A Rapid Late Enhancement MRI Protocol Improves Differentiation between Brain Tumor Recurrence and Treatment-Related Contrast Enhancement of Brain Parenchyma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
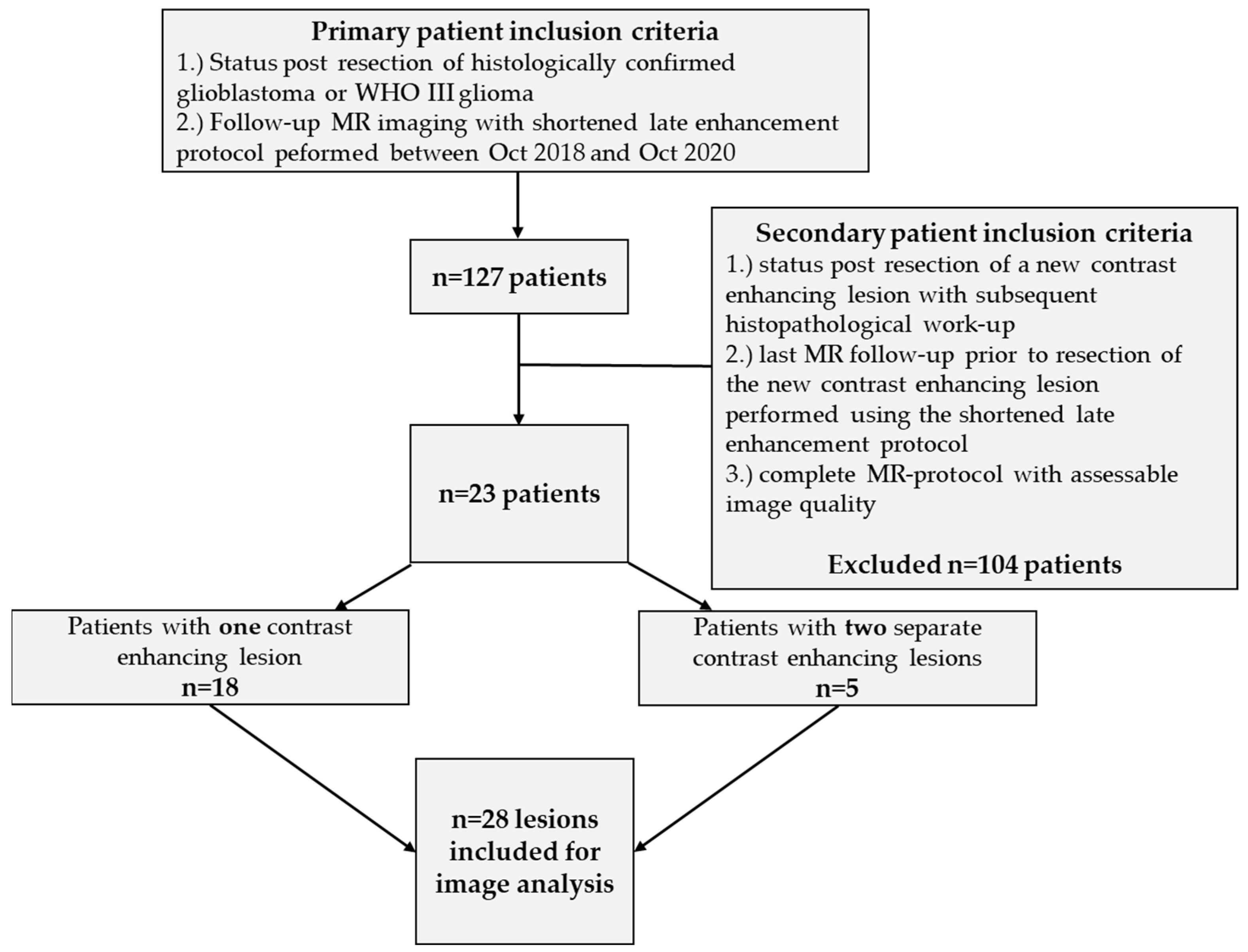
2.1. Patient Population
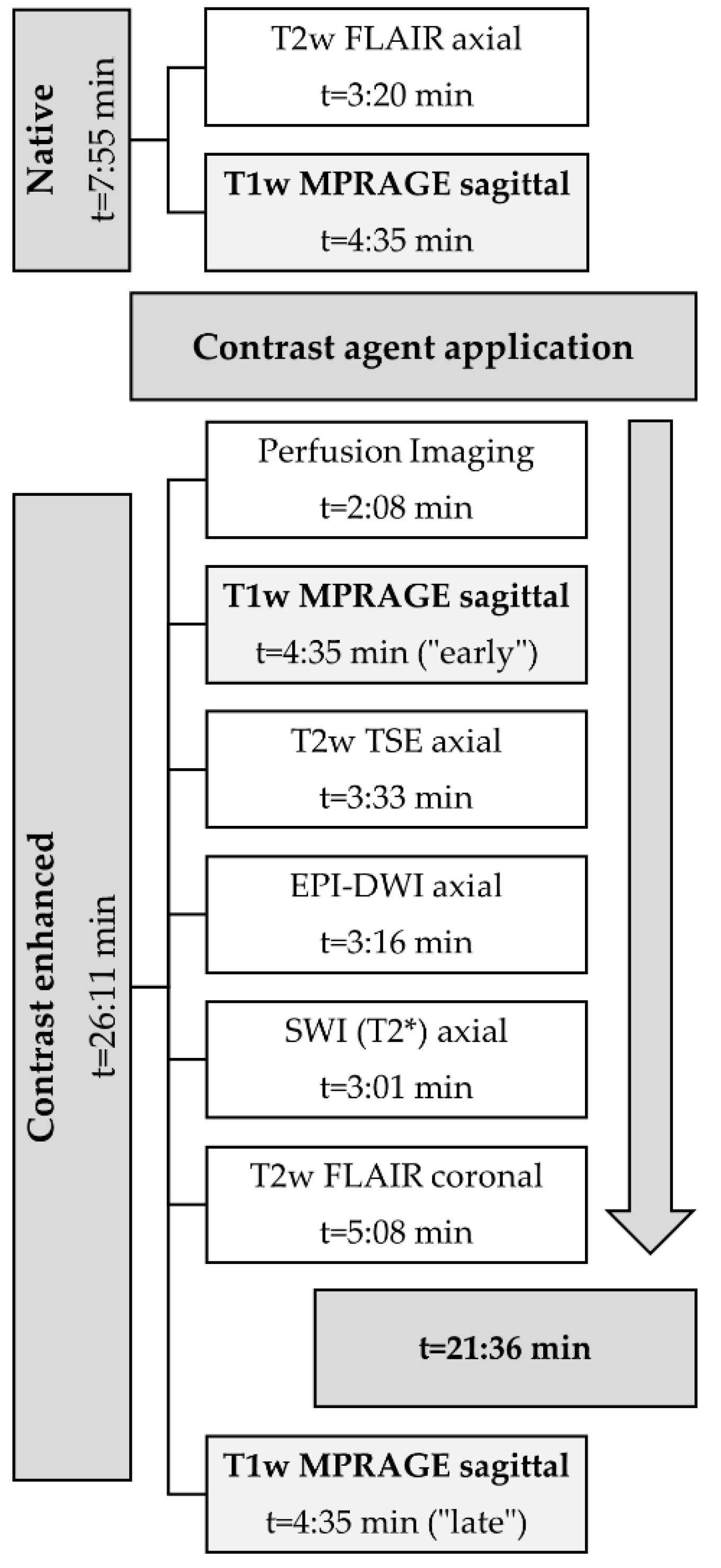
2.2. MRI-Protocol
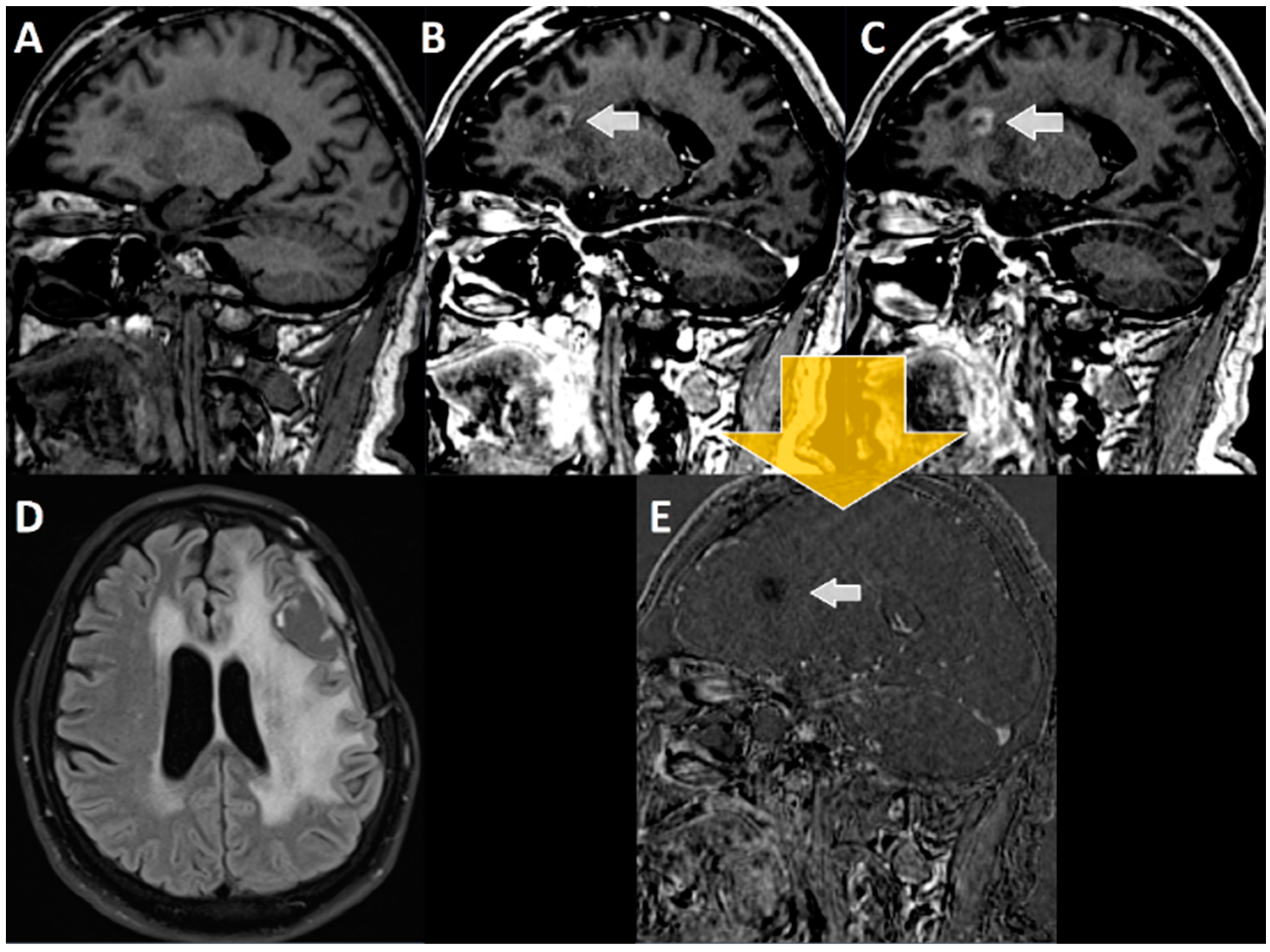
2.3. Evaluation of Late Enhancement
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Lesion ID | Patient ID and Initial Tumor Histology | Patient Age at Initial Diagnosis [Years] | Localization of the Initial Tumor (Prior to First Resection) | Time (in Months) between the End of RCT and Detection of the First Suspect Contrast Enhancing Lesion in Follow-Up MRI | Localization of Suspect Lesion in Follow-Up MRI (with Wash Out in Late Enhancement) | Time (in Months) between the Detection of the Suspect Lesion and MRI Using the Late Enhancement Protocol | Time (in Months) between the Initial Tumor Resection and Re-Resection after Late Enhancement- Follow Up | Histology of Resected Lesions Declared as Suspect in Late Enhancement Follow-Up MRI |
|---|---|---|---|---|---|---|---|---|
| 1 | GBM-9 | 62 | Precentral left | 1 | Craniorostral margin of the resection cavity | 22 | 25 | Residual/recurrent tumor tissue of glioblastoma and reactive changes |
| 2 | GBM-10 | 56 | Temporobasal left | 21 | Temporopolar left | 1 | 63 | Residual/recurrent tumor tissue of glioblastoma |
| 3 | GBM-24 | 64 | Temporoparietal left | Deceased during RCT | Craniomedial circumference of theresection cavity | 0 | 5 | Residual/recurrent tumor tissue of glioblastoma |
| 4 | GBM-32 | 57 | Temporal left | 3 | Entire circumferential of the resection cavity | 1 | 7 | Residual/recurrent tumor tissue of glioblastoma |
| 5 | GBM-37 | 41 | Precentral right | 1 | Frontal right | 2 | 100 | Residual/recurrent tumor tissue of glioblastoma |
| 6 | GBM-40 * | 63 | Temporal left | 10 | Anterolateral margin of resection cavity | 0 | 13 | Cell-rich anaplastic glial tumor with tumor tissue necrosis |
| 7 | GBM-40 * | 63 | Temporal left | 10 | Craniomedial of the resection cavity | 5 | 13 | Anaplastic glial tumor tissue compatible with residual/recurrent recurrent tumor tissue of known glioblastoma |
