Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision
Abstract
Simple Summary
Abstract
1. Introduction
2. Surgical Resection and Liver Transplantation
2.1. iCCA
2.2. Extrahepatic Cholangiocarcinoma
3. Systemic Therapies
3.1. Targeted Therapy
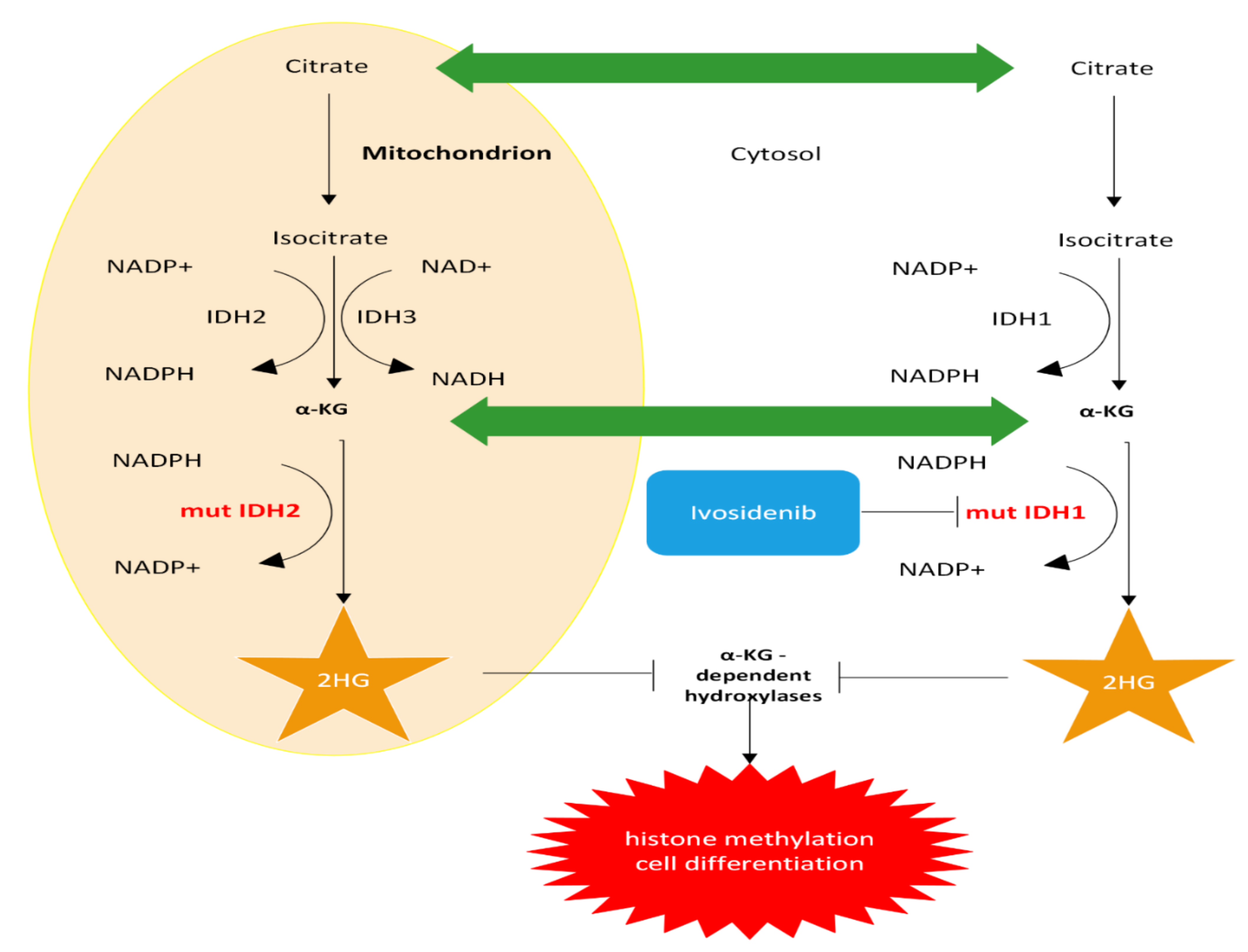
3.1.1. IDH1/2
3.1.2. FGFR2 Fusions or Rearrangements
3.1.3. NTRK Fusions
3.1.4. BRAF V600E
3.1.5. HER2/ERBB2 Amplifications
3.2. Immunotherapy
3.2.1. Monotherapy
3.2.2. Immunotherapy and Chemotherapy
3.2.3. Dual Immunotherapy
3.2.4. Immunotherapy and Targeted Therapy
4. Current and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Training Modules, Module Name. U. S. National Institutes of Health, National Cancer Institute. Pancreatic & Biliary Cancer. Available online: https://training.seer.cancer.gov/biliary/ (accessed on 29 July 2022).
