Myelodysplastic Syndromes with Isolated del(5q): Value of Molecular Alterations for Diagnostic and Prognostic Assessment
Abstract
Simple Summary
Abstract
1. Introduction
2. From “5q- Syndrome” to MDS-5q
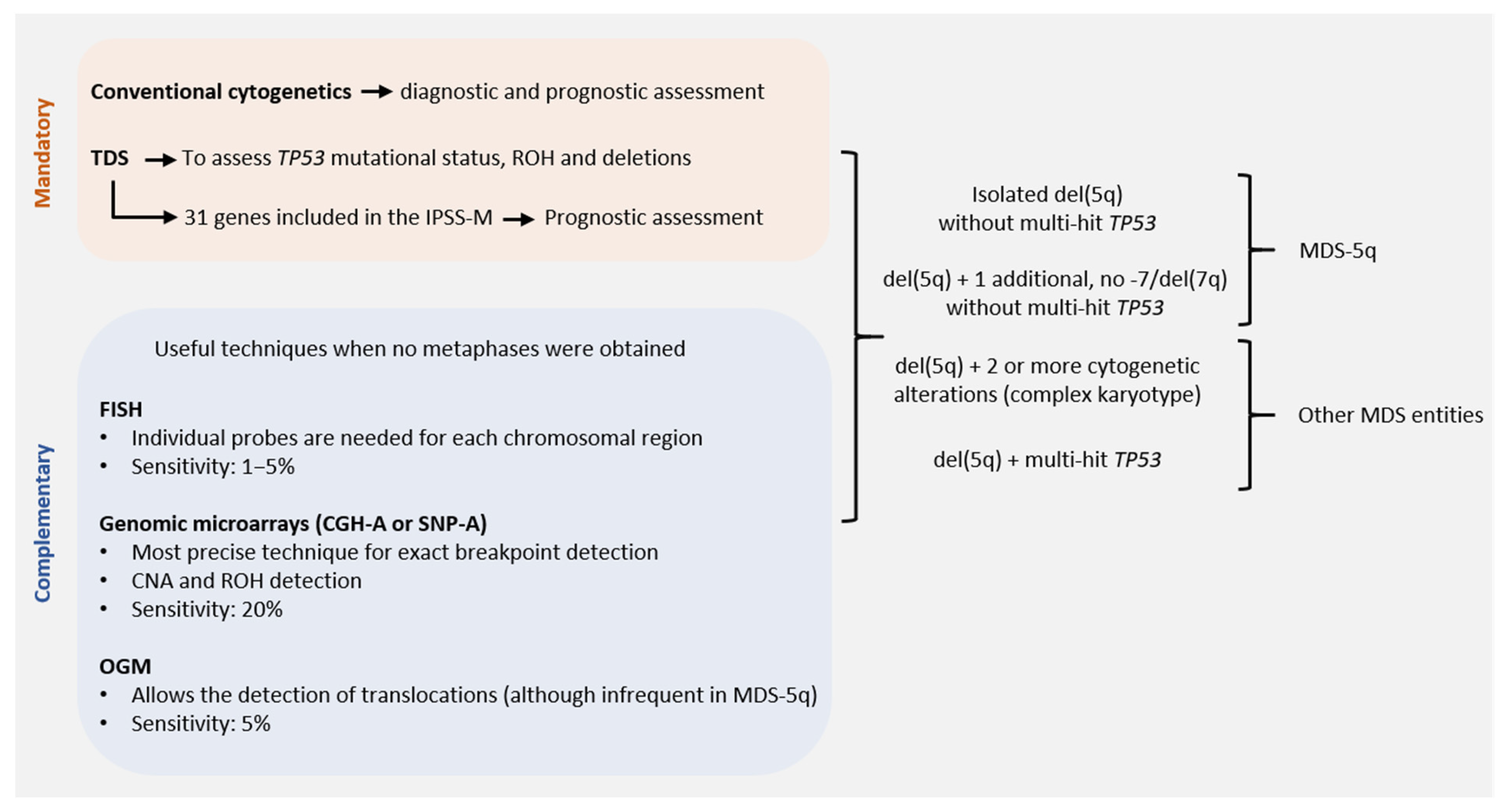
3. Role of Conventional Cytogenetics in MDS-5q
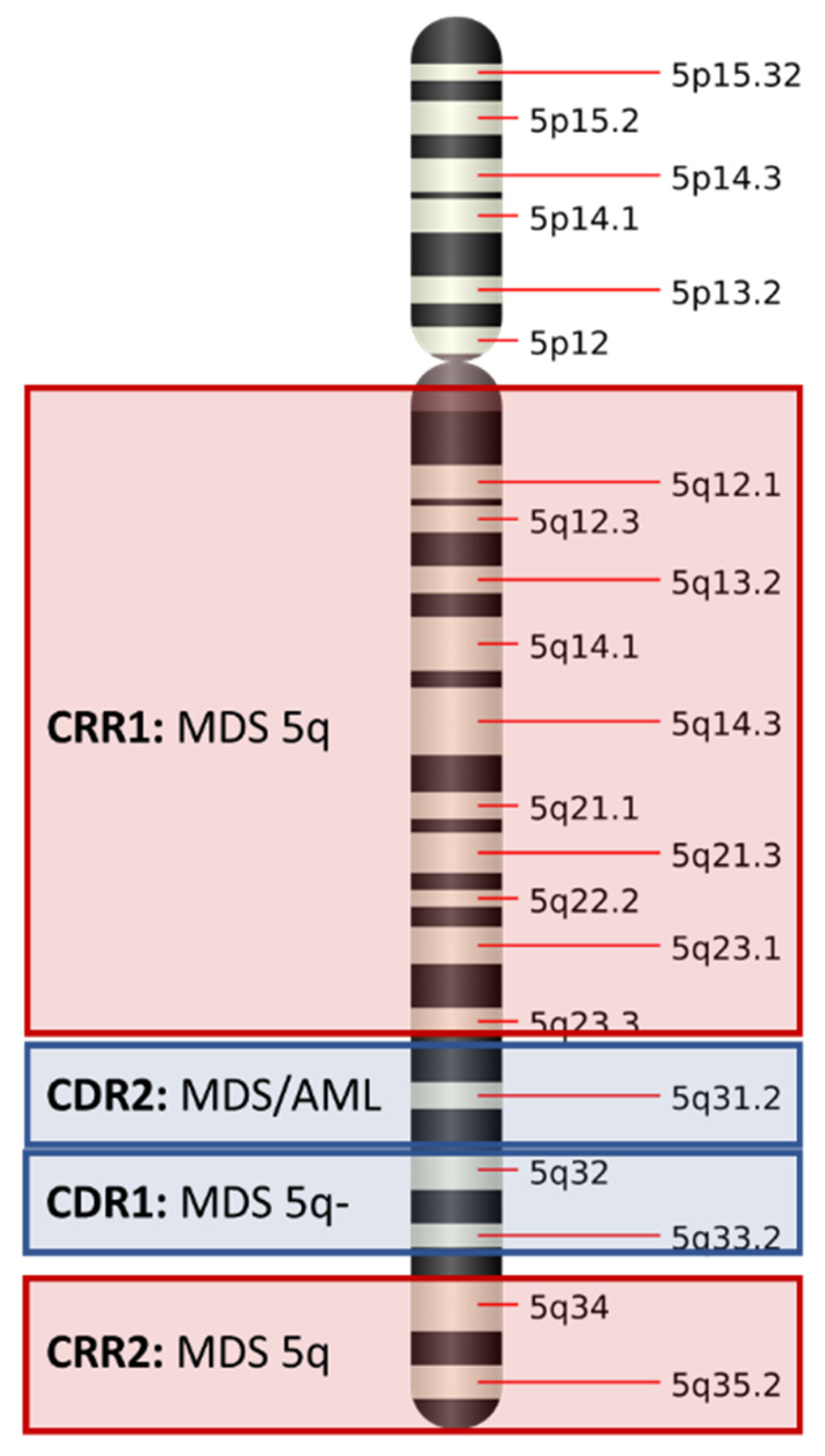
3.1. Commonly Deleted Regions in Chromosome 5q
3.2. Karyotyping: Present and Future Directions
4. Prognostic Impact of Somatic Mutations in MDS-5q
4.1. SF3B1 Mutation
4.2. DNMT3A, TET2 and ASXL1 Mutations
4.3. TP53 Mutations
4.4. CSNK1A1 Mutations
4.5. JAK2 Mutations
5. Clonal Evolution
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Haase, D. Cytogenetic Features in Myelodysplastic Syndromes. Ann. Hematol. 2008, 87, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Germing, U.; Schanz, J.; Pfeilstocker, M.; Nosslinger, T.; Hildebrandt, B.; Kundgen, A.; Lubbert, M.; Kunzmann, R.; Giagounidis, A.A.N.; et al. New Insights into the Prognostic Impact of the Karyotype in MDS and Correlation with Subtypes: Evidence from a Core Dataset of 2124 Patients. Blood 2007, 110, 4385–4395. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and Biological Implications of Driver Mutations in Myelodysplastic Syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of Genetic Lesions in 944 Patients with Myelodysplastic Syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berghe, H.; Cassiman, J.-J.; David, G.; Fryns, J.-P.; Michaux, J.-L.; Sokal, G. Distinct Haematological Disorder with Deletion of Long Arm of No. 5 Chromosome. Nature 1974, 251, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the Classification of the Acute Leukaemias. French-American-British (FAB) Co-Operative Group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Vardiman, J.W. (Eds.) World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2001. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues; IARC: Lyon, France, 2008. [Google Scholar]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Mallo, M.; Cervera, J.; Schanz, J.; Such, E.; García-Manero, G.; Luño, E.; Steidl, C.; Espinet, B.; Vallespí, T.; Germing, U.; et al. Impact of Adjunct Cytogenetic Abnormalities for Prognostic Stratification in Patients with Myelodysplastic Syndrome and Deletion 5q. Leukemia 2011, 25, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Schanz, J.; Tüchler, H.; Solé, F.; Mallo, M.; Luño, E.; Cervera, J.; Granada, I.; Hildebrandt, B.; Slovak, M.L.; Ohyashiki, K.; et al. New Comprehensive Cytogenetic Scoring System for Primary Myelodysplastic Syndromes (MDS) and Oligoblastic Acute Myeloid Leukemia After MDS Derived From an International Database Merge. JCO 2012, 30, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Mallo, M.; Arenillas, L.; Espinet, B.; Salido, M.; Hernandez, J.M.; Lumbreras, E.; del Rey, M.; Arranz, E.; Ramiro, S.; Font, P.; et al. Fluorescence in Situ Hybridization Improves the Detection of 5q31 Deletion in Myelodysplastic Syndromes without Cytogenetic Evidence of 5q-. Haematologica 2008, 93, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Mallo, M.; Del Rey, M.; Ibáñez, M.; Calasanz, M.J.; Arenillas, L.; Larráyoz, M.J.; Pedro, C.; Jerez, A.; Maciejewski, J.; Costa, D.; et al. Response to Lenalidomide in Myelodysplastic Syndromes with Del(5q): Influence of Cytogenetics and Mutations. Br. J. Haematol. 2013, 162, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Boultwood, J.; Fidler, C.; Lewis, S.; Kelly, S.; Sheridan, H.; Littlewood, T.; Buckle, V.; Wainscoat, J. Molecular Mapping of Uncharacteristically Small 5q Deletions in Two Patients with the 5q- Syndrome: Delineation of the Critical Region on 5q and Identification of a 5q- Breakpoint. Genomics 1994, 19, 425–432. [Google Scholar] [CrossRef]
- Boultwood, J.; Fidler, C.; Strickson, A.J.; Watkins, F.; Gama, S.; Kearney, L.; Tosi, S.; Kasprzyk, A.; Cheng, J.-F.; Jaju, R.J.; et al. Narrowing and Genomic Annotation of the Commonly Deleted Region of the 5q- Syndrome. Blood 2002, 99, 4638–4641. [Google Scholar] [CrossRef]
- Jaju, R.; Boultwood, J.; Oliver, F.; Kostrzewa, M.; Fidler, C.; Parker, N.; McPherson, J.; Morris, S.; Müller, U.; Wainscoat, J.; et al. Molecular Cytogenetic Delineation of the Critical Deleted Region in the 5q- Syndrome. Genes Chromosom. Cancer 1998, 22, 251–256. [Google Scholar] [CrossRef]
- Nybakken, G.E.; Bagg, A. The Genetic Basis and Expanding Role of Molecular Analysis in the Diagnosis, Prognosis, and Therapeutic Design for Myelodysplastic Syndromes. J. Mol. Diagn. 2014, 16, 145–158. [Google Scholar] [CrossRef]
