Lenvatinib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Search Results
Patient Characteristics
3.2. Therapeutic Efficacy Assessment
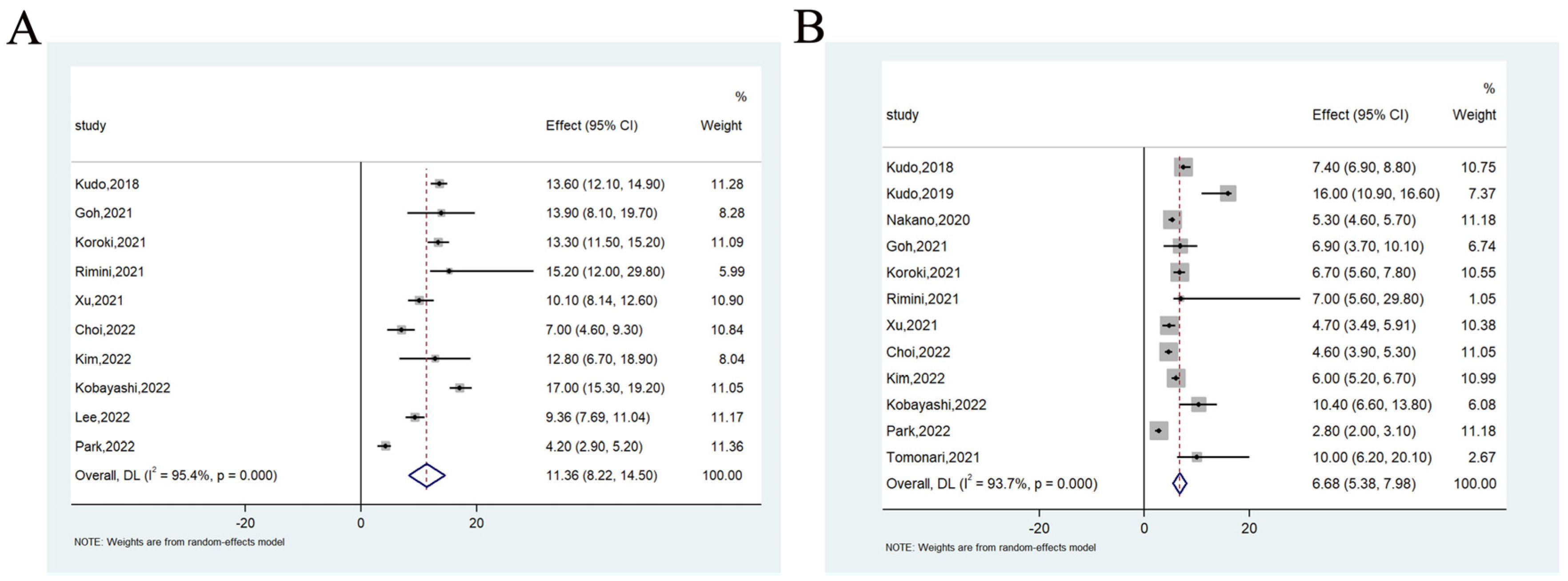
3.2.1. Overall Survival and Progression-Free Survival
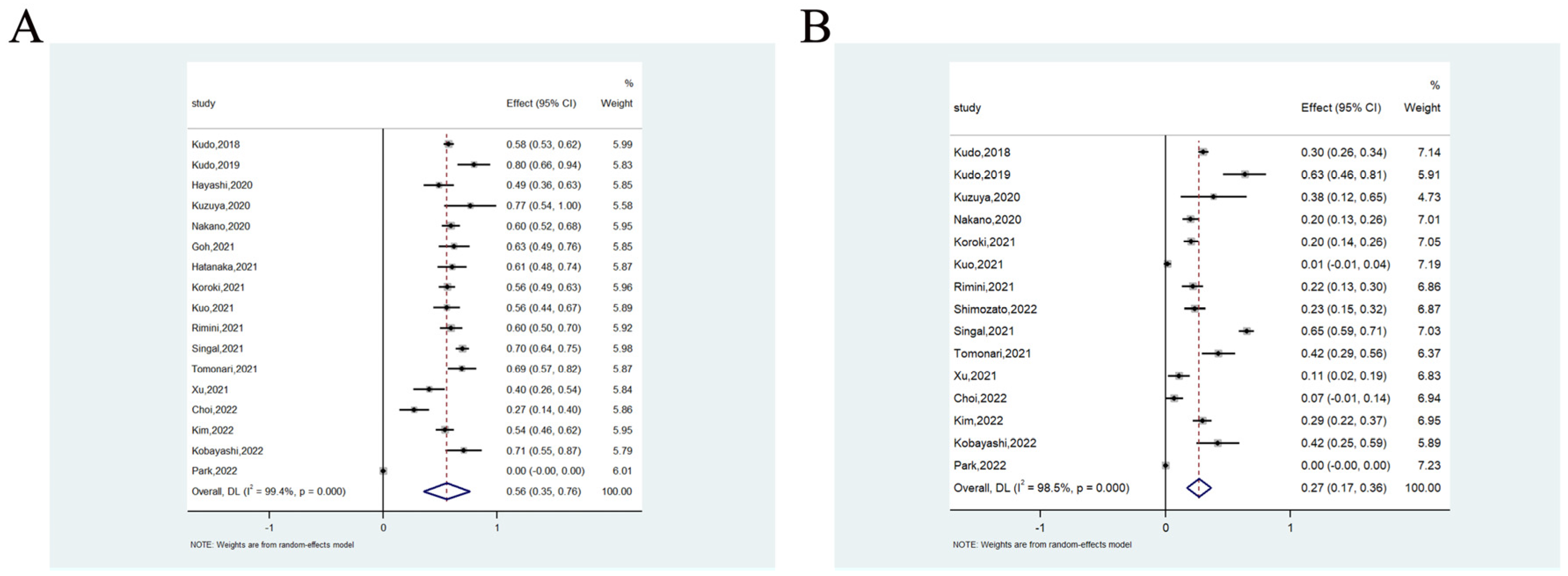
3.2.2. Objective Response Rate and Disease Control Rate
3.2.3. Adverse Events
3.3. Lenvatinib Versus Sorafenib
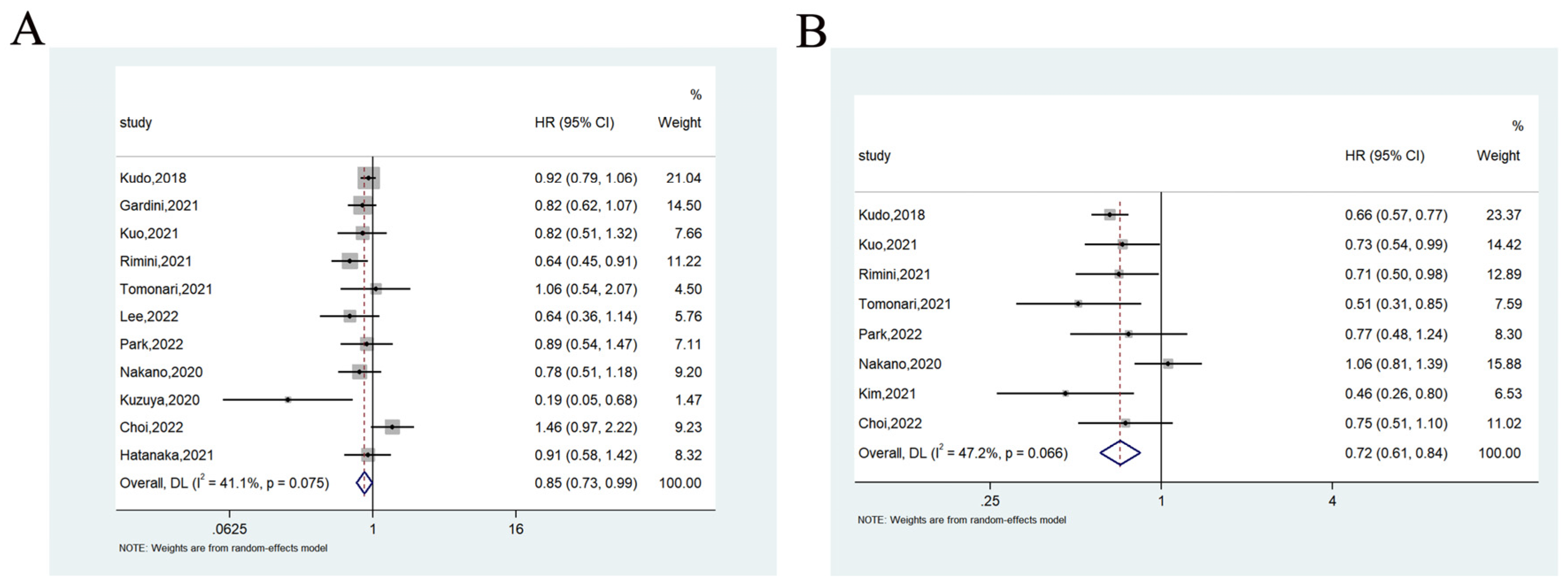
3.3.1. Overall Survival
3.3.2. Progression-Free Survival
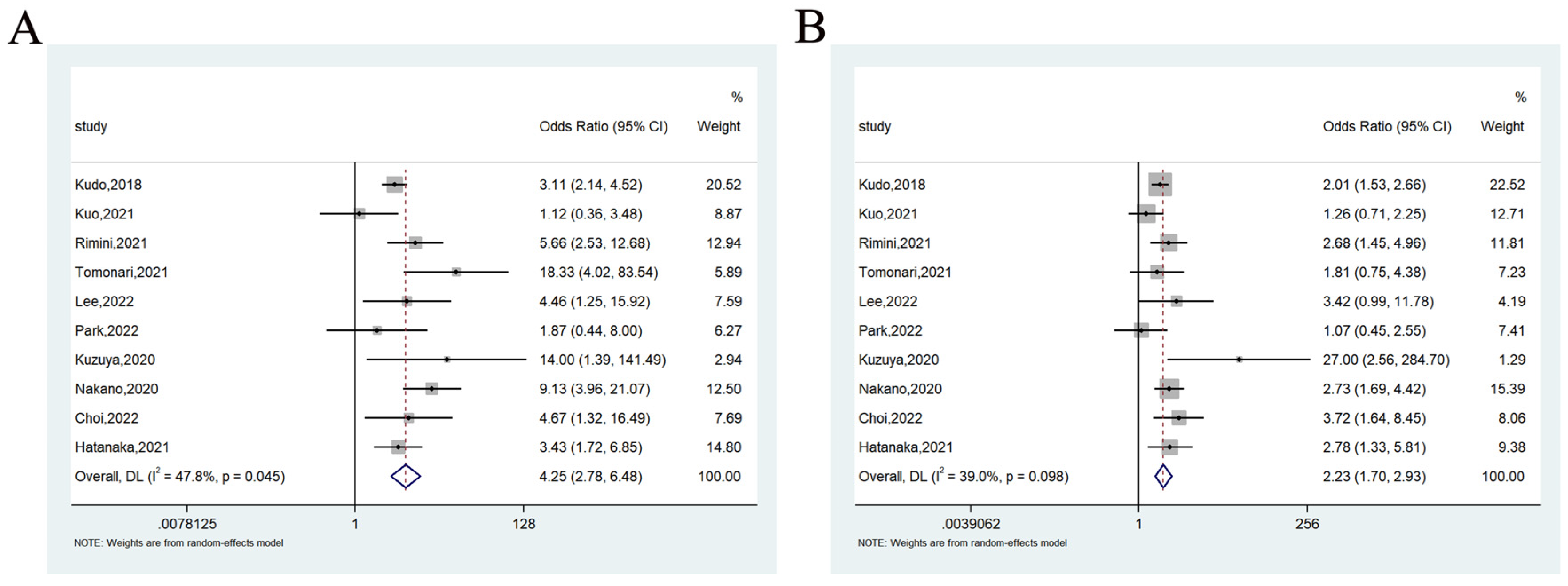
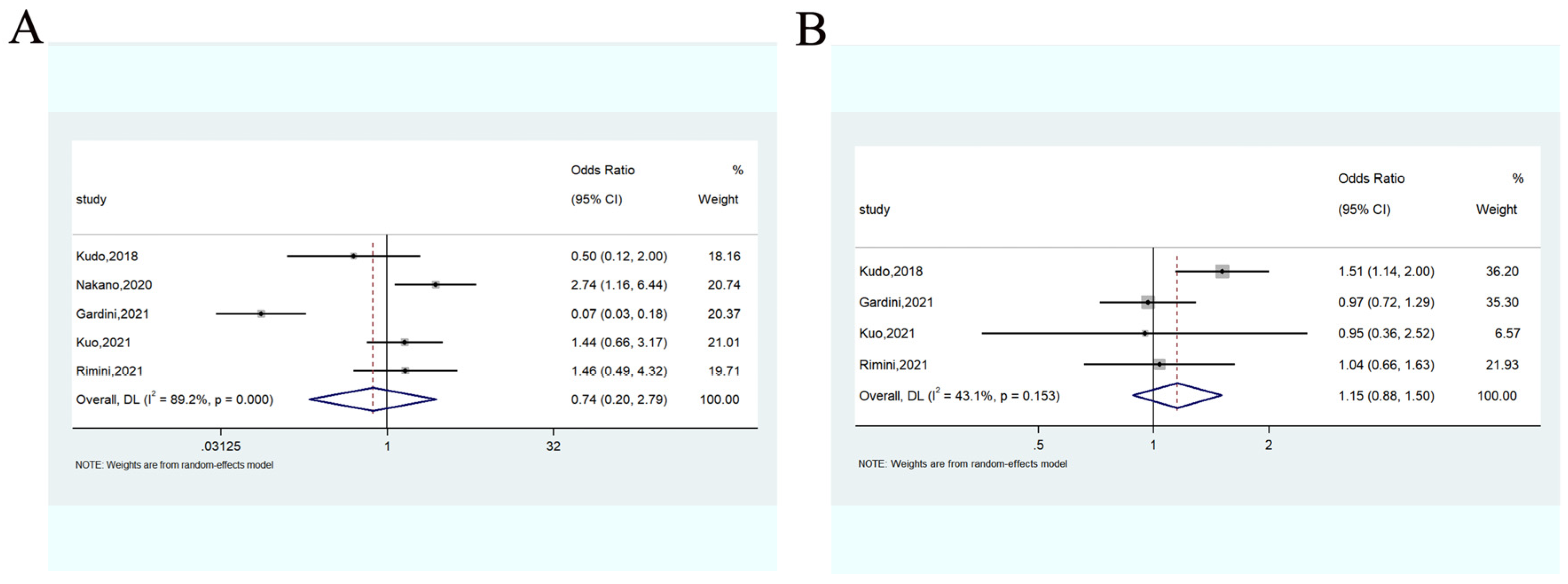
3.3.3. Objective Response Rate
3.3.4. Disease Control Rate
3.3.5. Adverse Events
3.4. Subgroup Analysis
3.4.1. Therapeutic Efficacy Assessment According to the Reflect Criteria
Overall and Progression-Free Survival
Objective Response Rate and Disease Control Rate
3.4.2. Therapeutic Efficacy Assessment According to the Study Background
Overall Survival and Progression-Free Survival
Objective Response Rate and Disease Control Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, M.; Zhu, J.; Qu, J.; Qin, K.; Zhao, D.; Wang, L.; Dong, L.; Zhang, X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 2020, 132, 110797. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Rehman, O.; Jaferi, U.; Padda, I.; Khehra, N.; Atwal, H.; Mossabeh, D.; Bhangu, R. Overview of lenvatinib as a targeted therapy for advanced hepatocellular carcinoma. Clin. Exp. Hepatol. 2021, 7, 249–257. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, Y.B.; Moon, H.; Choi, N.R.; Kim, M.A.; Jang, H.; Nam, J.Y.; Cho, E.J.; Lee, J.H.; Yu, S.J.; et al. Effectiveness of Lenvatinib Versus Sorafenib for Unresectable Hepatocellular Carcinoma in Patients with Hepatic Decompensation. Dig. Dis. Sci. 2022, 67, 4939–4949. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 18 May 2022).