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Lee, A.J.; Chun, Y.S. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates. Chin. Clin. Oncol. 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Farges, O.; Fuks, D.; Le Treut, Y.P.; Azoulay, D.; Laurent, A.; Bachellier, P.; Nuzzo, G.; Belghiti, J.; Pruvot, F.R.; Regimbeau, J.M. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011, 117, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Aloia, T.A.; Vauthey, J.N.; Abdalla, E.K.; Zhu, A.X.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. A proposed staging system for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2009, 16, 14–22. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Hepatobiliary Cancers Version 1. 2022. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438 (accessed on 29 July 2022).
- Matsuo, K.; Rocha, F.G.; Ito, K.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; Dematteo, R.P.; Gonen, M.; Endo, I.; Jarnagin, W.R. The Blumgart preoperative staging system for hilar cholangiocarcinoma: Analysis of resectability and outcomes in 380 patients. J. Am. Coll Surg. 2012, 215, 343–355. [Google Scholar] [CrossRef]
- Hong, S.M.; Pawlik, T.M.; Cho, H.; Aggarwal, B.; Goggins, M.; Hruban, R.H.; Anders, R.A. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 2009, 146, 250–257. [Google Scholar] [CrossRef]
- Ciardiello, D.; Maiorano, B.A.; Parente, P.; Rodriquenz, M.G.; Latiano, T.P.; Chiarazzo, C.; Pazienza, V.; Guerrera, L.P.; Amoruso, B.; Normanno, N.; et al. Immunotherapy for Biliary Tract Cancer in the Era of Precision Medicine: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 820. [Google Scholar] [CrossRef]
- Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; DrozDitBusset, M.; Sapisochin, G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Al-Rawi, H.; Esmail, A.; Xu, J.; Umoru, G.; Ibnshamsah, F.; Abudayyeh, A.; Victor, D.; Saharia, A.; McMillan, R.; et al. Gemcitabine and Cisplatin as Neo-Adjuvant for Cholangiocarcinoma Patients Prior to Liver Transplantation: Case-Series. Curr. Oncol. 2022, 29, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Nolte Fong, J.V.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; Abudayyeh, A.; Abdel-Wahab, N.; Diab, A.; Murakami, N.; Kaseb, A.O.; Chang, J.C.; Gaber, A.O.; et al. Utilization of Immunotherapy for the Treatment of Hepatocellular Carcinoma in the Peri-Transplant Setting: Transplant Oncology View. Cancers 2022, 14, 1760. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Umoru, G.; Westhart, K.; Abudayyeh, A.; Saharia, A.; Ghobrial, R.M. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr. Oncol. 2022, 29, 4267–4273. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A. P-168 Combination of gemcitabine plus cisplatin compared to non-gemcitabine and cisplatin regimens as neo-adjuvant treatment in liver transplant recipients with cholangiocarcinoma. Ann. Oncol. 2022, 33, S309–S310. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abudayyeh, A.; Victor, D.; McMillan, R.; Kodali, S.; et al. Gemcitabine Plus Cisplatin Versus Non-Gemcitabine and Cisplatin Regimens as Neoadjuvant Treatment for Cholangiocarcinoma Patients Prior to Liver Transplantation: An Institution Experience. Front. Oncol. 