Advanced Neuroimaging Approaches to Pediatric Brain Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Pediatric Brain Tumors
2.1. Overview of Pediatric Brain Tumors
2.2. Medulloblastoma
2.3. Glioma
2.4. Ependymoma
3. MRI Techniques
3.1. Introduction to MRI Modalities
3.2. Diffusion-Weighed Imaging (DWI)
3.3. Diffusion Tensor Imaging (DTI) and Tractography
3.4. Functional MRI (fMRI)
3.5. Arterial Spin Labeling (ASL) Perfusion Imaging
3.6. Magnetic Resonance Spectroscopy (MRS)
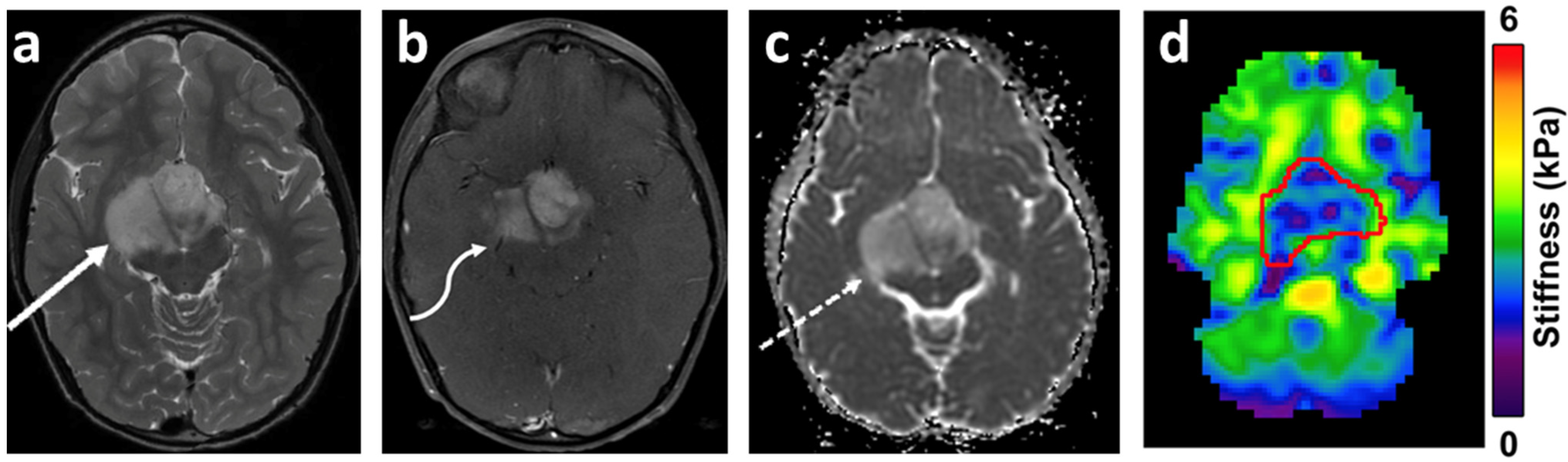
3.7. Magnetic Resonance Elastography (MRE)
3.8. Amide Proton Transfer (APT)-Weighted Imaging
3.9. Radiomics and Radiogenomics
3.10. Response Evaluation of Pediatric Brain Tumors
4. Positron Emission Tomography (PET) Imaging
4.1. Introduction to PET Imaging
4.2. Investigational Probes
5. Single-Photon Emission Computed Tomography (SPECT) Investigative Tracers
6. Multimodality Fusion Techniques and Treatment Planning
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.C.; Minino, A.M.; Anderson, R.N. Declines in Cancer Death Rates among Children and Adolescents in the United States, 1999–2014. In NCHS Data Brief; Department Of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MA, USA, 2016; pp. 1–8. [Google Scholar]
- Rineer, J.; Schreiber, D.; Choi, K.; Rotman, M. Characterization and outcomes of infratentorial malignant glioma: A population-based study using the Surveillance Epidemiology and End-Results database. Radiother. Oncol. 2010, 95, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lannering, B.; Sandström, P.-E.; Holm, S.; Lundgren, J.; Pfeifer, S.; Samuelsson, U.; Strömberg, B.; Gustafsson, G. For the Swedish Childhood CNS Tumor Working Group (VCTB) Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984–2005. Acta Paediatr. 2009, 98, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Hales, P.W.; D’Arco, F.; Cooper, J.; Pfeuffer, J.; Hargrave, D.; Mankad, K.; Clark, C. Arterial spin labelling and diffusion-weighted imaging in paediatric brain tumours. NeuroImage Clin. 2019, 22, 101696. [Google Scholar] [CrossRef]
- Thust, S.C.; Bent, M.J.V.D.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef]
- Lee, J.; Wang, N.; Turk, S.; Mohammed, S.; Lobo, R.; Kim, J.; Liao, E.; Camelo-Piragua, S.; Kim, M.; Junck, L.; et al. Discriminating pseudoprogression and true progression in diffuse infiltrating glioma using multi-parametric MRI data through deep learning. Sci. Rep. 2020, 10, 20331. [Google Scholar] [CrossRef]