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Takahashi, A.; Moriguchi, M.; Seko, Y.; Ishikawa, H.; Yo, T.; Kimura, H.; Fujii, H.; Shima, T.; Mitsumoto, Y.; Ishiba, H.; et al. Impact of Relative Dose Intensity of Early-phase Lenvatinib Treatment on Therapeutic Response in Hepatocellular Carcinoma. Anticancer Res. 2019, 39, 5149–5156. [Google Scholar] [CrossRef]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE 2020, 15, e0244370. [Google Scholar] [CrossRef]
- Maruta, S.; Ogasawara, S.; Ooka, Y.; Obu, M.; Inoue, M.; Itokawa, N.; Haga, Y.; Seki, A.; Okabe, S.; Azemoto, R.; et al. Potential of Lenvatinib for an Expanded Indication from the REFLECT Trial in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 382–396. [Google Scholar] [CrossRef]
- Sho, T.; Suda, G.; Ogawa, K.; Kimura, M.; Shimazaki, T.; Maehara, O.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open 2020, 4, 54–60. [Google Scholar] [CrossRef]
- Goh, M.J.; Oh, J.H.; Park, Y.; Kim, J.; Kang, W.; Sinn, D.H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; et al. Efficacy and Safety of Lenvatinib Therapy for Unresectable Hepatocellular Carcinoma in a Real-World Practice in Korea. Liver Cancer 2021, 10, 52–62. [Google Scholar] [CrossRef]
- Koroki, K.; Kanogawa, N.; Maruta, S.; Ogasawara, S.; Iino, Y.; Obu, M.; Okubo, T.; Itokawa, N.; Maeda, T.; Inoue, M.; et al. Posttreatment after Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2021, 10, 473–484. [Google Scholar] [CrossRef]
- Shimozato, N.; Namisaki, T.; Okano, A.; Ohana, M.; Kinoshita, D.; Kawasaki, T.; Aihara, Y.; Nakatani, T.; Kinoshita, H.; Ann, T.; et al. Efficacy and Safety of Lenvatinib for Patients With Advanced Hepatocellular Carcinoma: A Retrospective, Real-world Study Conducted in Japan. Anticancer Res. 2022, 42, 173–183. [Google Scholar] [CrossRef]
- Singal, A.G.; Nagar, S.P.; Hitchens, A.; Davis, K.L.; Iyer, S. Real-world effectiveness of lenvatinib monotherapy among unresectable hepatocellular carcinoma patients in the USA. Future Oncol. 2021, 17, 2759–2768. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fukushima, T.; Ueno, M.; Moriya, S.; Chuma, M.; Numata, K.; Tsuruya, K.; Hirose, S.; Kagawa, T.; Hattori, N.; et al. A prospective observational cohort study of lenvatinib as initial treatment in patients with BCLC-defined stage B hepatocellular carcinoma. BMC Cancer 2022, 22, 517. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Anticancer Res. 2020, 40, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kuromatsu, R.; Niizeki, T.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; Noda, Y.; Kamachi, N.; Koga, H.; et al. Primary Treatment with Molecular-Targeted Agents for Hepatocellular Carcinoma: A Propensity Score-matching Analysis. Hepatol. Commun. 2020, 4, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Casadei-Gardini, A.; Scartozzi, M.; Tada, T.; Yoo, C.; Shimose, S.; Masi, G.; Lonardi, S.; Frassineti, L.G.; Nicola, S.; Piscaglia, F.; et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: An inverse probability of treatment weighting analysis. Liver Int. 2021, 41, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.H.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Kim, D.Y.; Kim, S.U. Lenvatinib is independently associated with the reduced risk of progressive disease when compared with sorafenib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021, 36, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Ueno, T.; Namikawa, M.; Tojima, H.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. A change in the timing for starting systemic therapies for hepatocellular carcinoma: The comparison of sorafenib and lenvatinib as the first-line treatment. Acta Gastroenterol. Belg. 2021, 84, 65–72. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lu, S.N.; Chen, Y.Y.; Kee, K.M.; Yen, Y.H.; Hung, C.H.; Hu, T.H.; Chen, C.H.; Wang, J.H. Real-World Lenvatinib Versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front. Oncol. 2021, 11, 737767. [Google Scholar] [CrossRef]
- Rimini, M.; Shimose, S.; Lonardi, S.; Tada, T.; Masi, G.; Iwamoto, H.; Lai, E.; Burgio, V.; Hiraoka, A.; Ishikawa, T.; et al. Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: A multi-institutional matched case-control study. Hepatol. Res. 2021, 51, 1229–1241. [Google Scholar] [CrossRef]