2022, 12, 908687. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Saharia, A.; McMillan, R.; Ghobrial, R.M. Gemcitabine plus cisplatin versus non-gemcitabine and cisplatin regimens as neoadjuvant treatment for cholangiocarcinoma patients prior to liver transplantation. J. Clin. Oncol. 2022, 40 (Suppl. S16), e16202. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Okane, G.; Esmail, A.; Saharia, A.; Kodali, S.; Victor, D. P-162 Atezolizumab and bevacizumab pre-liver transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann. Oncol. 2022, 33, S308. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Victor, D.; Esmail, A.; Kodali, S.; Graviss, E.A.; Nguyen, D.T.; Moore, L.W.; Saharia, A.; McMillan, R.; Fong, J.N.; et al. Transarterial Chemoembolization (TACE) Plus Sorafenib Compared to TACE Alone in Transplant Recipients with Hepatocellular Carcinoma: An Institution Experience. Cancers 2022, 14, 650. [Google Scholar] [CrossRef]
- Esmail, A.; Kodali, S.; Graviss, E.; Nguyen, D.; Moore, L.; Saharia, A.; Uosef, A.; Victor, D.; Abdelrahim, M. P-163 Tyrosine kinase inhibitors (TKIs) plus transarterial chemoembolization (TACE) compared to TACE alone as downstaging therapy in transplant recipients with hepatocellular carcinoma. Ann. Oncol. 2022, 33, S308. [Google Scholar] [CrossRef]
- Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abdelrahim, M. P-169 Feasibility of gemcitabine plus cisplatin as neo-adjuvant in cholangiocarcinoma patients prior to liver transplantation. Ann. Oncol. 2022, 33, S310. [Google Scholar] [CrossRef]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.Y.; Vibert, E.; Cherqui, D.; Grant, D.R.; Hernandez-Alejandro, R.; et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef]
- De Martin, E.; Rayar, M.; Golse, N.; Dupeux, M.; Gelli, M.; Gnemmi, V.; Allard, M.A.; Cherqui, D.; Sa Cunha, A.; Adam, R.; et al. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transplant. 2020, 26, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Petrowsky, H.; Kaldas, F.M.; Farmer, D.G.; Durazo, F.A.; Finn, R.S.; Saab, S.; Han, S.H.; Lee, P.; Markovic, D.; et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J.Am. Coll Surg. 2011, 212, 514–520, discussion 520–521. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, G.; Lunsford, K.E.; Latt, N.L.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N. Expanding indications for liver transplantation in the era of liver transplant oncology. World J. Gastrointest. Surg. 2021, 13, 392–405.e1. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, J.K.; Groot Koerkamp, B.; Cieslak, K.P.; Doussot, A.; van Klaveren, D.; Allen, P.J.; Besselink, M.G.; Busch, O.R.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J. Am. Coll Surg. 2016, 223, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3, quiz e14. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Xie, H.; Bin-Riaz, I.; Sharma, P.; Durani, U.; Goyal, G.; Borah, B.; Borad, M.J.; Smoot, R.L.; Roberts, L.R.; et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019, 45, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, J.; Huang, J.; Zeng, Y.; Yuan, K. Neoadjuvant and Adjuvant Therapy in Intrahepatic Cholangiocarcinoma. J. Clin. Transl. Hepatol. 