| 8 | GBM-45 | 55 | Occipital right | 5 (only radiotherapy) | Ventral circumferential resection margin | 0 | 8 | Anaplastic glial tumor with tumor tissue necrosis is compatible with residual/recurrence of glioblastoma |
| 9 | GBM-47 * | 47 | Occipital left | 11 | Parietotemporal left | 0 | 14 | Moderately cell-rich recurrence of known glioblastoma |
| 10 | GBM-47 * | 47 | Occipital left | 20 | Rostromedial and dorsomedial circumference of resection cavity | 0 | 22 | Cell-rich anaplastic astrocytic tumor. Residual/recurrent tumor of glioblastoma |
| 11 | GBM-49 | 79 | Occipital left | 2 (only radiotherapy) | Temporopolar left | 0 | 3 | Anaplastic glial tumor with tumor tissue necrosis, compatible with residual/recurrent tumor tissue glioblastoma |
| 12 | GBM-50 | 46 | Corpus Callosum bilaterally | 5 | Truncus of the corpus callosum | 1 | 10 | Compatible with residual/recurrent tumor tissue of glioblastoma |
| 13 | GBM-54 | 63 | Temporomesial left | 13 | Subcortical temporolateral left | 0 | 16 | Compatible with residual/recur-rent tumor tissue of glioblastoma |
| 14 | GBM-55 | 69 | Parietal right | 14 | Pons | 0 | 17 | Compatible with residual/recurrent tumor tissue of glioblastoma |
| 15 | GBM-56 | 23 | Frontopolar right | 31 (only radiotherapy) | Parietal right | 0 | 36 | Compatible with residual/recurrent tumor tissue of glioblastoma |
| 16 | GBM-57 * | 57 | Temporal left | 3 | Temporobasal left | 0 | 6 | Cell-rich anaplastic astrocytic tumor compatible with residual/recurrent tumor tissue of glioblastoma |
| 17 | GBM-57 * | 57 | Temporal left | 14 | Dorsal to the resection cavity around the left cornu posterior | 0 | 17 | Cell-rich anaplastic astrocytic tumor with high proliferation activity |
| 18 | GBM-49 | 52 | Frontal right | 27 | Lateral to the right anterior cornu | 0 | 33 | Compatible with glioblastoma |
| 19 | GBM-60 | 70 | Parietooccipital right | 1 | Anteromedial to the resection cavity | 0 | 5 | Glial tissue with increased cell density and reactive changes |
| 20 | GBM-63 | 43 | Frontal left | not assessable, external image data | Posteromedial of the genu corporis callosi | not assessable, external image data | 24 | Compatible with residual/recurrent tumor tissue of glioblastoma |