- Tomonari, T.; Sato, Y.; Tani, J.; Hirose, A.; Ogawa, C.; Morishita, A.; Tanaka, H.; Tanaka, T.; Taniguchi, T.; Okamoto, K.; et al. Comparison of therapeutic outcomes of sorafenib and lenvatinib as primary treatments for hepatocellular carcinoma with a focus on molecular-targeted agent sequential therapy: A propensity score-matched analysis. Hepatol. Res. 2021, 51, 472–481. [Google Scholar] [CrossRef]
- Xu, Y.J.; Lai, Z.C.; He, M.K.; Bu, X.Y.; Chen, H.W.; Zhou, Y.M.; Xu, L.; Wei, W.; Zhang, Y.J.; Chen, M.S.; et al. Toripalimab Combined with Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211063848. [Google Scholar] [CrossRef]
- Choi, N.R.; Kim, J.Y.; Hong, J.H.; Hur, M.H.; Cho, H.; Park, M.K.; Kim, J.; Lee, Y.B.; Cho, E.J.; Lee, J.H.; et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2022, 22, 135. [Google Scholar] [CrossRef]
- Kim, B.K.; Cheon, J.; Kim, H.; Kang, B.; Ha, Y.; Kim, D.Y.; Hwang, S.G.; Chon, Y.E.; Chon, H.J. Atezolizumab/Bevacizumab vs. Lenvatinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Real-World, Multi-Center Study. Cancers 2022, 14, 1747. [Google Scholar] [CrossRef]
- Lee, S.W.; Yang, S.S.; Lien, H.C.; Peng, Y.C.; Ko, C.W.; Lee, T.Y. Efficacy of Lenvatinib and Sorafenib in the Real-World First-Line Treatment of Advanced-Stage Hepatocellular Carcinoma in a Taiwanese Population. J. Clin. Med. 2022, 11, 1444. [Google Scholar] [CrossRef]
- Suzuki, E.; Kaneko, S.; Okusaka, T.; Ikeda, M.; Yamaguchi, K.; Sugimoto, R.; Aramaki, T.; Asagi, A.; Yasui, K.; Sano, K.; et al. A multicenter Phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn J. Clin. Oncol. 2018, 48, 317–321. [Google Scholar] [CrossRef]
- Inghilesi, A.L.; Gallori, D.; Antonuzzo, L.; Forte, P.; Tomcikova, D.; Arena, U.; Colagrande, S.; Pradella, S.; Fani, B.; Gianni, E.; et al. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J. Gastroenterol. 2014, 20, 786–794. [Google Scholar] [CrossRef]
