Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Lifestyle Risk Factors
1.2. Anatomical Risk Factors
1.3. Geographical Risk Factors
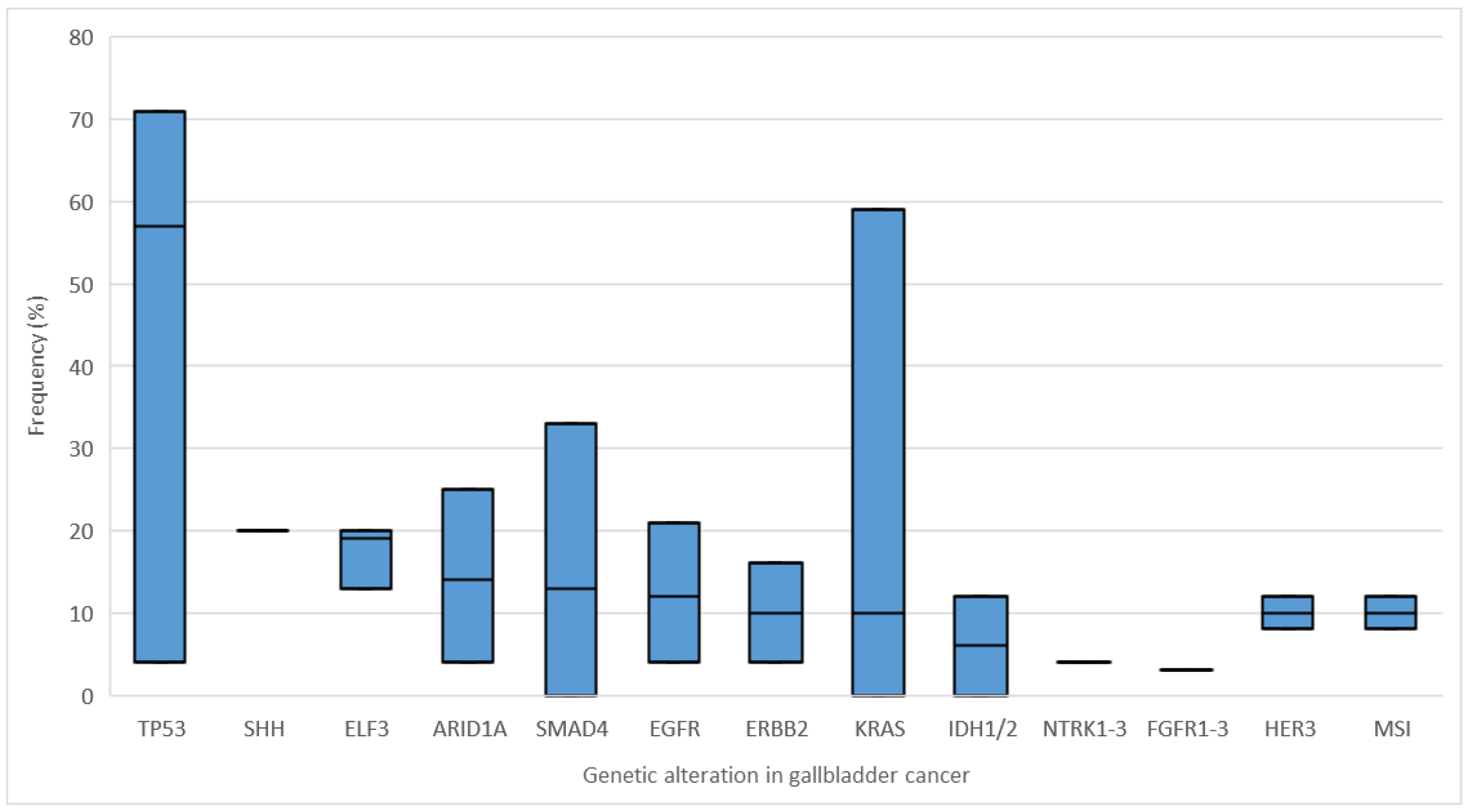
1.4. Genetic Alterations
2. Therapy
2.1. Neoadjuvant Therapy
2.2. Surgery
2.3. Adjuvant Therapy
2.4. Palliative Therapy
2.5. Targeted Therapy and Future Perspectives
2.5.1. HER2/Neu (ErbB2)
2.5.2. VEGF/VEGFR and Antiangiogenetic Therapy
2.5.3. EGFR (ErbB1; HER1)
2.5.4. MAPK (RAS/RAF/MEK/ERK) Pathway
2.5.5. PI3K/AKT/mTOR Pathway
2.5.6. PD-1/PD-L1, MSI-High, High TMB and ICI Therapy
2.5.7. DNA Damage Repair (DDR) Deficiency
2.5.8. Other Molecular Alterations and Future Perspectives of Targeted Therapy in GBC
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benavides, M.; Antón, A.; Gallego, J.; Gómez, M.A.; Jiménez-Gordo, A.; La Casta, A.; Laquente, B.; Macarulla, T.; Rodríguez-Mowbray, J.R.; Maurel, J. Biliary tract cancers: SEOM clinical guidelines. Clin. Transl. Oncol. 2015, 17, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Goetze, T.O. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J. Gastroenterol. 2015, 21, 12211–12217. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e194–e203. [Google Scholar] [CrossRef]
- Ethun, C.G.; Le, N.; Lopez-Aguiar, A.G.; Pawlik, T.M.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; Krasnick, B.A.; et al. Pathologic and prognostic implications of incidental versus nonincidental gallbladder cancer: A 10-institution study from the United States extrahepatic biliary malignancy consortium. In Proceedings of the American Surgeon. Am. Surg. 2017, 83, 679–686. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Marcano-Bonilla, L.; Roberts, L.R. Gallbladder cancer: Epidemiology and genetic risk associations. Chin. Clin. Oncol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Zhu, A.X.; Hong, T.S.; Hezel, A.F.; Kooby, D.A. Current Management of Gallbladder Carcinoma. Oncologist 2010, 15, 168–181. [Google Scholar] [CrossRef]
- Fairweather, M.; Balachandran, V.P.; D’Angelica, M.I. Surgical management of biliary tract cancers. Chin. Clin. Oncol. 2016, 5, 63. [Google Scholar] [CrossRef]
- Randi, G.; Malvezzi, M.; Levi, F.; Ferlay, J.; Negri, E.; Franceschi, S.; La Vecchia, C. Epidemiology of biliary tract cancers: An update. Ann. Oncol. 2009, 20, 146–159. [Google Scholar] [CrossRef]
- Lai, C.H.E.; Lau, W.Y. Gallbladder cancer—A comprehensive review. Surgeon 2008, 6, 101–110. [Google Scholar] [CrossRef]
- Li, Z.M.; Wu, Z.X.; Han, B.; Mao, Y.Q.; Chen, H.L.; Han, S.F.; Xia, J.L.; Wang, L.S. The association between BMI and gallbladder cancer risk: A meta-analysis. Oncotarget 2016, 7, 43669–43679. [Google Scholar] [CrossRef] [PubMed]
- Marcano-Bonilla, L.; Mohamed, E.A.; Mounajjed, T.; Roberts, L.R. Biliary tract cancers: Epidemiology, molecular pathogenesis and genetic risk associations. Chin. Clin. Oncol. 2016, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Maringhini, A.; Moreau, J.A.; Melton, L.J.; Hench, V.S.; Zinsmeister, A.R.; DiMagno, E.P. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann. Intern. Med. 1987, 107, 30–35. [Google Scholar] [CrossRef]
- Vitetta, L.; Sali, A.; Little, P.; Mrazek, L. Gallstones and gall bladder carcinoma. ANZ J. Surg. 2000, 70, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case–control study and meta-analysis. Cancer Med. 2016, 5, 3235–3310. [Google Scholar] [CrossRef]
- Said, K.; Glaumann, H.; Bergquist, A. Gallbladder disease in patients with primary sclerosing cholangitis. J. Hepatol. 2008, 48, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Kuruma, S.; Chiba, K.; Tabata, T.; Koizumi, S.; Kikuyama, M. Biliary carcinogenesis in pancreaticobiliary maljunction. J. Gastroenterol. 2017, 52, 158–163. [Google Scholar] [CrossRef]
- Funabiki, T.; Matsubara, T.; Miyakawa, S.; Ishihara, S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbeck Arch. Surg. 2009, 394, 159–169. [Google Scholar] [CrossRef]
- Lazcano-Ponce, E.C.; Miquel, J.F.; Munoz, N.; Herrero, R.; Ferrecio, C.; Wistuba, I.I.; Alonso de Ruiz, P.; Aristi Urista, G.; Nervi, F. Epidemiology and Molecular Pathology of Gallbladder Cancer. CA Cancer J. Clin. 2001, 51, 349–364. [Google Scholar] [CrossRef]
