Initial Experience of Intra-Arterial Chemotherapy Using a Novel External Carotid Arterial Sheath System Combined with Radiotherapy and Systemic Chemotherapy for Locally Advanced Tongue Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
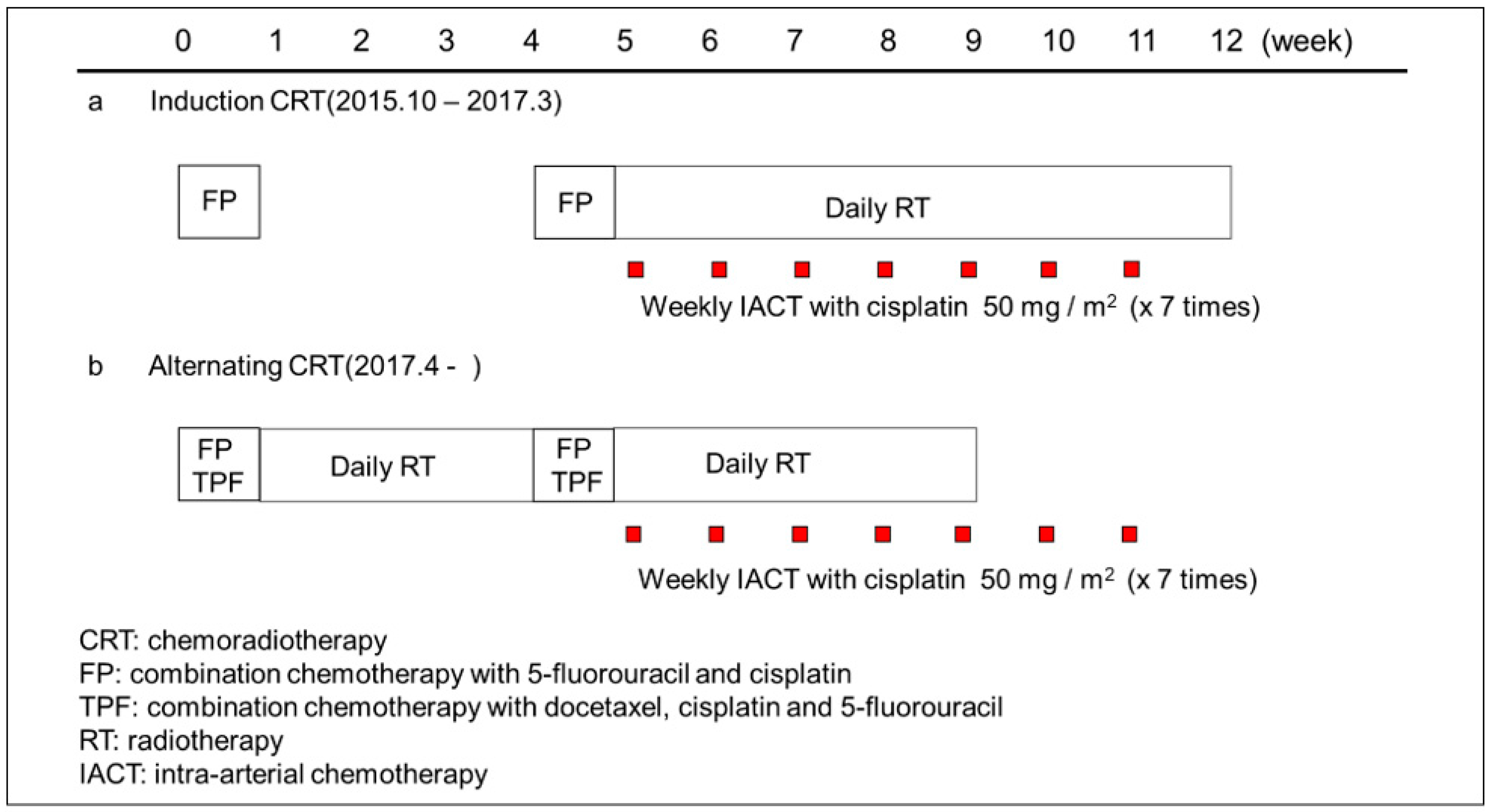
2.2. Treatment Procedure
2.3. Chemotherapy
2.4. Radiotherapy
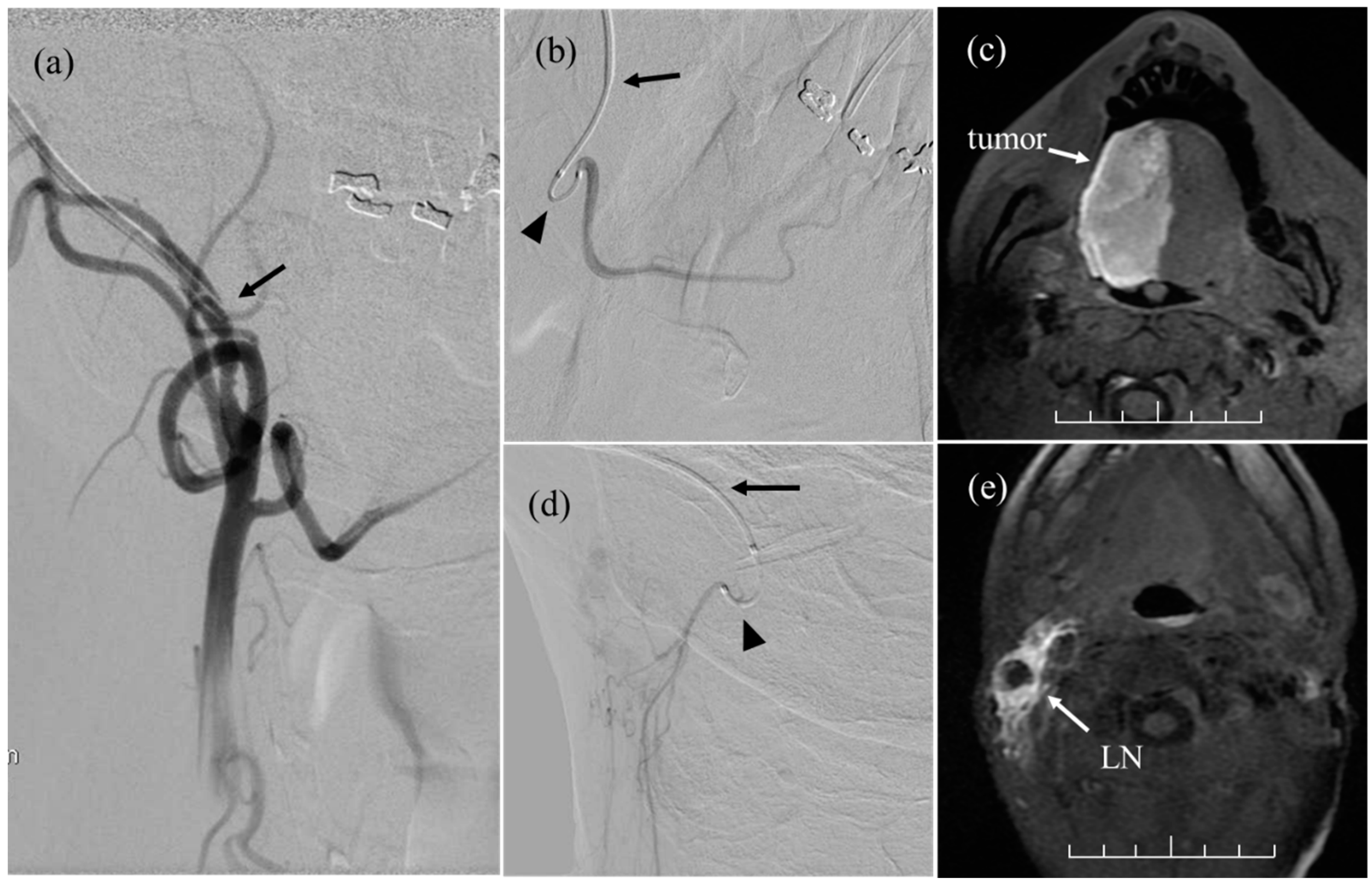
2.5. Intra-Arterial Chemotherapy
2.6. Patient Assessments
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
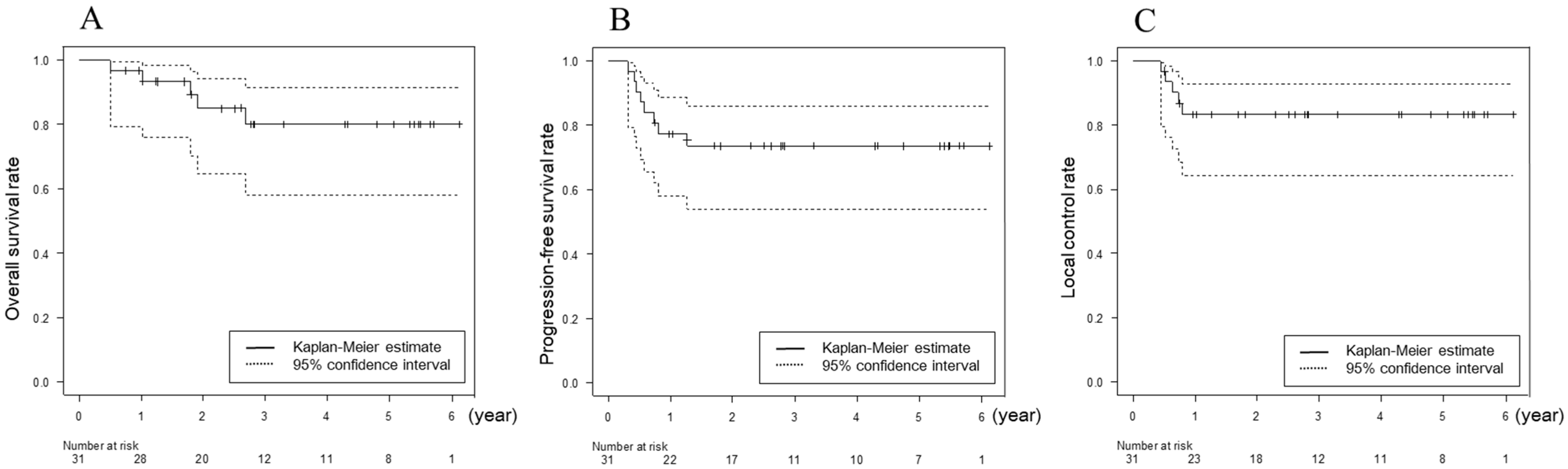
3.2. Response and Survival
3.3. Toxicity
3.4. Factors Related to OS, PFS, and LC
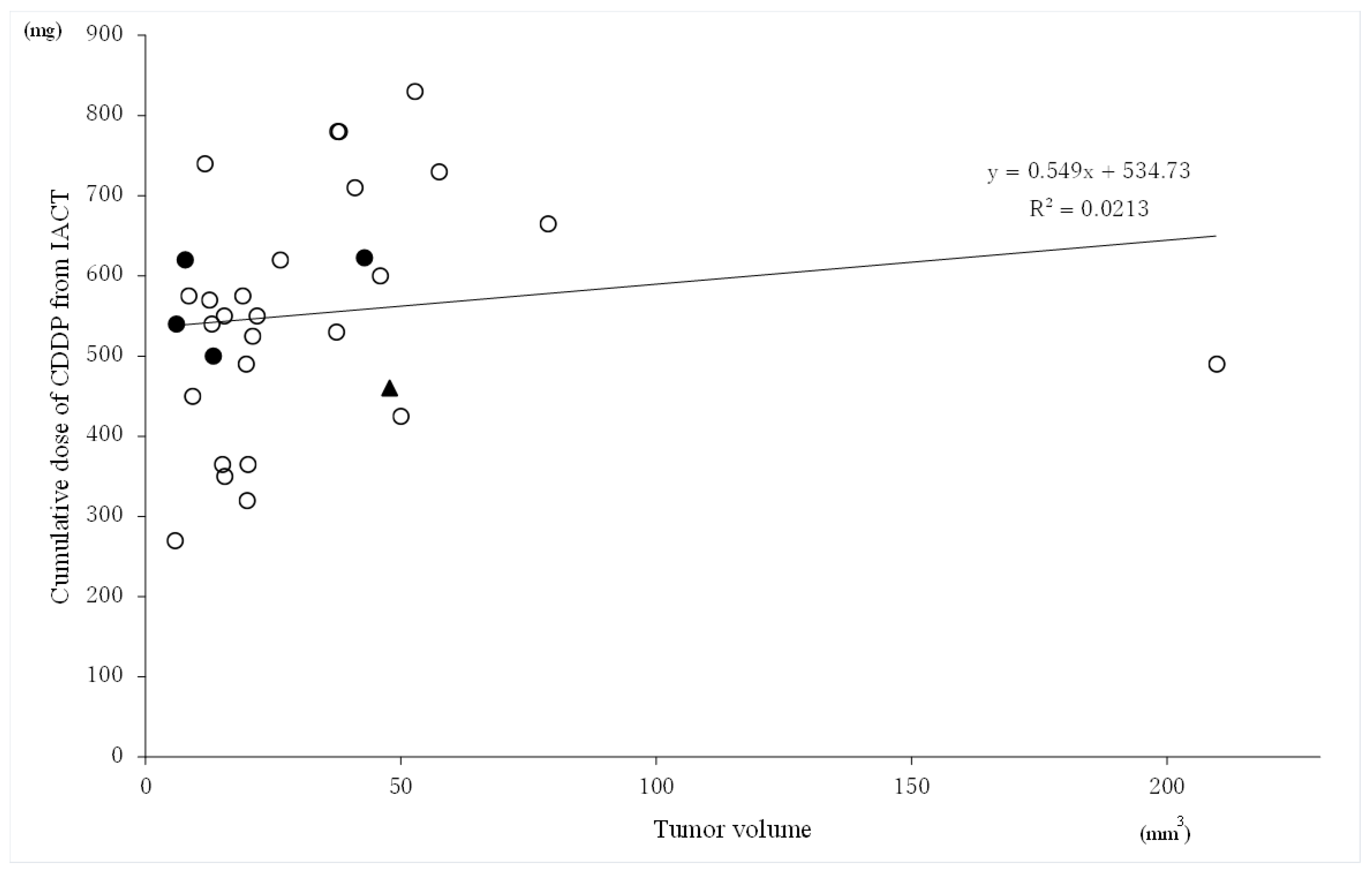
3.5. Relationship between the Tumor Volume and Cumulative Cisplatin Dose of CDDP from IACT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Paderno, A.; Morello, R.; Piazza, C. Tongue carcinoma in young adults: A review of the literature. Acta Otorhinolaryngol. Ital. 2018, 38, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.H.; Dikshit, M.; Bhaduri, D.; Aghamolaei, T.; Moosavy, S.H.; Azarpaykan, A. Interaction of alcohol use and specific types of smoking on the development of oral cancer. Int. J. High Risk Behav. Addict. 2014, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Fontanesi, J.; Wong, F.S.; Vicario, D.; Seagren, S.; Kumar, P.; Weisman, R.; Pellitteri, P.; Thomas, J.R.; Flick, P.; et al. A novel organ preservation protocol for advanced carcinoma of the larynx and pharynx. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 853–857. [Google Scholar] [CrossRef]
- Mroueh, R.; Haapaniemi, A.; Grénman, R.; Laranne, J.; Pukkila, M.; Almangush, A.; Salo, T.; Mäkitie, A. Improved outcomes with oral tongue squamous cell carcinoma in Finland. Head Neck 2017, 39, 1306–1312. [Google Scholar] [CrossRef]
- Fuwa, N.; Kodaira, T.; Furutani, K.; Tachibana, H.; Nakamura, T.; Nakahara, R.; Tomoda, T.; Inokuti, H.; Daimon, T. Arterial chemoradiotherapy for locally advanced tongue cancer: Analysis of retrospective study of therapeutic results in 88 patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1090–1100. [Google Scholar] [CrossRef]