- Jerez, A.; Gondek, L.P.; Jankowska, A.M.; Makishima, H.; Przychodzen, B.; Tiu, R.V.; O’Keefe, C.L.; Mohamedali, A.M.; Batista, D.; Sekeres, M.A.; et al. Topography, Clinical, and Genomic Correlates of 5q Myeloid Malignancies Revisited. JCO 2012, 30, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Arenillas, L.; Mallo, M.; Ramos, F.; Guinta, K.; Barragán, E.; Lumbreras, E.; Larráyoz, M.-J.; De Paz, R.; Tormo, M.; Abáigar, M.; et al. Single Nucleotide Polymorphism Array Karyotyping: A Diagnostic and Prognostic Tool in Myelodysplastic Syndromes with Unsuccessful Conventional Cytogenetic Testing: Snp Array In Myelodysplastic Syndromes. Genes Chromosom. Cancer 2013, 52, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Galván, A.B.; Mallo, M.; Arenillas, L.; Salido, M.; Espinet, B.; Pedro, C.; Florensa, L.; Serrano, S.; Solé, F. Does Monosomy 5 Really Exist in Myelodysplastic Syndromes and Acute Myeloid Leukemia? Leuk. Res. 2010, 34, 1242–1245. [Google Scholar] [CrossRef]
- Ebert, B.L.; Galili, N.; Tamayo, P.; Bosco, J.; Mak, R.; Pretz, J.; Tanguturi, S.; Ladd-Acosta, C.; Stone, R.; Golub, T.R.; et al. An Erythroid Differentiation Signature Predicts Response to Lenalidomide in Myelodysplastic Syndrome. PLoS Med. 2008, 5, e35. [Google Scholar] [CrossRef]
- Pellagatti, A.; Hellström-Lindberg, E.; Giagounidis, A.; Perry, J.; Malcovati, L.; Della Porta, M.G.; Jädersten, M.; Killick, S.; Fidler, C.; Cazzola, M.; et al. Haploinsufficiency of RPS14 in 5q- Syndrome Is Associated with Deregulation of Ribosomal- and Translation-Related Genes. Br. J. Haematol. 2008, 142, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Joslin, J.M.; Fernald, A.A.; Tennant, T.R.; Davis, E.M.; Kogan, S.C.; Anastasi, J.; Crispino, J.D.; Le Beau, M.M. Haploinsufficiency of EGR1, a Candidate Gene in the Del(5q), Leads to the Development of Myeloid Disorders. Blood 2007, 110, 719–726. [Google Scholar] [CrossRef]
- Kumar, M.S.; Narla, A.; Nonami, A.; Mullally, A.; Dimitrova, N.; Ball, B.; McAuley, J.R.; Poveromo, L.; Kutok, J.L.; Galili, N.; et al. Coordinate Loss of a MicroRNA and Protein-Coding Gene Cooperate in the Pathogenesis of 5q- Syndrome. Blood 2011, 118, 8. [Google Scholar] [CrossRef]
- Starczynowski, D.T.; Kuchenbauer, F.; Argiropoulos, B.; Sung, S.; Morin, R.; Muranyi, A.; Hirst, M.; Hogge, D.; Marra, M.; Wells, R.A.; et al. Identification of MiR-145 and MiR-146a as Mediators of the 5q– Syndrome Phenotype. Nat. Med. 2010, 16, 49–58. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef]