- Kuipers, H.; de Bitter, T.J.J.; de Boer, M.T.; van der Post, R.S.; Nijkamp, M.W.; de Reuver, P.R.; Fehrmann, R.S.N.; Hoogwater, F.J.H. Gallbladder cancer: Current insights in genetic alterations and their possible therapeutic implications. Cancers 2021, 13, 5257. [Google Scholar] [CrossRef]
- Dixit, R.; Pandey, M.; Tripathi, S.K.; Dwivedi, A.N.D.; Shukla, V.K. Comparative Analysis of Mutational Profile of Sonic hedgehog Gene in Gallbladder Cancer. Dig. Dis. Sci. 2017, 62, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kumari, N.; Krishnani, N. Molecular pathogenesis of gallbladder cancer: An update. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2019, 816–818, 111674. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Aloia, T.A.; Járufe, N.; Javle, M.; Maithel, S.K.; Roa, J.C.; Adsay, V.; Coimbra, F.J.F.; Jarnagin, W.R. Gallbladder Cancer: Expert consensus statement. Hpb 2015, 17, 681–690. [Google Scholar] [CrossRef]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar]
- Nemunaitis, J.M.; Brown-Glabeman, U.; Soares, H.; Belmonte, J.; Liem, B.; Nir, I.; Phuoc, V.; Gullapalli, R.R. Gallbladder cancer: Review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017, 23, 3978–3998. [Google Scholar] [CrossRef]
- Goldstein, D.; Lemech, C.; Valle, J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017, 2, e000152. [Google Scholar] [CrossRef]
- Voesch, S.; Bitzer, M.; Blödt, S.; Follmann, M.; Freudenberger, P.; Langer, T.; Lorenz, P.; Jansen, P.L.; Steubesand, N.; Galle, P.; et al. S3-Leitlinie: Diagnostik und Therapie des hepatozellulären Karzinoms und biliärer Karzinome—Version 2.0—Juni 2021, AWMF-Registernummer: 032-053OL. Z. Gastroenterol. 2022, 60, E131–E185. [Google Scholar] [CrossRef]
- Sharma, A.; Kalyan Mohanti, B.; Pal Chaudhary, S.; Sreenivas, V.; Kumar Sahoo, R.; Kumar Shukla, N.; Thulkar, S.; Pal, S.; Deo, S.V.; Pathy, S.; et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur. J. Cancer 2019, 123, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Guest, R.V.; Harrison, E.M.; Kendall, T.J.; Garden, O.J.; Wigmore, S.J. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br. J. Surg. 2018, 106, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Coburn, N.G.; Cleary, S.P.; Tan, J.C.C.; Law, C.H.L. Surgery for Gallbladder Cancer: A Population-Based Analysis. J. Am. Coll. Surg. 2008, 207, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Thandra, K.C.; Barsouk, A. Epidemiology of gallbladder cancer. Clin. Exp. Hepatol. 2019, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.R.; Papoulas, M.; Menon, K.V. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer—A systematic review. Eur. J. Surg. Oncol. 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Goetze, T.O.; Bechstein, W.O.; Bankstahl, U.S.; Keck, T.; Königsrainer, A.; Lang, S.A.; Pauligk, C.; Piso, P.; Vogel, A.; Al-Batran, S.E. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy o. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Engineer, R.; Goel, M.; Chopra, S.; Patil, P.; Purandare, N.; Rangarajan, V.; Ph, R.; Bal, M.; Shrikhande, S.; Shrivastava, S.K.; et al. Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Gallbladder Cancers: A New Paradigm. Ann. Surg. Oncol. 2016, 23, 3009–3015. [Google Scholar] [CrossRef]
- Verma, V.; Surkar, S.M.; Brooks, E.D.; Simone, C.B.; Lin, C. Chemoradiotherapy versus Chemotherapy Alone for Unresected Nonmetastatic Gallbladder Cancer: National Practice Patterns and Outcomes. J. Natl. Compr. Cancer Netw. 2018, 16, 59–65. [Google Scholar] [CrossRef]
- Phelip, J.M.; Vendrely, V.; Rostain, F.; Subtil, F.; Jouve, J.L.; Gasmi, M.; Michel, P.; Le Malicot, K.; Smith, D.; Seitz, J.F.; et al. Gemcitabine plus cisplatin versus chemoradiotherapy in locally advanced biliary tract cancer: Fédération Francophone de Cancérologie Digestive 9902 phase II randomised study. Eur. J. Cancer 2014, 50, 2975–2982. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.Y.; Lim, C.S.; Kang, M.J.; Kim, S.W. Systematic review on the surgical treatment for T1 gallbladder cancer. World J. Gastroenterol. 2011, 17, 174–180. [Google Scholar] [CrossRef]
