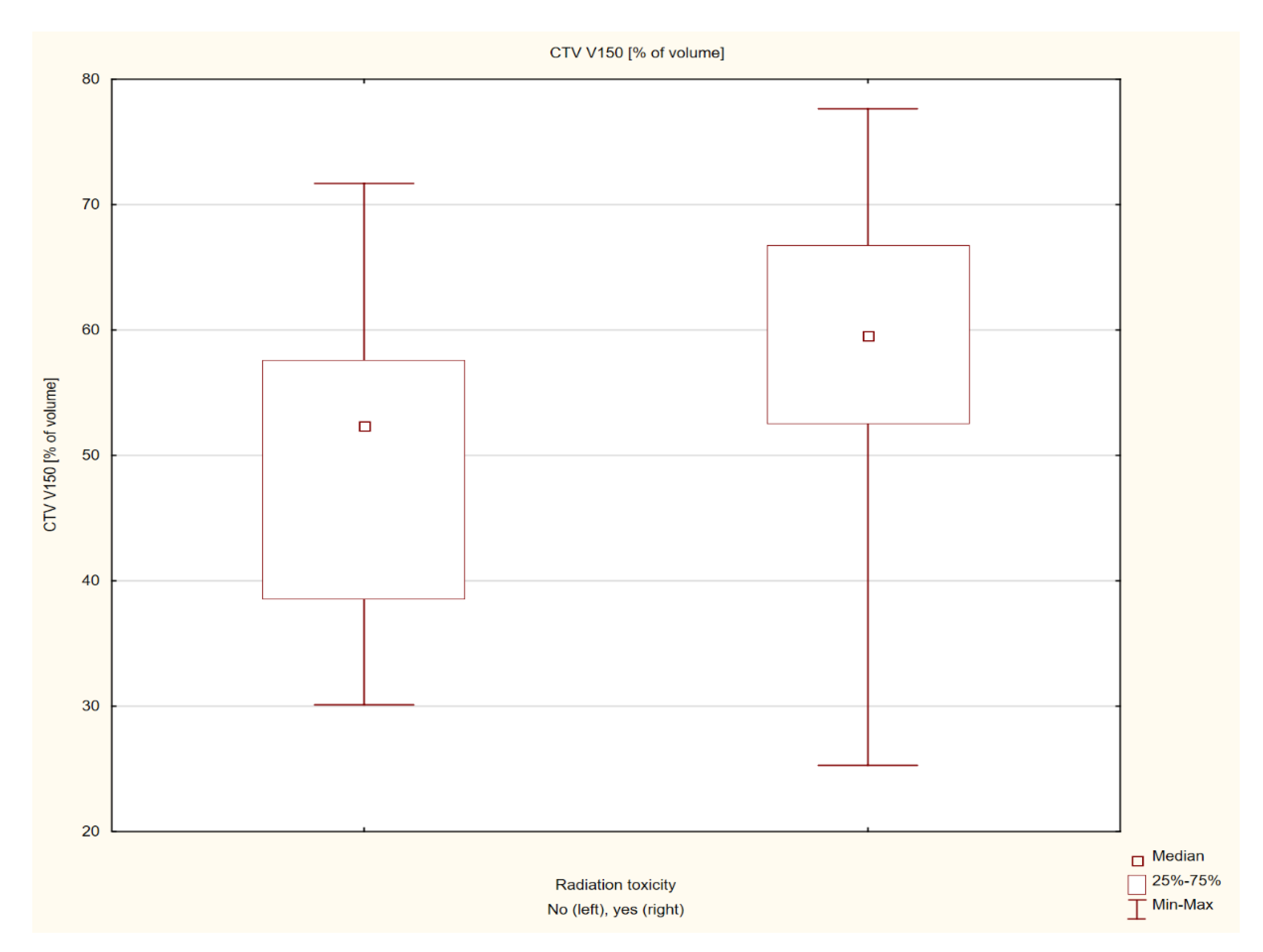
| CTV V150 [% of volume] | 53.6 ± 13.33 | 53.9 (25.3 −77.65) | |
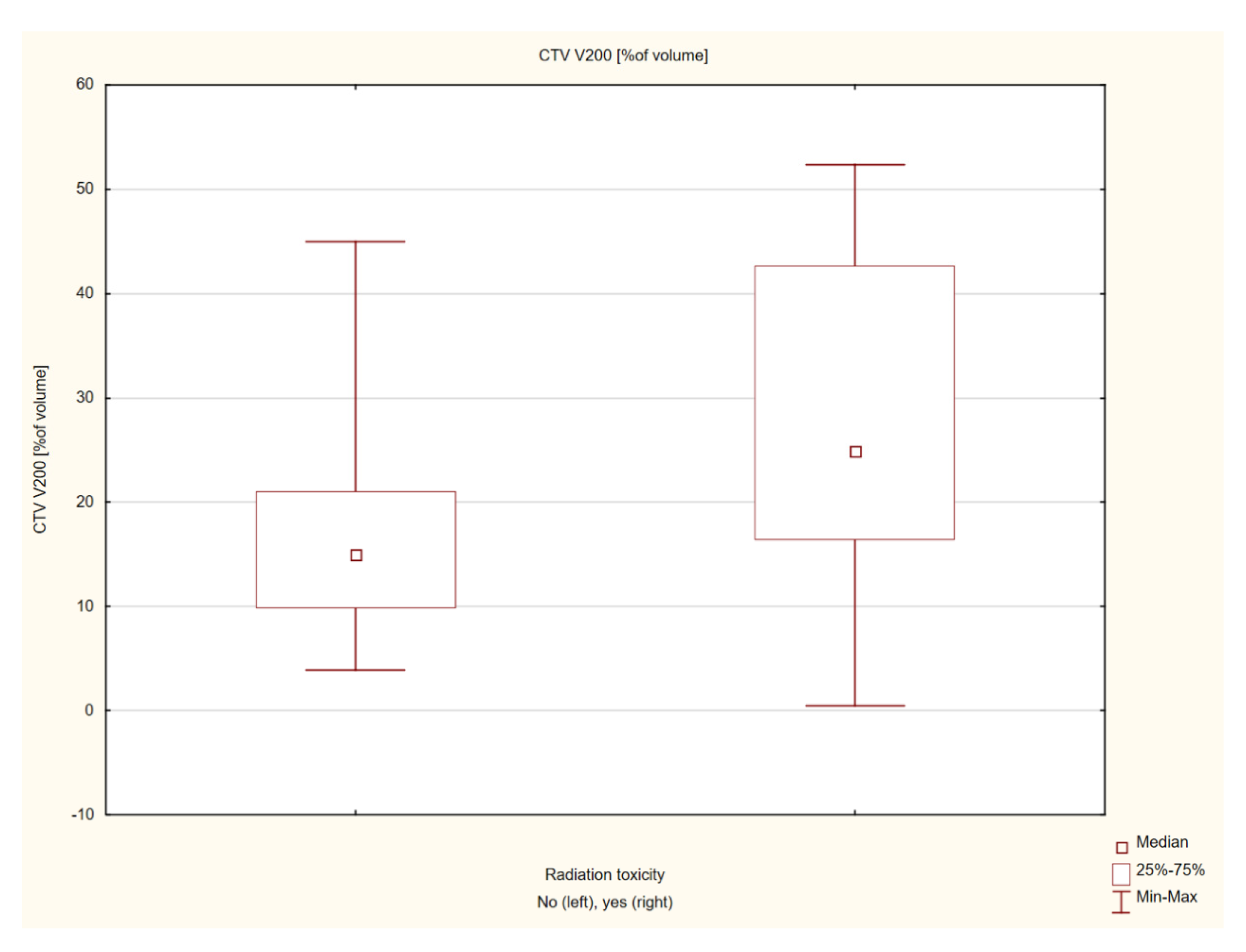
| CTV V200 [%of volume] | 22.86 ± 14.66 | 19.49 (0.5–52.31) | |
| OAR’s dose | Brain D1.00cc [Gy] | 1.53 ± 0.69 | 1.49 (0.3–2.94) |
| Brain D1.00cc [Gy] × number of fractions | 16.46 ± 9.65 | 14.40 (2.7–33.6) | |
| Brain D1.00cc [Gy] × number of fractions BED 3 | 25.95 ± 17.21 | 21.77 (2.97–58.66) | |
| Brain D1.00cc [Gy] × number of fractions BED 10 | 19.31 ± 11.88 | 17.76 (2.78–41.12) | |
| Brain D0.10cc [Gy] | 1.79 ± 0.83 | 1.72 (0.34–4.07) | |
| Brain D0.10cc [Gy] × number of fractions | 19.04 ± 10.86 | 17.25 (3.06–36.9) | |
| Brain D0.10cc [Gy] × number of fractions BED 3 | 31.62 ± 20.42 | 27.77 (3.41–67.13) | |
| Brain D0.10cc [Gy] × number of fractions BED 10 | 22.81 ± 13.68 | 20.54 (3.16–45.98 | |
| Bone D1.00cc [Gy] | 2.92 ± 0.93 | 2.95 (0.82–5.44) | |
| Bone D1.00cc [Gy] × number of fractions | 31.42 ± 14.49 | 37.20 (4.73–51.6) | |
| Bone D1.00cc [Gy] × number of fractions BED 3 | 63.25 ± 32.89 | 67.92 (9.4–137.65) | |
| Bone D1.00cc [Gy] × number of fractions BED 10 | 40.97 ± 19.83 | 46.42 (6.97–75.59) | |
| Bone D0.10cc [Gy] | 3.63 ± 1.37 | 3.51 (1.05–8.14) | |
| Bone D0.10cc [Gy] × number of fractions | 38.39 ± 17.91 | 42.6 (8.14–71.4) | |
| Bone D0.10cc [Gy] × number of fractions BED 3 | 86.50 ± 47.15 | 95.51 (12.75–184.57) | |