- Smith, A.C.; Neveling, K.; Kanagal-Shamanna, R. Optical Genome Mapping for Structural Variation Analysis in Hematologic Malignancies. Am. J. Hematol. 2022, 97, 975–982. [Google Scholar] [CrossRef]
- Yang, H.; Garcia-Manero, G.; Sasaki, K.; Montalban-Bravo, G.; Tang, Z.; Wei, Y.; Kadia, T.; Chien, K.; Rush, D.; Nguyen, H.; et al. High-Resolution Structural Variant Profiling of Myelodysplastic Syndromes by Optical Genome Mapping Uncovers Cryptic Aberrations of Prognostic and Therapeutic Significance. Leukemia 2022, 36, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Meggendorfer, M.; Haferlach, C.; Kern, W.; Haferlach, T. Molecular Analysis of Myelodysplastic Syndrome with Isolated Deletion of the Long Arm of Chromosome 5 Reveals a Specific Spectrum of Molecular Mutations with Prognostic Impact: A Study on 123 Patients and 27 Genes. Haematologica 2017, 102, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mercado, M.; Burns, A.; Pellagatti, A.; Giagounidis, A.; Germing, U.; Agirre, X.; Prosper, F.; Aul, C.; Killick, S.; Wainscoat, J.S.; et al. Targeted Re-Sequencing Analysis of 25 Genes Commonly Mutated in Myeloid Disorders in Del(5q) Myelodysplastic Syndromes. Haematologica 2013, 98, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Kulasekararaj, A.G.; Smith, A.E.; Mian, S.A.; Mohamedali, A.M.; Krishnamurthy, P.; Lea, N.C.; Gäken, J.; Pennaneach, C.; Ireland, R.; Czepulkowski, B.; et al. TP53 Mutations in Myelodysplastic Syndrome Are Strongly Correlated with Aberrations of Chromosome 5, and Correlate with Adverse Prognosis. Br. J. Haematol. 2013, 160, 660–672. [Google Scholar] [CrossRef]
- Hosono, N.; Makishima, H.; Mahfouz, R.; Przychodzen, B.; Yoshida, K.; Jerez, A.; LaFramboise, T.; Polprasert, C.; Clemente, M.J.; Shiraishi, Y.; et al. Recurrent Genetic Defects on Chromosome 5q in Myeloid Neoplasms. Oncotarget 2017, 8, 6483–6495. [Google Scholar] [CrossRef]
- Malcovati, L.; Stevenson, K.; Papaemmanuil, E.; Neuberg, D.; Bejar, R.; Boultwood, J.; Bowen, D.T.; Campbell, P.J.; Ebert, B.L.; Fenaux, P.; et al. SF3B1-Mutant Myelodysplastic Syndrome as a Distinct Disease Subtype—A Proposal of the International Working Group for the Prognosis of Myelodysplastic Syndromes (IWG-PM). Blood 2020, 136, 157–170. [Google Scholar] [CrossRef]