- Liu, C.; Rein, L.; Clarke, C.; Mogal, H.; Tsai, S.; Christians, K.K.; Gamblin, T.C. Comparison of overall survival in gallbladder carcinoma at academic versus community cancer centers: An analysis of the National Cancer Data Base. J. Surg. Oncol. 2020, 122, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, H.; Han, Y.; Hwang, Y.J.; Kim, S.G.; Kwon, H.J.; Vinuela, E.; Járufe, N.; Roa, J.C.; Han, I.W.; et al. Role of tumour location and surgical extent on prognosis in T2 gallbladder cancer: An international multicentre study. Br. J. Surg. 2020, 107, 1334–1343. [Google Scholar] [CrossRef]
- Aoki, T.; Sakamoto, Y.; Kohno, Y.; Akamatsu, N.; Kaneko, J.; Sugawara, Y.; Hasegawa, K.; Makuuchi, M.; Kokudo, N. Hepatopancreaticoduodenectomy for Biliary Cancer. Ann. Surg. 2018, 267, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Ebata, T.; Yokoyama, Y.; Igami, T.; Yamaguchi, J.; Onoe, S.; Watanabe, N.; Ando, M.; Nagino, M. Major hepatectomy with or without pancreatoduodenectomy for advanced gallbladder cancer. Br. J. Surg. 2019, 106, 626–635. [Google Scholar] [CrossRef] [PubMed]
- D’Angelica, M.; Dalal, K.M.; Dematteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann. Surg. Oncol. 2009, 16, 806–816. [Google Scholar] [CrossRef] [PubMed]
- de Savornin Lohman, E.A.J.; van der Geest, L.G.; de Bitter, T.J.J.; Nagtegaal, I.D.; van Laarhoven, C.J.H.M.; van den Boezem, P.; van der Post, C.S.; de Reuver, P.R. Re-resection in Incidental Gallbladder Cancer: Survival and the Incidence of Residual Disease. Ann. Surg. Oncol. 2020, 27, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Toge, K.; Sakata, J.; Hirose, Y.; Yuza, K.; Ando, T.; Soma, D.; Katada, T.; Miura, K.; Takizawa, K.; Kobayashi, T.; et al. Lymphatic spread of T2 gallbladder carcinoma: Regional lymphadenectomy is required independent of tumor location. Eur. J. Surg. Oncol. 2019, 45, 1446–1452. [Google Scholar] [CrossRef]
- Meng, H.; Wang, X.; Fong, Y.; Wang, Z.H.; Wang, Y.; Zhang, Z.T. Outcomes of radical surgery for gallbladder cancer patients with lymphatic metastases. Jpn. J. Clin. Oncol. 2011, 41, 992–998. [Google Scholar] [CrossRef]
- Ito, H.; Ito, K.; D’Angelica, M.; Gonen, M.; Klimstra, D.; Allen, P.; Dematteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Accurate staging for gallbladder cancer: Implications for surgical therapy and pathological assessment. Ann. Surg. 2011, 254, 320–325. [Google Scholar] [CrossRef]
- Vega, E.A.; De Aretxabala, X.; Qiao, W.; Newhook, T.E.; Okuno, M.; Castillo, F.; Sanhueza, M.; Diaz, C.; Cavada, G.; Jarufe, N.; et al. Comparison of oncological outcomes after open and laparoscopic re-resection of incidental gallbladder cancer. Br. J. Surg. 2020, 107, 289–300. [Google Scholar] [CrossRef]
- Tschuor, C.; Pickens, R.C.; Isenberg, E.E.; Motz, B.M.; Salibi, P.N.; Robinson, J.N.; Murphy, K.J.; Iannitti, D.A.; Baker, E.H.; Vrochides, D.; et al. Robotic Resection of Gallbladder Cancer: A Single-Center Retrospective Comparative Study to Open Resection. Am. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Use of indocyanine green (ICG) augmented near-infrared fluorescence imaging in robotic radical resection of gallbladder adenocarcinomas. Surg. Endosc. 2020, 34, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Sahara, K.; Tsilimigras, D.I.; Kikuchi, Y.; Ethun, C.G.; Maithel, S.K.; Abbott, D.E.; Poultsides, G.A.; Hatzaras, I.; Fields, R.C.; Weiss, M.; et al. Defining and Predicting Early Recurrence after Resection for Gallbladder Cancer. Ann. Surg. Oncol. 2021, 28, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Ma, N.; Cheng, H.; Qin, B.; Zhong, R.; Wang, B. Adjuvant therapy in the treatment of gallbladder cancer: A meta-analysis. BMC Cancer 2015, 15, 615. [Google Scholar] [CrossRef]
- Mantripragada, K.C.; Hamid, F.; Shafqat, H.; Olszewski, A.J. Adjuvant therapy for resected gallbladder cancer: Analysis of the national cancer data base. J. Natl. Cancer Inst. 2017, 109, djw202. [Google Scholar] [CrossRef]
- Fong, Z.V.; Brownlee, S.A.; Qadan, M.; Tanabe, K.K. The Clinical Management of Cholangiocarcinoma in the United States and Europe: A Comprehensive and Evidence-Based Comparison of Guidelines. Ann. Surg. Oncol. 2021, 28, 2660–2674. [Google Scholar] [CrossRef]