| 21 | GBM-64 | 71 | Frontal right | 3 | Resection margin | 5 | 10 | Compatible with residual/recurrent tumor tissue of glioblastoma |
| 22 | GBM-65 | 54 | Frontal left | 3 | Frontal left margin of resection cavity | 6 | 12 | Cell-dense portions of a glial tumor, as well as reactive/resorptive changes |
| 23 | GBM-66 | 43 | Temporal left | 4 | Resection margin | 0 | 20 | Residual/recurrent tumor tissue of glioblastoma |
| 24 | WHO3-11 | 62 | Temporal left | 5 | Temporal left | 0 | 8 | Coagulation necrosis as a radiation regressive change. No malignant cells of the known anaplastic astrocytoma |
| 25 | WHO3-26 * | 46 | Temporal right | 37 | Parietal right/temporo-occipital right | 0 | 40 | Residual/recurrent tumor tissue of the known anaplastic astrocytoma |
| 26 | WHO3-26 * | 46 | Temporal right | 47 | In the medial temporal lobe right | 0 | 49 | Residual/recurrent tumor tissue of anaplastic astrocytoma |
| 27 | WHO3-53 * | 62 | Frontal right | 0 | Ventromedial circumference of resection cavity | 9 | 13 | Necrotic tissue particles and connective tissue with reactive/resorptive changes |
| 28 | WHO3-53 * | 62 | Frontal right | 0 | Ventrocaudal circumference of resection cavity | 1 | 17 | Extensive therapy-induced reactive and resorptive changes |
References
- Gittleman, H.R.; Ostrom, Q.T.; Rouse, C.D.; Dowling, J.A.; de Blank, P.M.; Kruchko, C.A.; Elder, J.B.; Rosenfeld, S.S.; Selman, W.R.; Sloan, A.E.; et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adoles-cents, and children in the United States, 2000 to 2010. Cancer 2015, 121, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. Cancer Treat. Res. 2015, 163, 1–14. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.R.; Reulen, H.-J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.; Tully, P.A.; Barnes, E.H.; Lwin, Z.; Jeffree, R.; Drummond, K.J.; Gan, H.; Khasraw, M. Outcomes after second surgery for recurrent glioblastoma: A retrospective case-control study. J. Neuro-Oncol. 2018, 137, 409–415. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Stupp, R.; Warren, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Brandsma, D.; van den Bent, M.J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef]