2022, 10, 553–563. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. New Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Catalano, P.J.; Zalupski, M.M.; Lubner, S.J.; Menge, M.R.; Nimeiri, H.S.; Munshi, H.G.; Benson, A.B., III; O’Dwyer, P.J. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Borbath, I.; Ceratti, A.; Verslype, C.; Demols, A.; Delaunoit, T.; Laurent, S.; Deleporte, A.; Vergauwe, P.; Van Maanen, A.; Sempoux, C.; et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: A phase II study of the Belgian Group of Digestive Oncology. Ann. Oncol. 2013, 24, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Ghafoori, A.P.; Nelson, J.W.; Willett, C.G.; Chino, J.; Tyler, D.S.; Hurwitz, H.I.; Uronis, H.E.; Morse, M.A.; Clough, R.W.; Czito, B.G. Radiotherapy in the Treatment of Patients with Unresectable Extrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 654–659. [Google Scholar] [CrossRef]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Q.; Zhang, C.; Kuan, P.F.; Liu, Y.; Jeck, W.R.; Andersen, J.B.; Jiang, W.; Savich, G.L.; Tan, T.X.; et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 2013, 32, 3091–3100. [Google Scholar] [CrossRef]
- Mondesir, J.; Willekens, C.; Touat, M.; de Botton, S. IDH1 and IDH2 mutations as novel therapeutic targets: Current perspectives. J. Blood Med. 2016, 7, 171–180. [Google Scholar]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Graham, R.P.; Barr Fritcher, E.G.; Pestova, E.; Schulz, J.; Sitailo, L.A.; Vasmatzis, G.; Murphy, S.J.; McWilliams, R.R.; Hart, S.N.; Halling, K.C.; et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum.Pathol. 2014, 45, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat.Rev. 2021, 95, 102170. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Piha-Paul, S.A.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019, 37 (Suppl. S15). [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39 (Suppl. S3), 265. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat.Rev.Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Demols, A.; Rocq, L.; Charry, M.; Nève, N.D.; Verrellen, A.; Ramadhan, A.; Campenhout, C.V.; Clercq, S.D.; Salmon, I.; D’Haene, N. NTRK gene fusions in biliary tract cancers. J. Clin. Oncol. 2020, 38 (Suppl. S4), 574. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. New Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol 2018, 36, 536–542. [Google Scholar] [CrossRef]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.C.; Shin, D.W.; Kim, J.; Hwang, J.H. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci. Rep. 2020, 10, 12348. [Google Scholar] [CrossRef]
- Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; Italiano, A.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.K.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abudayyeh, A.; Victor, D.; McMillan, R.; Kodali, S.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar]