| Bone D0.10cc [Gy] × number of fractions BED 10 | 52.83 ± 26.38 | 56.01 (10.44–105.38) |
| Factor | Z | p | |
|---|---|---|---|
| General information | Age | 0.66713 | 0.504689 |
| Lesion size | −1.43029 | 0.152634 | |
| Time from surgery to irradiation | 3.01115 | 0.002603 | |
| Number of applicators | −0.62662 | 0.530910 | |
| Prescription dose | Total dose | −0.34345 | 0.731262 |
| Fraction dose | −0.64187 | 0.520960 | |
| Number of fractions | 0.36689 | 0.713702 | |
| Number of irradiation days | 0.54918 | 0.582880 | |
| Target dose | CTV D0.10cc [Gy] | −0.88261 | 0.377447 |
| CTV D0.10cc [Gy] × number of fractions | −1.45901 | 0.144564 | |
| CTV D90 [Gy] | −0.14411 | 0.885412 | |
| CTV D90 [Gy] × number of fractions | 0.21617 | 0.828857 | |
| CTV D90 [Gy] × number of fractions BED 10 | −0.14411 | 0.885412 | |
| CTV D100 [Gy] | 0.55848 | 0.576517 | |
| CTV D100 [Gy] × number of fractions | 0.30621 | 0.759444 | |
| CTV D100 [Gy] × number of fractions BED 10 | 0.12609 | 0.899663 | |
| CTV V100 [% of volume] | 1.67530 | 0.093877 | |
| CTV V150 [% of volume] | −1.92733 | 0.053939 | |
| CTV V200 [%of volume] | −2.03541 | 0.041811 | |
| OAR’s dose | Brain D1.00cc [Gy] | 1.49528 | 0.134841 |
| Brain D1.00cc [Gy] × number of fractions | 1.53106 | 0.125756 | |
| Brain D1.00cc [Gy] × number of fractions BED 3 | 1.74721 | 0.080602 | |
| Brain D1.00cc [Gy] × number of fractions BED 10 | 1.53106 | 0.125756 | |
| Brain D0.10cc [Gy] | 1.44112 | 0.149553 | |