- Robbins, K.T.; Storniolo, A.M.; Kerber, C.; Vicario, D.; Seagren, S.; Shea, M.; Hanchett, C.; Los, G.; Howell, S.B. Phase I study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J. Clin. Oncol. 1994, 12, 2113–2120. [Google Scholar] [CrossRef]
- Shimizu, T.; Sakakura, Y.; Hattori, T.; Yamaguchi, N.; Kubo, M.; Sakakura, K. Superselective intraarterial chemotherapy in combination with irradiation: Preliminary report. Am. J. Otolaryngol. 1990, 11, 131–136. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Wallace, S.; Dimery, I.; Goepfert, H. Intraarterial chemotherapy of head and neck tumors. AJNR Am. J. Neuroradiol. 1986, 7, 343–348. [Google Scholar]
- Robbins, K.T.; Kumar, P.; Wong, F.S.; Hartsell, W.F.; Flick, P.; Palmer, R.; Weir, A.B., III; Neill, H.B.; Murry, T.; Ferguson, R.; et al. Targeted chemoradiation for advanced head and neck cancer: Analysis of 213 patients. Head Neck 2000, 22, 687–693. [Google Scholar] [CrossRef]
- Suzuki, S.; Yasunaga, H.; Matsui, H.; Fushimi, K.; Saito, Y.; Yamasoba, T. Cerebral infarction after intraarterial and intravenous chemoradiotherapy for head and neck cancer: A retrospective analysis using a Japanese inpatient database. Head Neck 2016, 38, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Mitsudo, K.; Koizumi, T.; Iida, M.; Iwai, T.; Nakashima, H.; Oguri, S.; Kioi, M.; Hirota, M.; Koike, I.; Hata, M.; et al. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for stage III and IV oral cancer: Analysis of therapeutic results in 112 cases. Radiother. Oncol. 2014, 111, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Minamiyama, S.; Mitsudo, K.; Hayashi, Y.; Iida, M.; Iwai, T.; Nakashima, H.; Oguri, S.; Ozawa, T.; Koizumi, T.; Hirota, M.; et al. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for T2-4N0 tongue cancer: Control of occult neck metastasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ii, N.; Fuwa, N.; Toyomasu, Y.; Takada, A.; Nomura, M.; Kawamura, T.; Sakuma, H.; Nomoto, Y. A novel external carotid arterial sheath system for intra-arterial infusion chemotherapy of head and neck cancer. Cardiovasc. Interv. Radiol. 2017, 40, 1099–1104. [Google Scholar] [CrossRef]
- Nomura, M.; Fuwa, N.; Toyomasu, Y.; Takada, A.; Ii, N.; Nomura, J.; Yamada, H. A comparison of two types of microcatheters used for a novel external carotid arterial sheath system for intra-arterial chemotherapy of head and neck cancer. Jpn. J. Radiol. 2018, 36, 622–628. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Spiotto, M.T.; Jefferson, G.; Wenig, B.; Markiewicz, M.; Weichselbaum, R.R.; Koshy, M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer: A national cancer database analysis. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 691–699. [Google Scholar] [CrossRef]
- Iyer, N.G.; Tan, D.S.; Tan, V.K.; Wang, W.; Hwang, J.; Tan, N.-C.; Sivanandan, R.; Tan, H.-K.; Lim, W.T.; Ang, M.-K.; et al. Randomized Trial Comparing Surgery and Adjuvant Radiotherapy Versus Concurrent Chemoradiotherapy in Patients with Advanced, Nonmetastatic Squamous Cell Carcinoma of the Head and Neck: 10-Year Update and Subset Analysis. Cancer 2015, 121, 1599–1607. [Google Scholar] [CrossRef]
- Kravets, O.V.; Protsyk, V.S.; Burtyn, O.V.; Hurianov, V.G. Comparative analysis of the efficacy of definitive chemoradiation therapy and surgery followed by adjuvant radiation therapy in advanced-stage oral tongue cancer. Exp. Oncol. 2020, 42, 228–232. [Google Scholar]
- Mitsudo, K.; Hayashi, Y.; Minamiyama, S.; Ohashi, N.; Iida, M.; Iwai, T.; Oguri, S.; Koizumi, T.; Kioi, M.; Hirota, M.; et al. Chemoradiotherapy using retrograde superselective intra-arterial infusion for tongue cancer: Analysis of therapeutic results in 118 cases. Oral Oncol. 2018, 79, 71–77. [Google Scholar] [CrossRef]