- Ioka, T.; Ueno, M.; Oh, D.-Y.; Fujiwara, Y.; Chen, J.-S.; Doki, Y.; Mizuno, N.; Park, K.; Asagi, A.; Hayama, M.; et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J. Clin. Oncol. 2019, 37 (Suppl. S4), 387. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Zhao, Y.; Yang, Q.; Dong, L.; Liu, J.; Li, X.; Zhao, Z.; Mei, Q.; Han, W. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: Results from a phase II study. J. Immunother. Cancer 2020, 8, e000367. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Pehrsson, E.C.; Xie, C.; Duffy, A.G.; Mabry, D.; Wood, B.J.; Kleiner, D.E.; Steinberg, S.M.; Figg, W.D.; Redd, B.; et al. A Phase II Study of Pembrolizumab in Combination with Capecitabine and Oxaliplatin with Molecular Profiling in Patients with Advanced Biliary Tract Carcinoma. Oncologist 2022, 27, e273–e285. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Wu, H.; Gu, Y.; Shao, Y.; Shao, Q.; Zhu, F.; Li, X.; Qian, X.; Hu, J.; et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: A single-arm, open-label, phase II trial. J. Immunother. Cancer 2020, 8, e001240. [Google Scholar] [CrossRef]
- Chen, X.; Qin, S.; Gu, S.; Ren, Z.; Chen, Z.; Xiong, J.; Liu, Y.; Meng, Z.; Zhang, X.; Wang, L.; et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int. J. Cancer 2021, 149, 1944–1954. [Google Scholar] [CrossRef]
- Li, W.; Yu, Y.; Xu, X.; Guo, X.; Wang, Y.; Li, Q.; Wang, Y.; Cui, Y.; Liu, H.; Hao, Q.; et al. Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: Update analytic results of an open-label phase II clinical study (JS001-ZS-BC001). J. Clin. Oncol. 2021, 39 (Suppl. S15), e16170. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Kim, T.Y.; Bang, J.-H.; Nam, A.-R.; Lee, Y.; Zhang, Q.; Rebelatto, M.; Li, W.; et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2020, 38 (Suppl. S15), 4520. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Lum, C.; Behren, A.; Palmer, J.; Tebbutt, N.C.; et al. Combination immunotherapy with ipilimumab and nivolumab in patients with advanced biliary tract cancers. J. Clin. Oncol. 2020, 38 (Suppl. S15), 4588. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
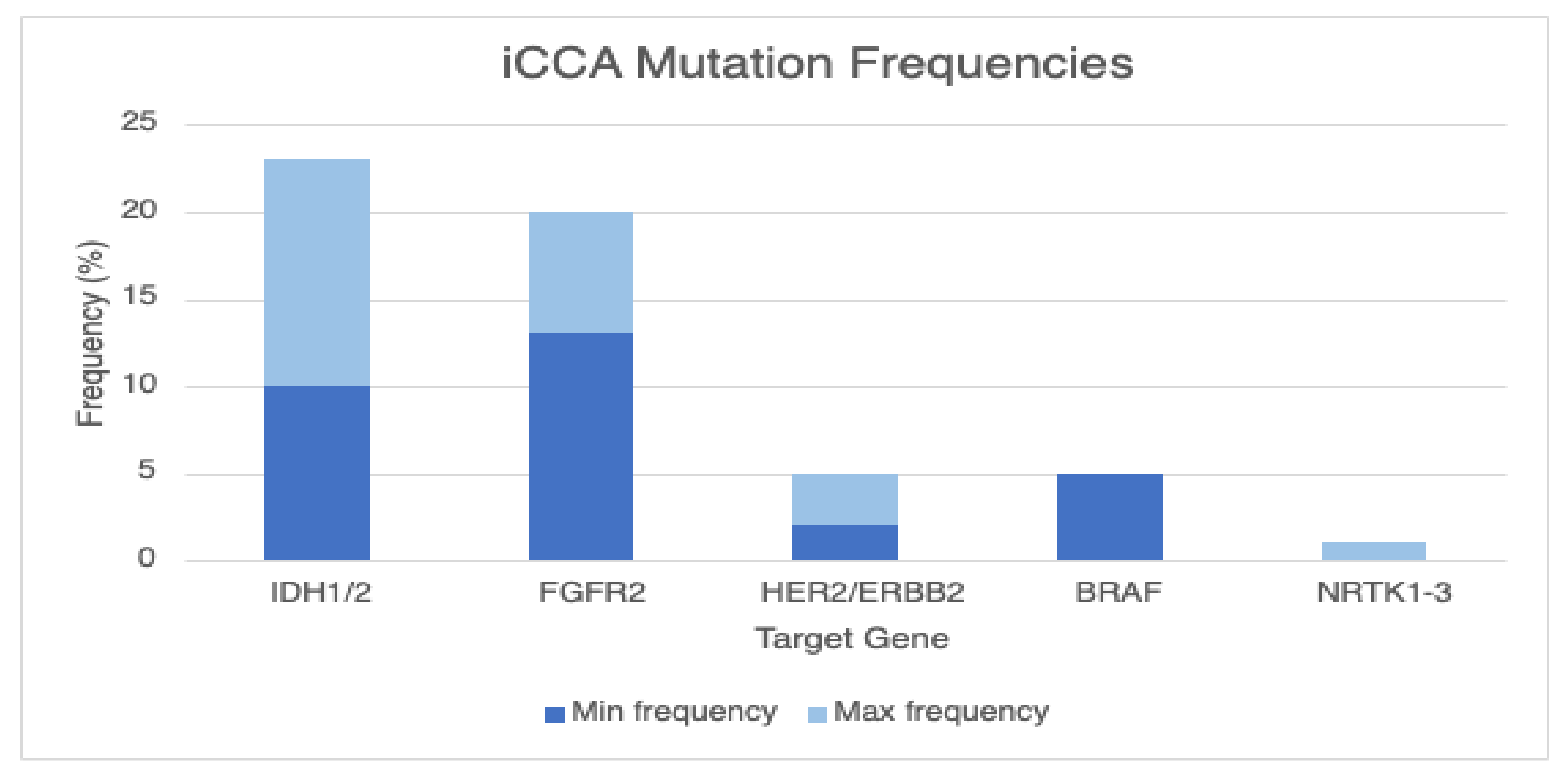

| Target | Agent | Clinical Trial | Primary Outcome |
|---|---|---|---|
| IDH1 | Ivosidenib | ClarIDHy (NCT02989857), Phase 3 | PFS: median 2.7 months [95% CI 1.6–4.2] vs. 1.4 months [1.4–1.6] |
| FGFR2 | Pemigatinib | FIGHT-202 (NCT02924376), Phase 3 | ORR: 35.5% [95% CI 26.5–45.4] |
| Infigratinib | NCT02150967 (not complete) | ORR: 23.1% [95% CI 15.6–32.2] | |
| MSI-high, MMR-deficient, or TMB-H | Pembrolizumab | KEYNOTE-028 (NCT02054806), Phase 1b, PDL-1 positive tumors only | ORR: 13.0% (3/23; 95% CI, 2.8–33.6%) |
| KEYNOTE-158 (NCT02628067), Phase 2 (not complete) | ORR: 5.8% (6/104; 95% CI, 2.1–12.1%) | ||
| ORR of PD-L1-positive: 6.6% (95% CI: 1.8–15.9%) | |||
| Basket TMB-H group: ORR: 29% (95% CI 21–39) | |||
| CCA subgroup ORR: 40.9% (95% CI, 20.7–63.6%) | |||
| BRAF | dabrafenib and trametinib | ROAR trial (NCT 02034110), Phase 2 | ORR: 47% (20/43; 95% CI 36–67%) |
| Subprotocol H trial (EAY131-H) | ORR: 38% (90% CI, 22.9% to 54.9%) | ||
| NTRK | Entrectinib * | STARTRK-1(NCT02097810) (basket Phase 1) | ORR: 57% (31/54; 95% CI 43.2–70.8%) |
| STARTRK-2 (NCT02568267) (basket Phase 2) | |||
| Larotrectinib * | NCT02122913, NCT02637687, and NCT02576431 | ORR: 75% (95% confidence interval [CI], 61 to 85) |
| Target | Agents | Clinical Trial | Notes |
|---|---|---|---|
| IDH1 | Ivosidenib + Gemcitabine + cisplatin | NCT04088188, phase I | |