- Jing, H.; Yang, F.; Peng, K.; Qin, D.; He, Y.; Yang, G.; Zhang, H. Multimodal MRI-Based Radiomic Nomogram for the Early Differentiation of Recurrence and Pseudoprogression of High-Grade Glioma. Biomed. Res. Int. 2022, 2022, 4667117. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef]
- Tran, D.K.; Jensen, R.L. Treatment-related brain tumor imaging changes: So-called “pseudoprogression” vs. tumor progression: Review and future research opportunities. Surg. Neurol. Int. 2013, 4, 129–135. [Google Scholar] [CrossRef]
- Zach, L.; Guez, D.; Last, D.; Daniels, D.; Grober, Y.; Nissim, O.; Hoffmann, C.; Nass, D.; Talianski, A.; Spiegelmann, R.; et al. Delayed contrast extravasation MRI: A new paradigm in neuro-oncology. Neuro-Oncology 2015, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zach, L.; Guez, D.; Last, D.; Daniels, D.; Grober, Y.; Nissim, O.; Hoffmann, C.; Nass, D.; Talianski, A.; Spiegelmann, R.; et al. Delayed contrast extravasation MRI for depicting tumor and non-tumoral tissues in primary and metastatic brain tumors. PLoS ONE 2012, 7, e52008. [Google Scholar] [CrossRef]
- Wagner, S.; Lanfermann, H.; Eichner, G.; Gufler, H. Radiation injury versus malignancy after stereotactic radiosurgery for brain metastases: Impact of time-dependent changes in lesion morphology on MRI. Neuro-Oncology 2017, 19, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Colen, R.R.; Zinn, P.O.; Hazany, S.; Do-Dai, D.; Wu, J.K.; Yao, K.; Zhu, J.J. Magnetic resonance imaging appearance and changes on intracavitary Gliadel wafer placement: A pilot study. World J. Radiol. 2011, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, S.; Spalek, K.; Nabavi, A.; Schultka, S.; Mehdorn, H.M.; Kesari, S.; Dörner, L. Temporal changes in magnetic resonance imaging characteristics of Gliadel wafers and of the adjacent brain parenchyma. Neuro-Oncology 2012, 14, 482–490. [Google Scholar] [CrossRef]
- Yun, T.J.; Park, C.K.; Kim, T.M.; Lee, S.H.; Kim, J.H.; Sohn, C.H.; Park, S.H.; Kim, I.H.; Choi, S.H. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: Differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology 2015, 274, 830–840. [Google Scholar] [CrossRef]