- Takayama, K.; Nakamura, T.; Takada, A.; Makita, C.; Suzuki, M.; Azami, Y.; Kato, T.; Hayashi, Y.; Ono, T.; Toyomasu, Y.; et al. Treatment results of alternating chemoradiotherapy followed by proton beam therapy boost combined with intra-arterial infusion chemotherapy for stage III-IVB tongue cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-H.; Lin, C.-Y.; Kang, C.-J.; Huang, S.-F.; Wang, H.-M.; Chen, E.Y.-C.; Chen, I.-H.; Liao, C.-T.; Cheng, A.-J.; Chang, J.T.-C. Combined-modality treatment for advanced oral tongue squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys 2007, 67, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Imanishi, Y.; Tomita, T.; Ozawa, H. Clinical review and statistical analysis of tongue squamous cell carcinoma. Jpn. J. Head Neck Cancer 2011, 37, 4–10. [Google Scholar]
- Suzuki, M.; Yoshino, K.; Fujii, T.; Kii, M.; Sugawa, T. Outcome of tongue cancer treated with surgery and postoperative radiotherapy. Nippon. Jibiinkoka Gakkai Kaiho 2014, 117, 907–913. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Uchi, R.; Wakasaki, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Clinical analysis of tongue squamous cell carcinomas based on the new TNM classification. J. Jpn. Soc. Head Neck Surg. 2020, 29, 273–278. [Google Scholar] [CrossRef][Green Version]
- Oikawa, Y.; Kugimoto, T.; Kashima, Y.; Okuyama, K.; Ohsako, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Shimamoto, H.; Michi, Y.; et al. Surgical treatment for oral tongue squamous cell carcinoma: A retrospective study of 432 patients. Glob. Health Med. 2021, 3, 157–162. [Google Scholar] [CrossRef]
- Ansarin, M.; De Berardinis, R.; Corso, F.; Giugliano, G.; Bruschini, R.; De Benedetto, L.; Zorzi, S.; Maffini, F.; Sovardi, F.; Pigni, C.; et al. Survival Outcomes in Oral Tongue Cancer: A Mono-Institutional Experience Focusing on Age. Front. Oncol. 2021, 11, 616653. [Google Scholar] [CrossRef]