- Heuser, M.; Meggendorfer, M.; Cruz, M.M.A.; Fabisch, J.; Klesse, S.; Köhler, L.; Göhring, G.; Ganster, C.; Shirneshan, K.; Gutermuth, A.; et al. Frequency and Prognostic Impact of Casein Kinase 1A1 Mutations in MDS Patients with Deletion of Chromosome 5q. Leukemia 2015, 29, 1942–1945. [Google Scholar] [CrossRef]
- Mossner, M.; Jann, J.-C.; Wittig, J.; Nolte, F.; Fey, S.; Nowak, V.; Obländer, J.; Pressler, J.; Palme, I.; Xanthopoulos, C.; et al. Mutational Hierarchies in Myelodysplastic Syndromes Dynamically Adapt and Evolve upon Therapy Response and Failure. Blood 2016, 128, 1246–1259. [Google Scholar] [CrossRef]
- Malcovati, L.; Karimi, M.; Papaemmanuil, E.; Ambaglio, I.; Jädersten, M.; Jansson, M.; Elena, C.; Gallì, A.; Walldin, G.; Della Porta, M.G.; et al. SF3B1 Mutation Identifies a Distinct Subset of Myelodysplastic Syndrome with Ring Sideroblasts. Blood 2015, 126, 233–241. [Google Scholar] [CrossRef]
- Acha, P.; Palomo, L.; Fuster-Tormo, F.; Xicoy, B.; Mallo, M.; Manzanares, A.; Grau, J.; Marcé, S.; Granada, I.; Rodríguez-Luaces, M.; et al. Analysis of Intratumoral Heterogeneity in Myelodysplastic Syndromes with Isolated Del(5q) Using a Single Cell Approach. Cancers 2021, 13, 841. [Google Scholar] [CrossRef]
- Woll, P.S.; Kjällquist, U.; Chowdhury, O.; Doolittle, H.; Wedge, D.C.; Thongjuea, S.; Erlandsson, R.; Ngara, M.; Anderson, K.; Deng, Q.; et al. Myelodysplastic Syndromes Are Propagated by Rare and Distinct Human Cancer Stem Cells In Vivo. Cancer Cell 2014, 25, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.A.; Rouault-Pierre, K.; Smith, A.E.; Seidl, T.; Pizzitola, I.; Kizilors, A.; Kulasekararaj, A.G.; Bonnet, D.; Mufti, G.J. SF3B1 Mutant MDS-Initiating Cells May Arise from the Haematopoietic Stem Cell Compartment. Nat. Commun. 2015, 6, 10004. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal Hematopoiesis of Indeterminate Potential and Its Distinction from Myelodysplastic Syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ueda, Y.; Xie, S.; Li, E. A Novel Dnmt3a Isoform Produced from an Alternative Promoter Localizes to Euchromatin and Its Expression Correlates with Activede Novo Methylation. J. Biol. Chem. 2002, 277, 38746–38754. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-E.; Hou, H.-A.; Tsai, C.-H.; Wu, S.-J.; Kuo, Y.