- Primrose, J.N.; Neoptolemos, J.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Takada, T.; Amano, H.; Yasuda, H.; Nimura, Y.; Matsushiro, T.; Kato, H.; Nagakawa, T.; Nakayama, T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002, 95, 1685–1695. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Al-Rawashdeh, W.; White, S.A.; Abu Hilal, M.; Salti, G.I.; Dahdaleh, F.S. Adjuvant radiotherapy improves long-term survival after resection for gallbladder cancer A population-based cohort study. Eur. J. Surg. Oncol. 2022, 48, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ben-Josef, E.; Guthrie, K.A.; El-Khoueiry, A.B.; Corless, C.L.; Zalupski, M.M.; Lowy, A.M.; Thomas, C.R.; Alberts, S.R.; Dawson, L.A.; Micetich, K.C.; et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J. Clin. Oncol. 2015, 33, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Arnold, D.; Bridgewater, J.; Goldstein, D.; Jensen, L.H.; Klümpen, H.J.; Lohse, A.W.; Nashan, B.; Primrose, J.; Schrum, S.; et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial)—A randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Nakachi, K.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Uesaka, K.; Yanagimoto, H.; Morinaga, S.; Wada, H.; et al. Adjuvant S-1 versus observation in curatively resected biliary tract cancer: A phase III trial (JCOG1202: ASCOT). J. Clin. Oncol. 2022, 40, 382. [Google Scholar] [CrossRef]
- Luvira, V.; Satitkarnmanee, E.; Pugkhem, A.; Kietpeerakool, C.; Lumbiganon, P.; Pattanittum, P. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst. Rev. 2021, 2021, 1465–1858. [Google Scholar] [CrossRef]
- Ji, J.H.; Song, H.N.; Kim, R.B.; Oh, S.Y.; Lim, H.Y.; Park, J.O.; Park, S.H.; Kim, M.J.; Lee, S.I.; Ryou, S.H.; et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn. J. Clin. Oncol. 2015, 45, 256–260. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Dwary, A.D.; Mohanti, B.K.; Deo, S.V.; Pal, S.; Sreenivas, V.; Raina, V.; Shukla, N.K.; Thulkar, S.; Garg, P.; et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: A randomized controlled study. J. Clin. Oncol. 2010, 28, 4581–4586. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D.; On behalf of the ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet. Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Ramaswamy, A.; Ostwal, V.; Sharma, A.; Bhargava, P.; Srinivas, S.; Goel, M.; Patkar, S.; Mandavkar, S.; Jadhav, P.; Parulekar, M.; et al. Efficacy of Capecitabine plus Irinotecan vs. Irinotecan Monotherapy as Second-line Treatment in Patients with Advanced Gallbladder Cancer: A Multicenter Phase 2 Randomized Clinical Trial (GB-SELECT). JAMA Oncol. 2021, 7, 436–439. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, K.H.; Lee, J.H.; Suh, K.J.; Kim, J.W.; Park, J.H.; Kim, Y.J.; Kim, J.S.; Kim, J.H.; Kim, J.W. A randomised phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5-FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. Eur. J. Cancer 2021, 154, 288–295. [Google Scholar] [CrossRef]
- Azizi, A.A.; Lamarca, A.; McNamara, M.G.; Valle, J.W. Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103328. [Google Scholar] [CrossRef]
- Williams, K.J.; Picus, J.; Trinkhaus, K.; Fournier, C.C.; Suresh, R.; James, J.S.; Tan, B.R. Gemcitabine with carboplatin for advanced biliary tract cancers: A phase II single institution study. Hpb 2010, 12, 418–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Julka, P.K.; Puri, T.; Rath, G.K. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 110–114. [Google Scholar]
- Alberts, S.R.; Al-Khatib, H.; Mahoney, M.R.; Burgart, L.; Cera, P.J.; Flynn, P.J.; Finch, T.R.; Levitt, R.; Windschitl, H.E.; Knost, J.A.; et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: A north central cancer treatment group phase II trial. Cancer 2005, 103, 111–118. [Google Scholar] [CrossRef]
- Alberts, S.R.; Fishkin, P.A.; Burgart, L.J.; Cera, P.J.; Mahoney, M.R.; Morton, R.F.; Johnson, P.A.; Nair, S.; Goldberg, R.M. CPT-11 for bile-duct and gallbladder carcinoma: A phase II North Central Cancer Treatment Group (NCCTG) study. Int. J. Gastrointest. Cancer 2002, 32, 107–114. [Google Scholar] [CrossRef]