| Brain D0.10cc [Gy] × number of fractions | 1.49503 | 0.134907 | |
| Brain D0.10cc [Gy] × number of fractions BED 3 | 1.60311 | 0.108912 | |
| Brain D0.10cc [Gy] × number of fractions BED 10 | 1.49503 | 0.134907 | |
| Bone D1.00cc [Gy] | 0.68459 | 0.493605 | |
| Bone D1.00cc [Gy] × number of fractions | 1.04481 | 0.296112 | |
| Bone D1.00cc [Gy] × number of fractions BED 3 | 1.04481 | 0.296112 | |
| Bone D1.00cc [Gy] × number of fractions BED 10 | 1.11687 | 0.264053 | |
| Bone D0.10cc [Gy] | 0.1802 | 0.856996 | |
| Bone D0.10cc [Gy] × number of fractions | 0.9368 | 0.34886 | |
| Bone D0.10cc [Gy] × number of fractions BED 3 | 0.86474 | 0.387181 | |
| Bone D0.10cc [Gy] × number of fractions BED 10 | 0.86474 | 0.387181 |
| Feature | Z | p |
|---|---|---|
| Gender | 0.75 | 0.387 |
| Histopathological type | 12.833 | 0.615 |
| TNM | 13 | 0.602 |
| Indications for brachytherapy—individual vs. adjuvant after surgery | 2.53 | 0.120 |
| Application—contact vs. interstitial | 4.5 | 0.034 |
| Location—auricle vs. adjacent area of the auricle | 0.76 | 0.383 |
| CTCAE (Degree of Severity) | Number of Patients (Percentage) |
|---|---|
| Grade 0 (none) | 17 (52%) |
| Grade 1 (mild) | 14 (42%) |
| Grade 2 (moderate) | 2 (6%) |
| Grade 3 (severe) | - |
| Grade 4 (life-threatening) | - |
| Grade 5 (death) | - |
| RTOG | Number of Patients (Percentage) |
|---|---|
| Grade 0 | 15 (45.5%) |
| Grade 1 | 15 (45.5%) |
| Grade 2 | 3 (9%) |
| Grade 3 | - |