-Y.; Tseng, M.-H.; Liu, M.-C.; Liu, C.-W.; Chou, W.-C.; Chen, C.-Y.; et al. Dynamics of DNMT3A Mutation and Prognostic Relevance in Patients with Primary Myelodysplastic Syndrome. Clin. Epigenet. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Winschel, C.; Ludeking, A.; Yun, H.; Friesen, I.; Damm, F.; Wagner, K.; Krauter, J.; Heuser, M.; Ganser, A. Rare Occurrence of DNMT3A Mutations in Myelodysplastic Syndromes. Haematologica 2011, 96, 1870–1873. [Google Scholar] [CrossRef]
- Scharenberg, C.; Giai, V.; Pellagatti, A.; Saft, L.; Dimitriou, M.; Jansson, M.; Jädersten, M.; Grandien, A.; Douagi, I.; Neuberg, D.S.; et al. Progression in Patients with Low- and Intermediate-1-Risk Del(5q) Myelodysplastic Syndromes Is Predicted by a Limited Subset of Mutations. Haematologica 2017, 102, 498–508. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Cluzeau, T.; Mansat-De Mas, V.; Dreyfus, F.; Beyne-Rauzy, O.; Quesnel, B.; Vey, N.; Gelsi-Boyer, V.; Raynaud, S.; et al. Impact of TET2 Mutations on Response Rate to Azacitidine in Myelodysplastic Syndromes and Low Blast Count Acute Myeloid Leukemias. Leukemia 2011, 25, 1147–1152. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.E.; Caughey, B.; Lindsley, R.C.; Mar, B.G.; Stojanov, P.; Getz, G.; Steensma, D.P.; Ritz, J.; Soiffer, R.; et al. Somatic Mutations Predict Poor Outcome in Patients with Myelodysplastic Syndrome After Hematopoietic Stem-Cell Transplantation. JCO 2014, 32, 2691–2698. [Google Scholar] [CrossRef]
- Thol, F.; Friesen, I.; Damm, F.; Yun, H.; Weissinger, E.M.; Krauter, J.; Wagner, K.; Chaturvedi, A.; Sharma, A.; Wichmann, M.; et al. Prognostic Significance of ASXL1 Mutations in Patients With Myelodysplastic Syndromes. JCO 2011, 29, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Lasho, T.L.; Finke, C.M.; Gangat, N.; Caramazza, D.; Holtan, S.G.; Pardanani, A.; Knudson, R.A.; Ketterling, R.P.; Chen, D.; et al. WHO-Defined ‘Myelodysplastic Syndrome with Isolated Del(5q)’ in 88 Consecutive Patients: Survival Data, Leukemic Transformation Rates and Prevalence of JAK2, MPL and IDH Mutations. Leukemia 2010, 24, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-J.; Amatruda, J.F.; Abrams, J.M. P53 Ancestry: Gazing through an Evolutionary Lens. Nat. Rev. Cancer 2009, 9, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Evan, G.I. P53—A Jack of All Trades but Master of None. Nat. Rev. Cancer 2009, 9, 821–829. [Google Scholar] [CrossRef]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of TP53 Allelic State for Genome Stability, Clinical Presentation and Outcomes in Myelodysplastic Syndromes. Nat. Med. 2020, 26, 1549–1556. [Google Scholar] [CrossRef]