| Ivosidenib | NCT02073994 | ||
| LY3410738 ± (Gemcitabine + cisplatin) or durvalumab | NCT04521686, phase I | LY3410738 is a potent, selective, and covalent inhibitor of IDH1-R132 | |
| AG-120 (oral Ivosidenib) | NCT02073994, phase I | ||
| IDH305 | NCT02381886, phase I | For IDH1R132-mutant tumors only | |
| FGFR2 | oral infigratinib or gemcitabine + cisplatin | NCT03773302, phase III | |
| futibatinib or gemcitabine + cisplatin | NCT04093362 phase III | ||
| derazantinib | NCT03230318, phase II | Derazantinib is a potent FGFR1‒3 kinase inhibitor | |
| atezolizumab + derazantinib | NCT03230318 *, phase II | ||
| E7090 | NCT04238715 *, phase II | E7090 is a selective tyrosine kinase inhibitor against FGFR1-3 | |
| Pemigatinib or Gemcitabine + Cisplatin | NCT03656536 (FIGHT-302), phase III | ||
| RLY-4008 | NCT04526106 (REFOCUS), phase I and II | RLY-4008 is a potent and highly selective FGFR2 inhibitor | |
| KIN-3248 | NCT05242822, phase I | KIN-3248 is an oral small molecule FGFR inhibitor | |
| Futibatinib | NCT02052778, phase I and II | ||
| Bemarituzumab | NCT05325866, phase I | Bemarituzumab (FPA144) is a humanized, afucosylated immunoglobulin G1 monoclonal antibody (mAb) against FGFR2b | |
| BRAF | ABM-1310 | NCT05501912 *, phase I | |
| ABM-1310 ± cobimetinib | NCT04190628, phase I | Cobimetinib is a MEK inhibitor approved for the treatment of advanced melanoma | |
| NTRK | Entrectinib | NCT02568267, phase I | Entrectinib is a pan-TrkA/B/C, ROS1, and ALK inhibitor |
| HER2 | tucatinib + trastuzumab ± (pembrolizumab or FOLFOX or CAPOX) | NCT04430738, phase I and II | tucatinib is a selective tyrosine kinase inhibitor of HER2 |
| A166 | NCT03602079, phase I and II | A166 is an antibody-drug conjugate composed of a novel cytotoxic drug (Duo-5, anti-microtubule agent) | |
| CT-0508 | NCT04660929, phase I | CT-0508 is a biologic composed of adenovirally-transduced autologous macrophages containin Anti-HER2 Chimeric Antigen Receptor (CAR macrophages) | |
| tebotelimab ± margetuximab | NCT03219268, phase I | Tebotelimab is a DART molecule designed to bind PD-1 and LAG-3. Margetuximab is an anti-HER2 antibody. | |
| Zanidatamab | NCT04466891 (HERIZON-BTC-01), phase II | ||
| Zanidatamab + XELOX or (FOLFOX ± bevacizumab) or (cisplatin + Gemcitabine or 5-FU) | NCT03929666 | ||
| DB-1303 | NCT05150691, phase I and II | DB-1303 is an antibody-drug conjugate |
| Monotherapy | ||||
|---|---|---|---|---|
| Target | Agent | Clinical Trial | Response Rate | OS and PFS |
| PD-1, MSI-high, MMR-deficient, or TMB-H | Pembrolizumab | KEYNOTE-028 (NCT02054806), Phase 1b, PDL-1 positive tumors only | ORR: 13.0% (3/23; 95% CI, 2.8–33.6%) | OS: 5.7(95% CI, 3.1–9.8) months; PFS: 1.8 (95% CI, 1.4–3.1) months |
| Nivolumab | NCT02829918 | ORR: 11% | OS: 14.2 (95% CI, 5.98 to not reached) months; PFS 3.68 (95% CI, 2.30–5.69) months | |
| JapicCTI-153098 | ORR: 3% | OS: 5.2 (90% CI, 4.5–8.7) months; PFS 1.4 (90% CI 1.4–1.4) months | ||
| PD-L1 | Durvalumab | NCT01938612 | ORR: 4.8% | OS: 8.1 (95% CI, 5.6–10.1) months; PFS 2 months |
| Dual Immunotherapy | ||||
| PD-L1 and CTLA4 | Nivolumab and Ipilimumab | CA209-538 | ORR: 23% | OS: 5.7 months; PFS 2.9 months |