- Iqbal, S.; Rankin, C.; Lenz, H.J.; Gold, P.J.; Ahmad, S.A.; El-Khoueiry, A.B.; Messino, M.J.; Holcombe, R.F.; Blanke, C.D. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother. Pharmacol. 2011, 68, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.R.; Sande, J.R.; Foster, N.R.; Quevedo, F.J.; McWilliams, R.R.; Kugler, J.W.; Fitch, T.R.; Jaslowski, A.J. Pemetrexed and gemcitabine for biliary tract and gallbladder carcinomas: A north central cancer treatment group (NCCTG) phase I and II trial, N9943. J. Gastrointest. Cancer 2007, 38, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ocean, A.J.; Christos, P.; Sparano, J.A.; Matulich, D.; Kaubish, A.; Siegel, A.; Sung, M.; Ward, M.M.; Hamel, N.; Espinoza-Delgado, I.; et al. Phase II trial of the ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehydethiosemicarbazone plus gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2011, 68, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pramanik, R.; Kumar, A.; Pathy, S.; Kumar, S.; Bhoriwal, S.; Thulkar, S.; Dash, N.R.; Pal, S.; Choudhary, P.; et al. Safety and Efficacy of Modified FOLFIRINOX in Unresectable or Metastatic Gallbladder Cancer: A Phase II Pilot Study. JCO Glob. Oncol. 2021, 7, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX versus CISGEM Chemotherapy for Patients with Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef]
- Phelip, J.M.; Edeline, J.; Blanc, J.F.; Barbier, E.; Michel, P.; Bourgeois, V.; Neuzillet, C.; Malka, D.; Manfredi, S.; Desrame, J. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig. Liver Dis. 2019, 51, 318–320. [Google Scholar] [CrossRef]
- Pollom, E.L.; Alagappan, M.; Park, L.S.; Whittemore, A.S.; Koong, A.C.; Chang, D.T. Does radiotherapy still have a role in unresected biliary tract cancer? Cancer Med. 2017, 6, 129–141. [Google Scholar] [CrossRef]
- Schepis, T.; Boškoski, I.; Tringali, A.; Bove, V.; Costamagna, G. Palliation in Gallbladder Cancer: The Role of Gastrointestinal Endoscopy. Cancers 2022, 14, 1686. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Ortega-Cava, C.; Raja, S.; Laiq, Z.; Bailey, T.; Luan, H.; Mohapatra, B.; Williams, S.; Ericsson, A.; Goswami, R.; Dimri, M.; et al. Continuous requirement of ErbB2 kinase activity for loss of cell polarity and lumen formation in a novel ErbB2/Neu-driven murine cell line model of metastatic breast cancer. J. Carcinog. 2011, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Neyaz, A.; Husain, N.; Gupta, S.; Kumari, S.; Arora, A.; Awasthi, N.P.; Malhotra, K.P.; Misra, S. Investigation of targetable predictive and prognostic markers in gallbladder carcinoma. J. Gastrointest. Oncol. 2018, 9, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Roa, I.; de Toro, G.; Schalper, K.; de Aretxabala, X.; Churi, C.; Javle, M. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients with Advanced Gallbladder Cancer. Gastrointest. Cancer Res. 2014, 7, 42. [Google Scholar] [PubMed]
- Kiguchi, K.; Carbajal, S.; Chan, K.; Beltrán, L.; Ruffino, L.; Shen, J.; Matsumoto, T.; Yoshimi, N.; DiGiovanni, J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001, 61, 6971–6976. [Google Scholar] [PubMed]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Harding, J.; Piha-paul, S.; Murphy, J.; Cleary, J.; Moreno, V.; Berger, M. Targeting HER2 Mutant Advanced Biliary Tract Cancers with Neratinib: Results from the SUMMIT ‘Basket’ Trial; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Jeong, H.; Jeong, J.H.; Kim, K.P.; Lee, S.S.; Oh, D.W.; Park, D.H.; Song, T.J.; Park, Y.; Hong, S.M.; Ryoo, B.Y.; et al. Feasibility of HER2-targeted therapy in advanced biliary tract cancer: A prospective pilot study of trastuzumab biosimilar in combination with gemcitabine plus cisplatin. Cancers 2021, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Z.; Huang, Y.; Zheng, H.; Sun, Q.; Yang, Q.; Zhang, Y.; Zhang, L.; Wang, W. Pretreatment with gemcitabine/5-fluorouracil enhances the cytotoxicity of trastuzumab to HER2-negative human gallbladder cancer cells in vitro and in vivo. Biomed. Res. Int. 2019, 2019, 9205851. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Futur. Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. 2022, 40, 4006. [Google Scholar] [CrossRef]
- Mody, K.; Baldeo, C.; Bekaii-Saab, T. Antiangiogenic therapy in colorectal cancer. Cancer J. 2018, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bareschino, M.A.; Schettino, C.; Colantuoni, G.; Rossi, E.; Rossi, A.; Maione, P.; Ciardielloi, F.; Gridell, C. The Role of Antiangiogenetic Agents in the Treatment of Breast Cancer. Curr. Med. Chem. 2011, 18, 5022–5032. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.N.; Cao, W.G.; Wang, X.; Wang, Q.; Gu, B.X.; Yang, Q.C.; Bin Hu, J.; Liu, H.; Zheng, S. Prognostic impact of vascular endothelial growth factor-A expression in resected gallbladder carcinoma. Tumour Biol. 2011, 32, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, J.; Jiang, F.; Cai, K.; Ren, G. The effect and mechanism of vascular endothelial growth factor (VEGF) on tumor angiogenesis in gallbladder carcinoma. Iran. J. Public Health 2019, 48, 713–721. [Google Scholar] [CrossRef]