- Jädersten, M.; Saft, L.; Smith, A.; Kulasekararaj, A.; Pomplun, S.; Göhring, G.; Hedlund, A.; Hast, R.; Schlegelberger, B.; Porwit, A.; et al. TP53 Mutations in Low-Risk Myelodysplastic Syndromes with Del(5q) Predict Disease Progression. J. Clin. Oncol. 2011, 29, 1971–1979. [Google Scholar] [CrossRef]
- Smith, A.; Jiang, J.; Kulasekararaj, A.G.; Mian, S.; Mohamedali, A.; Gaken, J.; Ireland, R.; Czepulkowski, B.; Best, S.; Mufti, G.J. CSNK1A1 Mutations and Isolated Del(5q) Abnormality in Myelodysplastic Syndrome: A Retrospective Mutational Analysis. Lancet Haematol. 2015, 2, e212–e221. [Google Scholar] [CrossRef]
- Knippschild, U.; Gocht, A.; Wolff, S.; Huber, N.; Löhler, J.; Stöter, M. The Casein Kinase 1 Family: Participation in Multiple Cellular Processes in Eukaryotes. Cell Signal. 2005, 17, 675–689. [Google Scholar] [CrossRef]
- Schittek, B.; Sinnberg, T. Biological Functions of Casein Kinase 1 Isoforms and Putative Roles in Tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef]
- Krönke, J.; Fink, E.C.; Hollenbach, P.W.; MacBeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide Induces Ubiquitination and Degradation of CK1α in Del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Schneider, R.K.; Ademà, V.; Heckl, D.; Järås, M.; Mallo, M.; Lord, A.M.; Chu, L.P.; McConkey, M.E.; Kramann, R.; Mullally, A.; et al. Role of Casein Kinase 1A1 in the Biology and Targeted Therapy of Del(5q) MDS. Cancer Cell 2014, 26, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bello, E.; Pellagatti, A.; Shaw, J.; Mecucci, C.; Kušec, R.; Killick, S.; Giagounidis, A.; Raynaud, S.; Calasanz, M.J.; Fenaux, P.; et al. CSNK1A1 Mutations and Gene Expression Analysis in Myelodysplastic Syndromes with Del(5q). Br. J. Haematol. 2015, 171, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular Pathogenesis of the Myeloproliferative Neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Nangalia, J.; Green, A.R. Myeloproliferative Neoplasms: From Origins to Outcomes. Blood 2017, 130, 2475–2483. [Google Scholar] [CrossRef]