| Durvalumab and Tremelimumab | NCT01938612 | ORR 10.8% | OS: 10.1 months | |
| PD-L1 and TGF-β RII | Bintrafusp alfa | NCT02699514 | ORR: 20% | OS: 12.5 months; PFS 2.5 months |
| NCT03833661 | ORR: 10.1% | PFS 1.8 (95% CI 1.7–1.8) months, OS of 7.6 (95% CI: 4.8–0.5) months. | ||
| PD-1 and | PEG-intron | NCT02982720 | Terminated early | |
| ICI and chemo | ||||
| PD-1 | Nivolumab and gem/cis | JapicCTI-153098 | ORR: 37% | OS: 15.4 months; PFS 4.2 months |
| NCT03311789 | ORR 55.6% | OS: 8.5 months; PFS 6.1 months | ||
| Camrelizumab, gemcitabine/oxaliplatin | NCT03486678 | ORR: 54% | OS: 11.8 months; PFS 6.1 months | |
| Camrelizumab, gemcitabine/oxaliplatin or FOLFOX | NCT03092895 | ORR: 16.3% | OS: 12.4 months; PFS 5.3 months | |
| Toripalimab, gemcitabine/S-1 | NCT03796429 | ORR: 27% | OS: 16 months; PFS 7 months | |
| Pembrolizumab and CAPOX | NCT03111732 | ORR: 27.3% | OS: 6.1 (95% CI 3.1–9.4); PFS: 4.5 (95% CI 2.5 to 9.6) months | |
| PD-L1 | Durvalumab, gem/cis | NCT03046862 | ORR: 73.3% | OS: 18.1 months; PFS 11 months |
| CTLA4 | PEGCISGEM ± Atezolizumab | NCT03267940 | AEs: 100% in both | none (early termination) |
| PD-L1 and CTLA4 | Durvalumab + gem/cis ± tremelimumab | NCT03046862 | ORR: 73.4% | OS: 20.7 months; PFS 11.9 months |
| Other combinations | ||||
| PD-1 and VEGFR2 | Pembrolizumab and Ramucirumab | NCT02443324 | ORR: 4% | OS: 6.4 months; PFS 1.6 months |
| PD-1 and tyrosine kinase | Pembrolizumab/nivolumab and Ramucirumab | NCT03892577 | ORR: 30% | OS: 11 months; PFS 5 months |
| PD-1 and FGFR2 | Pembrolizumab and pemigatinib (dose escalation) | NCT02393248 | - | |
| PD-1 and benzamide histone deacetylase | Nivolimumab and Entinostat | NCT03250273 | ORR: 0% | OS: 6.378 (95% CI 3.748-NR at 36 months) months |
| PD-1 and cell-based | Pembrolizumab and CD8+ T-cells | NCT02757391 | - | |
| PD-L1 | Durvalumab, tremelimumab, radiotherapy | NCT03482102 | ORR: 25% |
| Immunotherapy | ClinicalTrials.gov ID | Agents Used | Study Phase | Number Enrolled |
|---|---|---|---|---|
| Pembrolizumab | NCT04550624 * | Pembrolizumab + Lenvatinib | Phase 2 | 40 |
| NCT04306367 | Pembrolizumab + Olaparib | Phase 2 | 29 | |
| NCT05220722 | Pembrolizumab/Nivolumab/Ipilimumab + Pressure Enabled Delivery of SD-101 | Phase 1, 2 | 89 | |
| NCT03895970 * | Pembrolizumab + Lenvatinib | Phase 2 | 50 | |
| NCT03781934 * | Pembrolizumab/Lenvatinib + MIV-818 | Phase 1, 2 | 102 | |
| NCT02628067 | Pembrolizumab (MK-3475-158/KEYNOTE-158) | Phase 2 | 1595 | |
| NCT04430738 | (Tucatinib + trastuzumab) ± pembrolizumab ± (FOLFOX or CAPOX) in HER2+ cancers | Phase 1, 2 | 120 | |
| NCT05215574 | NGM831 ± Pembrolizumab | Phase 1 | 79 | |
| NCT04913337 | NGM707 ± Pembrolizumab | Phase 1, 2 | 179 | |
| NCT03849469 | XmAb®22841 ± Pembrolizumab | Phase 1 | 242 | |
| NCT05007106 | Pembrolizumab ± Vibostolimab ± (5-Fluorouracil + Cisplatin) ± Paclitaxel | Phase 2 | 480 | |
| NCT03329950 | CDX-1140 (CD40) ± (Pembrolizumab or CDX-301) | Phase 1 | 260 | |
| NCT03058289 | Intratumorally Dosed INT230-6 (cisplatin and vinblastine sulfate) ± (pembrolizumab or ipilimumab) | Phase 1, 2 | 180 | |
| NCT03872947 | TRK-950 + (Gemcitabine + Cisplatin or Pembrolizumab) | Phase 1 | 181 | |