- Welland, S.; Leyh, C.; Finkelmeier, F.; Jefremow, A.; Shmanko, K.; Gonzalez-Carmona, M.A.; Kandulski, A.; Jeliazkova, P.; Best, J.; Fründt, T.W.; et al. Real-World Data for Lenvatinib in Hepatocellular Carcinoma (ELEVATOR): A Retrospective Multicenter Study. Liver Cancer 2022, 11, 219–232. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, L.; Li, N.; Zheng, B.; Liu, M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. J. Comp. Eff. Res. 2020, 9, 553–562. [Google Scholar] [CrossRef]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef]
- Wu, H.; Ding, X.; Zhang, Y.; Li, W.; Chen, J. Incidence and risk of hypertension with lenvatinib in treatment of solid tumors: An updated systematic review and meta-analysis. J. Clin. Hypertens 2022, 24, 667–676. [Google Scholar] [CrossRef]
- Tlemsani, C.; Mir, O.; Boudou-Rouquette, P.; Huillard, O.; Maley, K.; Ropert, S.; Coriat, R.; Goldwasser, F. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target. Oncol. 2011, 6, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; De Ciuceis, C.; Porteri, E.; Agabiti-Rosei, C.; Agabiti-Rosei, E. Use of Antihypertensive Drugs in Neoplastic Patients. High Blood Press Cardiovasc. Prev. 2017, 24, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bair, S.M.; Choueiri, T.K.; Moslehi, J. Cardiovascular complications associated with novel angiogenesis inhibitors: Emerging evidence and evolving perspectives. Trends Cardiovasc. Med. 2013, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Gadaleta-Caldarola, G.; Brandi, G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open. 2022, 7, 100591. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhu, M.X.; Zhang, P.F.; Huang, X.Y.; Wan, J.K.; Yao, X.Z.; Hu, Z.T.; Chai, X.Q.; Peng, R.; Yang, X.; et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022, 77, 163–176. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, N.; Shi, L.; Li, F.; Meng, F.; Xie, X.; Xu, Z.; Wang, F. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: A retrospective, real-world study. Heliyon 2022, 8, e09538. [Google Scholar] [CrossRef]