- Ingram, W.; Lea, N.C.; Cervera, J.; Germing, U.; Fenaux, P.; Cassinat, B.; Kiladjian, J.J.; Varkonyi, J.; Antunovic, P.; Westwood, N.B.; et al. The JAK2 V617F Mutation Identifies a Subgroup of MDS Patients with Isolated Deletion 5q and a Proliferative Bone Marrow. Leukemia 2006, 20, 1319–1321. [Google Scholar] [CrossRef][Green Version]
- Sangiorgio, V.F.I.; Geyer, J.T.; Margolskee, E.; Al-Kawaaz, M.; Mathew, S.; Tam, W.; Orazi, A. Myeloid Neoplasms with Isolated Del(5q) and JAK2 V617F Mutation: A “Grey Zone” Combination of Myelodysplastic and Myeloproliferative Features? Haematologica 2020, 105, e276–e279. [Google Scholar] [CrossRef]
- da Silva-Coelho, P.; Kroeze, L.I.; Yoshida, K.; Koorenhof-Scheele, T.N.; Knops, R.; van de Locht, L.T.; de Graaf, A.O.; Massop, M.; Sandmann, S.; Dugas, M.; et al. Clonal Evolution in Myelodysplastic Syndromes. Nat. Commun. 2017, 8, 15099. [Google Scholar] [CrossRef]

| Gene | Pathway/Function | Frequency | Clinical and Biological Correlations |
|---|---|---|---|
| SF3B1 | Splicing factor | 19–20% |
|
| DNMT3A | DNA methylation | 18% |
|
| TP53 | Checkpoint/cell cycle | 18% |
|
| TET2 | DNA methylation | 12% |
|
| CSNK1A1 | Proliferation, apoptosis, DNA damage response | 7–10% |
|
| ASXL1 | Chromatin modification | 6% |
|
| JAK2 | Tyrosine kinase | 6% |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acha, P.; Mallo, M.; Solé, F. Myelodysplastic Syndromes with Isolated del(5q): Value of Molecular Alterations for Diagnostic and Prognostic Assessment. Cancers 2022, 14, 5531. https://doi.org/10.3390/cancers14225531
Acha P, Mallo M, Solé F. Myelodysplastic Syndromes with Isolated del(5q): Value of Molecular Alterations for Diagnostic and Prognostic Assessment. Cancers. 2022; 14(22):5531. https://doi.org/10.3390/cancers14225531
Chicago/Turabian StyleAcha, Pamela, Mar Mallo, and Francesc Solé. 2022. "Myelodysplastic Syndromes with Isolated del(5q): Value of Molecular Alterations for Diagnostic and Prognostic Assessment" Cancers 14, no. 22: 5531. https://doi.org/10.3390/cancers14225531
APA StyleAcha, P., Mallo, M., & Solé, F. (2022). Myelodysplastic Syndromes with Isolated del(5q): Value of Molecular Alterations for Diagnostic and Prognostic Assessment. Cancers, 14(22), 5531. https://doi.org/10.3390/cancers14225531