- Tomura, N.; Kokubun, M.; Saginoya, T.; Mizuno, Y.; Kikuchi, Y. Differentiation between Treatment-Induced Necrosis and Recurrent Tumors in Patients with Metastatic Brain Tumors: Comparison among (11)C-Methionine-PET, FDG-PET, MR Permeability Imaging, and MRI-ADC-Preliminary Results. AJNR Am. J. Neuroradiol. 2017, 38, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.D.; Kuruva, M.; Shim, H.; Mullins, M.E. Clinical Applications of Magnetic Resonance Spectroscopy in Brain Tumors: From Diagnosis to Treatment. Radiol. Clin. N. Am. 2021, 59, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Giesel, F.L.; Stieltjes, B. MRI for identification of progression in brain tumors: From morphology to function. Expert Rev. Neurother. 2008, 8, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Heilan, S.; Falini, A.; Jäger, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro-Oncology 2017, 19, 118–127. [Google Scholar] [CrossRef]
- Larsen, V.A.; Simonsen, H.J.; Law, I.; Larsson, H.B.; Hansen, A.E. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 2013, 55, 361–369. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xiao, H.; Chen, X.; Lei, Y.; Feng, Z.; Ma, X.; Ma, L. Perfusion magnetic resonance imaging in the differentiation between glioma recurrence and pseudoprogression: A systematic review, meta-analysis and meta-regression. Quant. Imaging Med. Surg. 2022, 12, 4805–4822. [Google Scholar] [CrossRef]


| MRI without Late Enhancement | Histology Positive for Tumor Progression or Recurrence | Histology Negative for Tumor Progression or Recurrence | ||
|---|---|---|---|---|
| tumor progress or recurrence rated in MRI | True positives (a) | False negatives (b) | ||
| 21 (R1) | 23 (R2) | 4 (R1) | 2 (R2) | |
| tumor progress or recurrence rated in MRI | False positives (c) | True negatives (d) | ||
| 3 (R1) | 3 (R2) | 0 (R1) | 0 (R2) | |
| Sensitivity | 84% (R1) | 92% (R2) | ||
| Specificity | 0% (R1) | 0% (R2) | ||
| PPV | 87.5% (R1) | 88.5% (R2) | ||
| NPV | 0% (R1) | 0% (R2) | ||
| Phi contingency coefficient (rPhi) | −0.141 (R1) | −0.096 (R2) | ||
| RATZ-index | −0.167 (R1) | −0.12 (R2) | ||