- Rizzo, A.; Nannini, M.; Novelli, M.; Dalia Ricci, A.; Scioscio, V.D.; Pantaleo, M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936932. [Google Scholar] [CrossRef]


| Study (Years) | Country | Regimen | MFUT | Patients | Age | Female | ECOG PS: 0/1/2 | Child–Pugh Class: A/B/C | HBV/HCV | MVI | EHS | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-arm study | ||||||||||||
| Takahashi, 2019 [14] | Japan | Lenvatinib | 6.6 | 50 | Median = 78 | 12 | 37/12/1 | 47/3/0 | 9/18 | 8 | 13 | 6 a |
| Hayashi, 2020 [15] | Japan | Lenvatinib | 6.9 | 53 | Median = 73 | 11 | NA | 53/0/0 | 7/15 | 10 | 18 | 6 a |
| Maruta, 2020 [16] | Japan | Lenvatinib | 6.7 | 95 | 47 (≤50 years) | 20 | 90 (0 or 1) | 84/11/0 | 13/41 | 25 | 33 | 7 a |
| Sho, 2019 [17] | Japan | Lenvatinib | NA | 18 | Median = 75 | 0 | 11/7/0 | 18/0/0 | 3/2 | NA | 4 | 7 a |
| Goh, 2021 [18] | Korea | Lenvatinib | 7.2 | 48 | Median = 57 | 7 | 38/NA/NA | 48/0/0 | NA | 20 | 39 | 7 a |
| Koroki, 2021 [19] | Japan | Lenvatinib | NA | 178 | 94 (≤73 years) | 33 | 169 (0 or 1) | 150/NA/NA | 25/70 | 45 | 64 | 7 a |
| Shimozato, 2022 [20] | Japan | Lenvatinib | NA | 98 | Median = 76 | 20 | 91/7/0 | 98/0/0 | 19/34 | 14 | 20 | 6 a |
| Singal, 2021 [21] | USA | Lenvatinib | 9.1 | 233 | Median = 62.9 | 75 | 75/147/NA | 104/92/17 | 36/84 | NA | NA | 7 a |
| Kobayashi,2022 [22] | Japan | Lenvatinib | NA | 31 | Median = 77 | 2 | 31/0/0 | 31/0/0 | 3/6 | 0 | NA | 6 a |
| Double-arm study | ||||||||||||
| Kudo, 2018 [8] | Multinational | Lenvatinib vs. Sorafenib | 27.7 | 478 476 | Mean = 61.3 Mean = 61.2 | 73 75 | 304/174/0 301/175/0 | 475/3/0 471/5/0 | 251/91 228/126 | 329 336 | 291 295 | 2 b |
| Kudo, 2019 [23] | Japan | Lenvatinib vs. TACE | 23 | 30 60 | Mean = 68.2 Mean = 72.4 | 6 18 | 30/0/0 60/0/0 | 30/0/0 60/0/0 | 7/12 29/10 | 0 0 | 0 0 | 7 a |
| Kuzuya, 2020 [24] | Japan | Lenvatinib vs. Sorafenib | NA | 13 13 | Median = 70 Median = 67 | 2 2 | 12/1/0 8/5/0 | 13/0/0 13/0/0 | 2/2 2/5 | NA | 3 7 | 6 a |
| Nakano, 2020 [25] | Japan | Lenvatinib vs. Sorafenib | 7.3 10.5 | 146 146 | Mean = 72.8 Mean = 72.8 | 21 25 | NA | 134/12/0 137/9/0 | 25/77 24/81 | 21 21 | 56 55 | 6 a |