- Singh, P.; Jain, S.L.; Sakhuja, P.; Agarwal, A. Expression of VEGF-A, HER2/neu, and KRAS in gall bladder carcinoma and their correlation with clinico-pathological parameters. Indian J. Pathol. Microbiol. 2021, 64, 687–692. [Google Scholar]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.P.; Horvath, L.; et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II consortium study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A multicenter phase II study of gemcitabine, capecitabine, and bevacizumab for locally advanced or metastatic biliary tract cancer. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2010, 102, 68–72. [Google Scholar] [CrossRef]
- Yi, J.H.; Thongprasert, S.; Lee, J.; Doval, D.C.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Lim, H.Y. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: A multicentre, multinational study. Eur. J. Cancer 2012, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Arrivas Bajardi, E.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2015, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407-e136. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, P.; Chandra, P.; Ticku, S.; Pallavi, B.; Rudresh, K.; Mansabdar, P. Epidermal growth factor receptor: Role in human cancer. Indian J. Dent. Res. 2017, 28, 687–694. [Google Scholar]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Gomes, R.V.; Vidigal, P.T.; Damasceno, K.A.; Rodrigues, M.; Resende, V. Epidermal growth factor receptor (EGFR) in Biliary tract cancer. Hpb 2016, 18, e466. [Google Scholar] [CrossRef][Green Version]
- Barreto, S.G.; Dutt, A.; Chaudhary, A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann. Oncol. 2014, 25, 1086–1097. [Google Scholar] [CrossRef]
- Hadi, R.; Pant, M.C.; Husain, N.; Singhal, A.; Khurana, R.; Agarwal, G.R.; Masood, S.; Awashthi, N.P. EGFR and HER-2/neu Expression in Gallbladder Carcinoma: An Institutional Experience. Gulf J. Oncol. 2016, 1, 12–19. [Google Scholar]
- Zhang, M.; Cai, S.; Zuo, B.; Gong, W.; Tang, Z.; Zhou, D.; Weng, M.; Qin, Y.; Wang, S.; Liu, J.; et al. Arctigenin induced gallbladder cancer senescence through modulating epidermal growth factor receptor pathway. Tumor Biol. 2017, 39, 1010428317698359. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II study of erlotinib in patients with advanced biliary cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.Y.; Micetich, K.C.; Kayaleh, O.R.; Lenz, H.J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yuan, Y.; Ge, W.; Fan, Y.; Liu, X.; Wu, D.; Hu, H. EGFR target therapy combined with GEMOX for advanced biliary tract cancers: A meta-analysis based on RCTs. J. Cancer 2018, 9, 1476–1485. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018, 109, 215–224. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef]
- Goeppert, B.; Frauenschuh, L.; Renner, M.; Roessler, S.; Stenzinger, A.; Klauschen, F.; Warth, A.; Vogel, M.N.; Mehrabi, A.; Hafezi, M.; et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod. Pathol. 2014, 27, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.J.; Weng, H.; Ye, Y.Y.; Hu, Y.P.; Bao, R.F.; Cao, Y.; Wang, X.A.; Zhang, F.; Xiang, S.S.; Li, H.F.; et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roa, I.; Garcia, H.; Game, A.; De Toro, G.; De Aretxabala, X.; Javle, M. Somatic Mutations of PI3K in Early and Advanced Gallbladder Cancer: Additional Options for an Orphan Cancer. J. Mol. Diagn. 2016, 18, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, A.; Webster, K.A.; Papa, A.; Padmani, B.; Clohessy, J.G.; Bronson, R.T.; Pandolfi, P.P. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget 2014, 5, 894–900. [Google Scholar] [CrossRef]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar]
- Costello, B.A.; Borad, M.J.; Qi, Y.; Kim, G.P.; Northfelt, D.W.; Erlichman, C.; Alberts, S.R. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Investig. New Drugs 2014, 32, 710–716. [Google Scholar] [CrossRef]