| MRI with late enhancement | ||||
| tumor progress or recurrence rated in MRI | True positives (a) | False negatives (b) | ||
| 25 (R1) | 25 (R2) | 0 (R1) | 0 (R2) | |
| no tumor progression or recurrence in MRI | False positives (c) | True negatives (d) | ||
| 0 (R1) | 0 (R2) | 3 (R1) | 3 (R2) | |
| Sensitivity | 100% (R1) | 100% (R2) | ||
| Specificity | 100% (R1) | 100% (R2) | ||
| PPV | 100% (R1) | 100% (R2) | ||
| NPV | 100% (R1) | 100% (R2) | ||
| Phi contingency coefficient (rPhi) | 1 (R1) | 1 (R2) | ||
| RATZ-index | 1 (R1) | 1 (R2) | ||
| Odds ratio for detecting suspect changes | 1.1 (95% CI: 0.98–1.3 for R1 and R2) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satvat, N.; Korczynski, O.; Müller-Eschner, M.; Othman, A.E.; Schöffling, V.; Keric, N.; Ringel, F.; Sommer, C.; Brockmann, M.A.; Reder, S. A Rapid Late Enhancement MRI Protocol Improves Differentiation between Brain Tumor Recurrence and Treatment-Related Contrast Enhancement of Brain Parenchyma. Cancers 2022, 14, 5523. https://doi.org/10.3390/cancers14225523
Satvat N, Korczynski O, Müller-Eschner M, Othman AE, Schöffling V, Keric N, Ringel F, Sommer C, Brockmann MA, Reder S. A Rapid Late Enhancement MRI Protocol Improves Differentiation between Brain Tumor Recurrence and Treatment-Related Contrast Enhancement of Brain Parenchyma. Cancers. 2022; 14(22):5523. https://doi.org/10.3390/cancers14225523
Chicago/Turabian StyleSatvat, Neda, Oliver Korczynski, Matthias Müller-Eschner, Ahmed E. Othman, Vanessa Schöffling, Naureen Keric, Florian Ringel, Clemens Sommer, Marc A. Brockmann, and Sebastian Reder. 2022. "A Rapid Late Enhancement MRI Protocol Improves Differentiation between Brain Tumor Recurrence and Treatment-Related Contrast Enhancement of Brain Parenchyma" Cancers 14, no. 22: 5523. https://doi.org/10.3390/cancers14225523
APA StyleSatvat, N., Korczynski, O., Müller-Eschner, M., Othman, A. E., Schöffling, V., Keric, N., Ringel, F., Sommer, C., Brockmann, M. A., & Reder, S. (2022). A Rapid Late Enhancement MRI Protocol Improves Differentiation between Brain Tumor Recurrence and Treatment-Related Contrast Enhancement of Brain Parenchyma. Cancers, 14(22), 5523. https://doi.org/10.3390/cancers14225523