| NCT04924062 * | Gemcitabine/Cisplatin ± Pembrolizumab (MK-3475-966/KEYNOTE-966)-China Extension Study | Phase 3 | 160 | |
| NCT04003636 | Gemcitabine/Cisplatin ± Pembrolizumab (MK-3475) (MK-3475-966/KEYNOTE-966) | Phase 3 | 1048 | |
| NCT04157985 | PD-1/PD-L1 Inhibitors (pembrolizu/nivolimu/atezolimu/ipilimu/cemiplimab) 1-year vs. more | Phase 3 | 578 | |
| Nivolumab | NCT05220722 | Pembrolizumab/Nivolumab/Ipilimumab + Pressure Enabled Delivery of SD-101 | Phase 1, 2 | 89 |
| NCT03829436 | TPST-1120 ± Nivolumab | Phase 1 | 138 | |
| NCT02834013 | Nivolumab ± Ipilimumab | Phase 2 | 818 | |
| NCT03684811 | FT 2102 ± Nivolumab ± (Gemcitabine + Cisplatin) | Phase 1|Phase 2 | 200 | |
| NCT04648319 * | Nivolumab + SBRT | Phase 2 | 40 | |
| NCT03872947 | TRK-950 + (Gemcitabine + Cisplatin) or Nivolumab or Pembrolizumab | Phase 1 | 181 | |
| NCT04157985 | PD-1/PD-L1 Inhibitors (pembrolizu/nivolimu/atezolimu/ipilimu/cemiplimab) 1-year vs. more | Phase 3 | 578 | |
| Durvalumab | NCT04301778 | Durvalumab + CSF-1R Inhibitor (SNDX-6532) | Phase 2 | 30 |
| NCT04989218 | Durvalumab + Tremelimumab + Gem + Cis | Phase 1|Phase 2 | 20 | |
| NCT04238637 * | Y-90 SIRT + Durvalimumab ± Tremelimumab | Phase 2 | 50 | |
| NCT03991832 * | Olaparib + Durvalumab in IDH-Mutated Solid tumors | Phase 2 | 78 | |
| NCT04298008 * | AZD6738 + Durvalumab | Phase 2 | 26 | |
| NCT04308174 * | Neoadjuvant Gem + Cis ± Durvalumab in Resectable Biliary Tract Cancer | Phase 2 | 45 | |
| NCT03257761 | Guadecitabine + Durvalumab | Phase 1 | 55 | |
| NCT04298021 * | AZD6738 + Durvalumab or Olaparib | Phase 2 | 74 | |
| NCT03473574 * | Durvalumab + Tremelimumab + Gemcitabine ± Cisplatin | Phase 2 | 128 | |
| NCT02821754 | Durvalumab + Tremelimumab ± TACE or RFA or Cryo | Phase 2 | 53 | |
| Atezolizumab | NCT05174650 | Atezolizumab + Derazantinib | Phase 2 | 37 |
| NCT03201458 | Atezolizumab ± Cobimetinib | Phase 2 | 76 | |
| NCT05211323 | Gem + Cs ± (Bevacizumab + Atezolizumab) | Phase 2 | 88 | |
| NCT04708067 | Hypofractionated Radiation Therapy + Bintrafusp Alfa | Phase 1 | 15 | |
| NCT04941287 | Atezolizumab + CDX-1127 (Varlilumab) ± Cobimetinib | Phase 2 | 64 | |
| NCT05000294 | Atezolizumab + Tivozanib | Phase 1|Phase 2 | 29 | |
| NCT04989218 | Durvalumab + Tremelimumabwith Platinum-based Chemotherapy in Intrahepatic Cholangiocarcinoma (ICC) | Phase 1|Phase 2 | 20 | |
| NCT04157985 | PD-1/PD-L1 Inhibitors (pembrolizu/nivolimu/atezolimu/ipilimu/cemiplimab) 1-year vs. more | Phase 3 | 578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Esmail, A.; Mazzaferro, V.; Abdelrahim, M. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers 2022, 14, 5074. https://doi.org/10.3390/cancers14205074
Zhang Y, Esmail A, Mazzaferro V, Abdelrahim M. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers. 2022; 14(20):5074. https://doi.org/10.3390/cancers14205074
Chicago/Turabian StyleZhang, Yuqi, Abdullah Esmail, Vincenzo Mazzaferro, and Maen Abdelrahim. 2022. "Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision" Cancers 14, no. 20: 5074. https://doi.org/10.3390/cancers14205074
APA StyleZhang, Y., Esmail, A., Mazzaferro, V., & Abdelrahim, M. (2022). Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers, 14(20), 5074. https://doi.org/10.3390/cancers14205074