- Ahn, D.H.; Li, J.; Wei, L.; Doyle, A.; Marshall, J.L.; Schaaf, L.J.; Phelps, M.A.; Villalona-Calero, M.A.; Bekaii-Saab, T. Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci. Rep. 2015, 5, 12122. [Google Scholar] [CrossRef]
- Duan, J.; Wang, Y.; Jiao, S. Checkpoint blockade-based immunotherapy in the context of tumor microenvironment: Opportunities and challenges. Cancer Med. 2018, 7, 4517–4529. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Zhang, F.; Zhou, W.; Jiang, X.; Yang, Y.; Qu, K.; Wang, Y.; Ma, Q.; Wang, T.; et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: A whole-exome sequencing analysis. Gut 2019, 68, 1024–1033. [Google Scholar] [CrossRef]
- Gong, K.; Gong, Z.J.; Lu, P.X.; Ni, X.L.; Shen, S.; Liu, H.; Wang, J.W.; Zhang, D.X.; Liu, H.B.; Suo, T. PLAC8 overexpression correlates with PD-L1 upregulation and acquired resistance to chemotherapies in gallbladder carcinoma. Biochem. Biophys. Res. Commun. 2019, 516, 983–990. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E.; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Piha-Paul, S.A.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019, 37, 4079. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Koyama, T.; Helwig, C.; Watanabe, M.; Doi, T. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in Asian patients with advanced solid tumors. J. Clin. Oncol. 2018, 36, 762. [Google Scholar] [CrossRef]
- Xie, C.; Duffy, A.G.; Mabry-Hrones, D.; Wood, B.; Levy, E.; Krishnasamy, V.; Khan, J.; Wei, J.S.; Agdashian, D.; Tyagi, M.; et al. Tremelimumab in Combination with Microwave Ablation in Patients with Refractory Biliary Tract Cancer. Hepatology 2019, 69, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Abdel-Wahab, R.; Ali, S.M.; Borad, M.J.; Shroff, R.T.; Kwong, L.; Vauthey, J.-N.; Koay, E.J.; Zuo, M.; Rashid, A.; Schrock, A.B.; et al. Variations in DNA repair genomic alterations and tumor mutation burden in biliary tract cancer (BTC) subtypes. J. Clin. Oncol. 2018, 36, 263. [Google Scholar] [CrossRef]
- Guillotin, D.; Martin, S.A. Exploiting DNA mismatch repair deficiency as a therapeutic strategy. Exp. Cell Res. 2014, 329, 110–115. [Google Scholar] [CrossRef]
- Kumari, N.; Shukla, P.; Behari, A.; Kapoor, V. Evaluation of Molecular Targets and Mismatch Repair Deficiency in Gallbladder Cancer. 2021. Available online: https://assets.researchsquare.com/files/rs-528091/v1/4ccb4f09-565b-40d1-97db-3e8836642d82.pdf?c=1634551718 (accessed on 1 November 2022). [CrossRef]
- Javle, M.M.; Catenacci, D.; Jain, A.; Young, L.; Wang, K.; Chung, J.; Hezel, A.F.; Schrock, A.B.; Goyal, L.; Gay, L.M.; et al. Precision medicine for gallbladder cancer using somatic copy number amplifications (SCNA) and DNA repair pathway gene alterations. J. Clin. Oncol. 2017, 35, 4076. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, J.; Li, R.; Han, Z.; Li, L.; Li, G.; Yang, B.; Yin, Q.; Wang, Y.; Ke, Y.; et al. Effectiveness of Olaparib Treatment in a Patient with Gallbladder Cancer with an ATM -Inactivating Mutation. Oncologist 2020, 25, 375–379. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Bonucci, C.; Tober, N.; Palloni, A.; Mollica, V.; Maggio, I.; Deserti, M.; Tavolari, S.; Brandi, G. PARP Inhibitors in Biliary Tract Cancer: A New Kid on the Block? Medicines 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.S.; Park, H.S.; Lee, H.; Pai, R.; Tarnawski, A.S.; Kim, K.R.; Jang, K.Y. Co-Expression of Cox-2, C-Met and β-catenin in Cells Forming Invasive front of Gallbladder Cancer. Cancer Res. Treat. 2005, 37, 171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doi, T.; Yamamoto, N.; Naito, Y.; Kuboki, Y.; Koyama, T.; Piao, Y.; Tsujimoto, N.; Asou, H.; Inoue, K.; Kondo, S. Merestinib monotherapy or in combination for japanese patients with advanced and/or metastatic cancer: A phase 1 study. Cancer Med. 2021, 10, 6579–6589. [Google Scholar] [CrossRef]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochim. Biophys. Acta-Gene Regul. Mech. 2015, 1849, 506–516. [Google Scholar] [CrossRef]
- Ishak, G.; Leal, M.F.; dos Santos, N.P.C.; Demachki, S.; Nunes, C.A.M.; do Nascimento Borges, B.; Calcagno, D.Q.; Smith, M.C.; Assumpção, P.P.; Burbano, R.R. Deregulation of MYC and TP53 through genetic and epigenetic alterations in gallbladder carcinomas. Clin. Exp. Med. 2015, 15, 421–426. [Google Scholar] [CrossRef]