- Doweck, I.; Thomas, K.T.; Samant, S.; Vieira, F. Intra-arterial chemoradiation for T3–4 oral cavity cancer: Treatment outcomes in comparison to oropharyngeal and hypopharyngeal carcinoma. World J. Surg. Oncol. 2008, 6, 2. [Google Scholar] [CrossRef]
- Rasch, C.R.; Hauptmann, M.; Schornagel, J.; Wijers, O.; Buter, J.; Gregor, T.; Wiggenraad, R.; de Boer, J.P.; Ackerstaff, A.H.; Kroger, R.; et al. Intra-arterial versus Intravenous Chemoradiation for Advanced Head and Neck Cancer: Results of a Randomized Phase 3 Trial. Cancer 2010, 116, 2159–2165. [Google Scholar] [CrossRef]
- Robbins, K.T.; Howell, S.B.; Williams, J.S. Intra-arterial chemotherapy for head and neck cancer: Is there a verdict? Cancer 2010, 116, 2068–2070. [Google Scholar] [CrossRef]
- Takahashi, K.; Ebihatra, K.; Honda, Y.; Nishikawa, K.; Kita, M. Antitumor Activity of cis-Dichlorodiamineplatinum(II) and Its Effect on Cell Cycle Progression. Jpn. J. Cancer Chemother. 1982, 9, 624–631. [Google Scholar]
- Hafiza, W.A.G.W.N.; Latifah, S.Y. Potential implications of GRP58 expression and susceptibility of cervical cancer to cisplatin and thymoquinone-based therapy. Oncol. Targets Ther. 2014, 7, 1375–1387. [Google Scholar]
- Ma, J.; Liu, Y.; Yang, X.; Zhang, C.-P.; Zhang, Z.-Y.; Zhong, L.-P. Induction chemotherapy in patients with resectable head and neck squamous cell carcinoma: A meta-analysis. World J. Surg. Oncol. 2013, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Faisal, M.; Bakar, M.A.; Muhammad, T.; Qadeer, S.; Mohtasham, S.; Hussain, R.; Jamshed, A. Locally advanced oral tongue cancer: Is organ preservation a safe option in resource-limited high-volume setting? Ann. Maxillofac. Surg. 2020, 10, 158–163. [Google Scholar]
| Characteristics | No. of Patients (%) |
|---|---|
| Gender | |
| Male | 23 (74) |
| Female | 8 (26) |
| Age (years) | |
| Range | 25–76 |
| Median | 49 |
| T classification | |
| T3 | 13 (42) |
| T4a | 17 (55) |
| T4b | 1 (3) |
| N classification | |
| N0 | 5 (16) |
| N1 | 4 (13) |
| N2b | 14 (45) |
| N2c | 7 (23) |
| N3b | 1 (3) |