| Gardini, 2021 [26] | Multinational | Lenvatinib vs. Sorafenib | 15.8 30.7 | 360 562 | Mean = 66.7 Mean = 66.2 | 67 105 | 110 (1 or 2) 197 (1 or 2) | 324/NA 498 | 93/138 164/206 | 196 156 | 146 255 | 7 a |
| Kim, 2021 [27] | Korean | Lenvatinib vs. Sorafenib | NA | 44 61 | Median = 56 Median = 64 | 17 10 | 41 (0 or 1) 59 (0 or 1) | 36 (A and B) 56 (A and B) | 27/NA 45/NA | 26 23 | 25 32 | 7 a |
| Hatanaka, 2021 [28] | Japan | Lenvatinib vs. Sorafenib | 9.8 10 | 56 375 | Median = 73.5 Median = 71.0 | 14 75 | NA | 50/6/0 304/71/0 | 3/32 40/224 | NA | NA | 6 a |
| Kuo, 2021 [29] | Taiwan (China) | Lenvatinib vs. Sorafenib | NA | 70 140 | Mean = 65 Mean = 65.7 | 20 40 | NA | 68/2/0 138/2/0 | 36/22 75/34 | 33 62 | 28 64 | 7 a |
| Rimini, 2021 [30] | Multinational | Lenvatinib vs. Sorafenib | NA | 92 92 | 23 (<65 years) 33 (<65 years) | 17 11 | 70/22/0 65/27/0 | 87/5/0 85/7/0 | 18/38 15/41 | 8 10 | 44 44 | 7 a |
| Tomonari, 2021 [31] | Japan | Lenvatinib vs. Sorafenib | 9.6 | 52 52 | Median = 70.0 Median = 71.0 | 16 17 | 38/14/0 37/15/0 | 52/0/0 52/0/0 | 15/18 10/19 | 11 9 | 10 9 | 7 a |
| Xu, 2021 [32] | China | Lenvatinib vs. Toripalimab plus HAIC | NA | 47 47 | 19 (≤50 years) 21 (≤50 years) | 6 5 | 4/32/11 5/35/7 | 47/0/0 47/0/0 | 42/NA 43/0 | NA | 18 15 | 6 a |
| Choi, 2022 [33] | Korea | Lenvatinib vs. Sorafenib | 4.7 6.7 | 44 88 | Median = 58.0 Median = 58.0 | 4 8 | 23/9/NA 48/29/NA | 29/13/2 63/19/6 | 44/NA 88/NA | NA | 31 59 | 6 a |
| Kim, 2022 [34] | Korea | Lenvatinib vs. Atezolizumab/Bevacizumab | 7.2 7.7 | 146 86 | Median = 62 Median = 62 | 22 16 | 105/41(1 or 2) 36/50 (1 or 2) | 127/19/0 82/4/0 | 90/19 62/3 | 76 43 | 91 37 | 7 a |
| Lee, 2022 [35] | Taiwan (China) | Lenvatinib vs. Sorafenib | NA | 22 44 | Mean = 63.95 Mean = 63.77 | 4 8 | NA | 22/0/0 44/0/0 | 12/6 24/13 | 13 25 | 11 23 | 7 a |
| Park, 2022 [9] | Korea | Lenvatinib vs. Sorafenib | 3.7 | 34 60 | Mean = 62 Mean = 65 | 5 13 | NA | 0/30/4 0/56/4 | 26/NA 43/NA | 21 40 | 23 37 | 6 a |