| Grade 4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, M.; Cisek, P.; Baranowska, I.; Kordzińska-Cisek, I.; Komaniecka, N.; Hymos, A.; Grywalska, E.; Niedźwiedzka-Rystwej, P. Brachytherapy in the Treatment of Non-Melanoma Skin Peri-Auricular Cancers—A Retrospective Analysis of a Single Institution Experience. Cancers 2022, 14, 5614. https://doi.org/10.3390/cancers14225614
Bilski M, Cisek P, Baranowska I, Kordzińska-Cisek I, Komaniecka N, Hymos A, Grywalska E, Niedźwiedzka-Rystwej P. Brachytherapy in the Treatment of Non-Melanoma Skin Peri-Auricular Cancers—A Retrospective Analysis of a Single Institution Experience. Cancers. 2022; 14(22):5614. https://doi.org/10.3390/cancers14225614
Chicago/Turabian StyleBilski, Mateusz, Paweł Cisek, Izabela Baranowska, Izabela Kordzińska-Cisek, Nina Komaniecka, Anna Hymos, Ewelina Grywalska, and Paulina Niedźwiedzka-Rystwej. 2022. "Brachytherapy in the Treatment of Non-Melanoma Skin Peri-Auricular Cancers—A Retrospective Analysis of a Single Institution Experience" Cancers 14, no. 22: 5614. https://doi.org/10.3390/cancers14225614
APA StyleBilski, M., Cisek, P., Baranowska, I., Kordzińska-Cisek, I., Komaniecka, N., Hymos, A., Grywalska, E., & Niedźwiedzka-Rystwej, P. (2022). Brachytherapy in the Treatment of Non-Melanoma Skin Peri-Auricular Cancers—A Retrospective Analysis of a Single Institution Experience. Cancers, 14(22), 5614. https://doi.org/10.3390/cancers14225614