| Stage classification | |
| III | 5 (16) |
| IVA | 24 (77) |
| IVB | 2 (7) |
| Reasons for not performing surgery | |
| Refusal | 29 (94) |
| Inoperable disease | 2 (6) |
| Total | 31 (100) |
| Toxicities | No of Patients by Toxicity Grade (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Acute | ||||
| Neutropenia | 4 (13) | 8 (26) | 10 (32) | 2 (7) |
| Anemia | 9 (29) | 17 (55) | 3 (10) | 0 |
| Thrombocytopenia | 21 (68) | 2 (7) | 3 (10) | 0 |
| Nausea | 3 (12) | 9 (29) | 4 (13) | _ |
| Oral mucositis | 2 (7) | 14 (45) | 14 (45) | 0 |
| Dry mouth | 11 (36) | 12 (39) | 3 (10) | _ |
| Dysphagia | 3 (10) | 14 (45) | 0 | 0 |
| Radiation dermatitis | 27 (87) | 4 (13) | 0 | 0 |
| Renal failure | 0 | 0 | 0 | 0 |
| Fever | 10 (32) | 1 (3) | 1 (3) | 0 |
| Catheter related infection | 0 | 0 | 0 | 0 |
| Late | ||||
| Ostenoradionecrosis | 0 | 0 | 0 | 0 |
| Variables | Level | No. | Overall Survival HR (95%CI) | p Value | Progression-Free Survival HR (95%CI) | p Value | Local Control HR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|---|
| Age (y) | <50 ≥50 | 15 16 | 1 3.71 (0.41–33.25) | 0.241 | 1 7.74(0.95–63.19) | 0.056 | undefined | 0.999 |
| Sex | Male Female | 23 8 | 1 2.49 (0.41–15.02) | 0.322 | 1 1.96(0.47–8.20) | 0.359 | 1 1.86 (0.31–11.16) | 0.496 |
| T classification | 3 4 | 13 18 | 1 1.09 (0.18–6.52) | 0.929 | 1 0.67(0.17–2.69) | 0.573 | 1 0.40 (0.07–2.41) | 0.317 |
| N classification | 0 or 1 ≥2 | 9 22 | 1 0.40 (0.07–2.42) | 0.318 | 1 0.30(0.08–1.22) | 0.093 | 1 1.38 (0.15–12.35) | 0.774 |
| Stage | III IVA-IVB | 5 26 | 1 0.48 (0.05–4.36) | 0.514 | 1 0.39 (0.08–1.97) | 0.253 | 1 0.50 (0.06–4.57) | 0.543 |
| Tumor volume (cm3) | <20 ≥20 | 15 16 | 1 0.60 (0.10–3.59) | 0.575 | 1 0.50 (0.12–2.10) | 0.344 | 1 0.56 (0.09–3.35) | 0.524 |
| RT dose (Gy) | <56 ≥56 | 13 18 | 1 3.32 (0.37–29.72) | 0.284 | 1 1.22 (0.29–5.10) | 0.789 | 1 3.01 (0.34–26.94) | 0.325 |