| Adverse Events | All Grades | Grades 3 and 4 | ||||
|---|---|---|---|---|---|---|
| No. of Studies Included | Total Events | Total Patients | No. of Studies Included | Total Events | Total Patients | |
| Diarrhea | 19 | 493 | 2015 | 14 | 53 | 1726 |
| Hypertension | 18 | 604 | 1664 | 14 | 174 | 1397 |
| PPES | 18 | 552 | 1869 | 12 | 35 | 1220 |
| Fatigue | 17 | 586 | 1642 | 12 | 80 | 1353 |
| Proteinuria | 17 | 394 | 1633 | 13 | 83 | 1366 |
| Decreased appetite | 16 | 573 | 1568 | 12 | 76 | 1301 |
| Hypothyroidism | 16 | 343 | 1602 | 12 | 9 | 1335 |
| Decreased PC | 10 | 187 | 1030 | 7 | 36 | 909 |
| Rash | 10 | 82 | 1048 | 9 | 2 | 1030 |
| Elevated AST | 10 | 118 | 1045 | 9 | 47 | 995 |
| Hepatic encephalopathy | 7 | 33 | 431 | 5 | 12 | 363 |
| Bilirubin elevation | 7 | 79 | 476 | 7 | 14 | 476 |
| Dysphonia | 6 | 138 | 780 | 6 | 2 | 780 |
| Nausea | 6 | 133 | 812 | 5 | 5 | 762 |
| Vomiting | 6 | 99 | 817 | 6 | 7 | 817 |
| Abdominal pain | 5 | 113 | 668 | 5 | 9 | 668 |
| Decreased weight | 5 | 203 | 698 | 4 | 38 | 680 |
| Fever | 3 | 4 | 83 | 2 | 0 | 65 |
| Stomatitis | 3 | 8 | 101 | 2 | 0 | 83 |
| Alopecia | 3 | 14 | 572 | 3 | 0 | 572 |
| Constipation | 2 | 78 | 520 | 2 | 3 | 520 |
| Increased SCR | 2 | 4 | 62 | 2 | 0 | 62 |
| Decreased albumin | 2 | 4 | 83 | 1 | 0 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, Y.; Yu, J.; Wu, H.; Zhou, Y. Lenvatinib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5525. https://doi.org/10.3390/cancers14225525
Wang S, Wang Y, Yu J, Wu H, Zhou Y. Lenvatinib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(22):5525. https://doi.org/10.3390/cancers14225525
Chicago/Turabian StyleWang, Shijie, Yiting Wang, Jiangtao Yu, Huaxing Wu, and Yanming Zhou. 2022. "Lenvatinib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis" Cancers 14, no. 22: 5525. https://doi.org/10.3390/cancers14225525
APA StyleWang, S., Wang, Y., Yu, J., Wu, H., & Zhou, Y. (2022). Lenvatinib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 14(22), 5525. https://doi.org/10.3390/cancers14225525