- Ooi, A.; Suzuki, S.; Nakazawa, K.; Itakura, J.; Imoto, I.; Nakamura, H.; Dobashi, Y. Gene amplification of Myc and its coamplification with ERBB2 and EGFR in gallbladder adenocarcinoma. Anticancer Res. 2009, 29, 19–26. [Google Scholar]
- Massó-Vallés, D.; Beaulieu, M.E.; Soucek, L. MYC, MYCL, and MYCN as therapeutic targets in lung cancer. Expert Opin. Ther. Targets 2020, 24, 101–114. [Google Scholar] [CrossRef]
| NCT Number | Study Phase | Condition | Study Size | Treatment Agent | Primary End Point | Institution | Completition |
|---|---|---|---|---|---|---|---|
| NCT03673072 | III | Incidental GBC and BTC | 300 | Gemcitabine + cisplatin perioperative vs. adjuvant | OS | Krankenhaus Nordwest, Germany | November 2024 |
| NCT02867865 | II/III | GBC | 314 | Gemcitabine + cisplatin alone vs. RT + gemcitabine + cisplatin | OS | Tata Memorial Hospital, India | August 2022 |
| NCT04308174 | II | BTC | 45 | Gemcitabine + cisplatin vs. gemcitabine + cisplatin + durvalumab | R0 resection rate | Asan Medical Center, Korea | December 2023 |
| NCT04559139 | II/III | GBC | 186 | Gemcitabine + cisplatin perioperative vs. adjuvant | OS | Emory University, Winship Cancer Institute, United States | September 2023 |
| NCT04480190 | I | BTC | 12 | Gemcitabine + cisplatin followed by RCT (5FU + RT) | Therapy completion | University of Cincinnati Medical Center, United States | February 2029 |
| NCTN | Phase | Condition | Study Size | Substance | Results | Reference |
|---|---|---|---|---|---|---|
| NCT00660140 | II | BTC + GBC | 49 | gemcitabine + carboplatin | PFS 7.8 months, OS 10.6 months | [77] |
| Not applicable | II | GBC | 20 | gemcitabine + carboplatin | ORR 36.7%, PFS 33.8 weeks | [78] |
| NCT00009893 | II | BTC + GBC | 42 | gemcitabine + 5FU + leucovorin | PFS 4.6 months, OS 9.7 months | [79] |
| NCT00003276 | II | BTC + GBC | 39 | irinotecan | ORR 8% | [80] |
| NCT00033540 | II | BTC + GBC | 57 | gemcitabine + capecitabin | ORR 25%, OS 7 months | [81] |
| NCT00059865 | II | BTC + GBC | 63 | gemcitabine + pemetrexed | No benefit of combined regimen compared to gemcitabine | [82] |
| NCT00075504 | II | BTC + GBC | 33 | triapine + gemcitabine | ORR 9%; no benefit with triapine | [83] |
| Molecular Alteration | Frequency | Therapeutic Agents |
|---|---|---|
| HER2/Neu overexpression/amplification | 9.8–27.3% | trastuzumab, lapatinib, neratinib, pertuzumab, afatinib, tucatinib |
| VEGF high expression | 48% | bevacizumanb, sorafenib, sunitinib, ramucirumab, vandetanib |
| EGFR overexpression | 44–77% | erlotinib, cetuximab, panitumumab, gefitinib, afatinib, dacomitinib, osimertinib, olmutinib |
| MAPK pathway alteration | Up to 45% | trametinib, selumetinib, sorafenib, tipifamib |
| PI3K/AKT/mTOR pathway alterations | 10% | everolimus |
| MSI-high/high TMB/high PD-L1 expression | 12% | pembrolizumab, nivolumab, durvalumab, tremelimumab |
| DDR deficiency | Up to 27% | olaparib, niraparib, rucaparib |
| IDH1/IDH2 mutation | 2% | ivosidenib |
| NTRK fusion | 4% | entrectinib, larotrectinib |
| FGFR2 translocation | 3% | pemigatinib, infigratinib, derazantinib, erdafitinib |
| c-MET overexpression | Up to 74% | small molecules targeting MET receptors (e.g., crizotinib, tivantinib, savolitinib, tepotinib, cabozantinib, and foretinib), MET receptor monoclonal antibodies (e.g., onartuzumab), and antibodies against HGF (e.g., ficlatuzumab, rilotumumab) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturm, N.; Schuhbaur, J.S.; Hüttner, F.; Perkhofer, L.; Ettrich, T.J. Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions. Cancers 2022, 14, 5580. https://doi.org/10.3390/cancers14225580
Sturm N, Schuhbaur JS, Hüttner F, Perkhofer L, Ettrich TJ. Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions. Cancers. 2022; 14(22):5580. https://doi.org/10.3390/cancers14225580
Chicago/Turabian StyleSturm, Niklas, Jasmin Selina Schuhbaur, Felix Hüttner, Lukas Perkhofer, and Thomas Jens Ettrich. 2022. "Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions" Cancers 14, no. 22: 5580. https://doi.org/10.3390/cancers14225580
APA StyleSturm, N., Schuhbaur, J. S., Hüttner, F., Perkhofer, L., & Ettrich, T. J. (2022). Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions. Cancers, 14(22), 5580. https://doi.org/10.3390/cancers14225580