| Systemic chemotherapy (times) | 1 2 | 6 25 | 1 0.25 (0.04–1.52) | 0.133 | 1 0.19 (0.05–0.76) | 0.019 | 1 0.32 (0.05–1.94) | 0.216 |
| Regimen of systemic chemotherapy | FP TPF | 16 15 | 1 0.57 (0.10–3.44) | 0.543 | 1 0.29 (0.06–1.44) | 0.129 | 1 0.59 (0.10–3.54) | 0.563 |
| CDDP dose by IACT (mg) | <550 ≥550 | 15 16 | 1 0.52 (0.09–3.14) | 0.478 | 1 0.86 (0.21–3.43) | 0.826 | 1 0.58 (0.10–3.45) | 0.546 |
| IACT (times) | <7 ≥7 | 10 21 | 1 0.60 (0.10–3.62) | 0.580 | 1 1.41 (0.28–7.00) | 0.673 | 1 1.83 (0.20–16.38) | 0.590 |
| Progression-Free Survival Factor | Level | HR (95%CI) | p Value |
|---|---|---|---|
| Age (y) | <50 ≥50 | 1 5.25 (0.63–43.78) | 0.126 |
| N classification | 0 or 1 ≥2 | 1 0.46 (0.11–1.86) | 0.278 |
| Systemic chemotherapy (times) | 1 2 | 1 0.31 (0.08–1.25) | 0.099 |
| Study (Publish) | Year of Collection | Sample Size | Stage | Treatment | Survival |
|---|---|---|---|---|---|
| Fan [22] (2007) | 1995–2002 | 201 | III, IV | OP + CRT | 48% (3-y OS) (III: 64%, IV: 37%) |
| Sakamoto [23] (2011) | 1996–2007 | 32 | III, IV | OP ± CRT | III:77.1% (5-y DFS), IV:39.7% (5-y DFS) |
| Suzuki [24] (2014) | 2000–2010 | 89 | III, IV | OP ± RT | III:71.5% (5-y OS), IV:61.5% (5-y OS) III:78.6% (5-y CSS), IV:69.1% (5-y CSS) |
| Mroueh [5] (2017) | 2005–2009 | 90 | III, IV | OP ± RT/CRT | 61% (5-y OS) III:69% (5-y DSS), IV:51% (5-y DSS) |
| Yasumatsu [25] (2020) | 2007–2016 | 46 | III, IV | OP ± CRT | III:70% (3-y DSS), IVA:64.2% (3-y DSS) |
| Kravets [19] (2020) | 2004–2013 | 114 | III, IV | OP + RT/CRT | 57% (5-y OS), 56.5% (5-y DFS) |
| Oikawa [26] (2021) | 2008–2017 | 89 | III, IV | OP ± CRT | III:84.1% (5-y DSS), IV:79.0% (5-y DSS) |
| Ansarin [27] (2021) | 2000–2018 | 353 | III, IV | OP ±CRT | 55% (5-y OS), 60% (5-y CSS), 50% (5-y DFS) |
| Fuwa [6] (2008) | 1993–2002 | 88 | III, IV | IACT + CRT | 57 % (3-y OS), 72 % (3-y LC) |
| Doweck [28] (2008) | 1993–2000 | 22 oral cavity * | III, IV | IACT + RT | 37% (5-y OS), 69% (5-y LC) |
| Takayama [21] (2016) | 2009–2012 | 33 | III, IV | IACT + CRT by protonbeam | 87.0% (3-y OS), 74.1% (3-y PFS), 86.6% (3-y LC) |
| Mitsudo [20] (2018) | 2006–2015 | 95 | III, IV | IACT + RT | III:94.7% (3-y OS), IV:64.9% (3-y OS) III:89.7% (3-y LRC), IV:72.1% (3-y LRC) |
| Nomura (present study) | 2015–2021 | 31 | III, IV | IACT + CRT | 81.6% (3-y OS), 74.2% (3-y PFS), 83.4% (3-y LC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, M.; Fuwa, N.; Ito, S.; Toyomasu, Y.; Takada, A.; Kobayashi, D.; Fuke, T.; Taniguchi, M.; Ii, N.; Uraki, J.; et al. Initial Experience of Intra-Arterial Chemotherapy Using a Novel External Carotid Arterial Sheath System Combined with Radiotherapy and Systemic Chemotherapy for Locally Advanced Tongue Cancer. Cancers 2022, 14, 5529. https://doi.org/10.3390/cancers14225529
Nomura M, Fuwa N, Ito S, Toyomasu Y, Takada A, Kobayashi D, Fuke T, Taniguchi M, Ii N, Uraki J, et al. Initial Experience of Intra-Arterial Chemotherapy Using a Novel External Carotid Arterial Sheath System Combined with Radiotherapy and Systemic Chemotherapy for Locally Advanced Tongue Cancer. Cancers. 2022; 14(22):5529. https://doi.org/10.3390/cancers14225529
Chicago/Turabian StyleNomura, Miwako, Nobukazu Fuwa, Shintaro Ito, Yutaka Toyomasu, Akinori Takada, Daisuke Kobayashi, Tomohito Fuke, Masanori Taniguchi, Noriko Ii, Junji Uraki, and et al. 2022. "Initial Experience of Intra-Arterial Chemotherapy Using a Novel External Carotid Arterial Sheath System Combined with Radiotherapy and Systemic Chemotherapy for Locally Advanced Tongue Cancer" Cancers 14, no. 22: 5529. https://doi.org/10.3390/cancers14225529
APA StyleNomura, M., Fuwa, N., Ito, S., Toyomasu, Y., Takada, A., Kobayashi, D., Fuke, T., Taniguchi, M., Ii, N., Uraki, J., & Yamada, H. (2022). Initial Experience of Intra-Arterial Chemotherapy Using a Novel External Carotid Arterial Sheath System Combined with Radiotherapy and Systemic Chemotherapy for Locally Advanced Tongue Cancer. Cancers, 14(22), 5529. https://doi.org/10.3390/cancers14225529