- Li, M.; Ren, X.; Dong, G.; Wang, J.; Jiang, H.; Yang, C.; Zhao, X.; Zhu, Q.; Cui, Y.; Yu, K.; et al. Corrigendum: Distinguishing Pseudoprogression From True Early Progression in Isocitrate Dehydrogenase Wild-Type Glioblastoma by Interrogating Clinical, Radiological and Molecular Features. Front. Oncol. 2021, 11, 700599. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
- Le Fèvre, C.; Constans, J.-M.; Chambrelant, I.; Antoni, D.; Bund, C.; Leroy-Freschini, B.; Schott, R.; Cebula, H.; Noël, G. Pseudoprogression versus true progression in glioblastoma patients: A multiapproach literature review. Part 2—Radiological features and metric markers. Crit. Rev. Oncol. 2021, 159, 103230. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Wells, E.M.; Packer, R.J. Pediatric Brain Tumors. Contin. Minneap. Minn. 2015, 21, 373–396. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Phillips, P.C. Pediatric Brain Tumors. Contin. Minneap. Minn. 2017, 23, 1727–1757. [Google Scholar] [CrossRef] [PubMed]
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Iv, M.; Zhou, M.; Shpanskaya, K.; Perreault, S.; Wang, Z.; Tranvinh, E.; Lanzman, B.; Vajapeyam, S.; Vitanza, N.; Fisher, P.; et al. MR Imaging–Based Radiomic Signatures of Distinct Molecular Subgroups of Medulloblastoma. Am. J. Neuroradiol. 2018, 40, 154–161. [Google Scholar] [CrossRef]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef]
- Patay, Z.; Desain, L.A.; Hwang, S.N.; Coan, A.; Li, Y.; Ellison, D.W.; April, A.C. MR Imaging Characteristics of Wingless-Type-Subgroup Pediatric Medulloblastoma. Am. J. Neuroradiol. 2015, 36, 2386–2393. [Google Scholar] [CrossRef]
- Lin, C.Y.; Erkek, S.; Tong, Y.; Yin, L.; Federation, A.J.; Zapatka, M.; Haldipur, P.; Kawauchi, D.; Risch, T.; Warnatz, H.-J.; et al. Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature 2016, 530, 57–62. [Google Scholar] [CrossRef]
- DeSouza, R.M.; Jones, B.R.; Lowis, S.P.; Kurian, K.M. Pediatric medulloblastoma—update on molecular classification driving targeted therapies. Front. Oncol. 2014, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- AlRayahi, J.; Zapotocky, M.; Ramaswamy, V.; Hanagandi, P.; Branson, H.; Mubarak, W.; Raybaud, C.; Laughlin, S. Pediatric Brain Tumor Genetics: What Radiologists Need to Know. Radio Graph. 2018, 38, 2102–2122. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.-Y. Targeting the Hedgehog Pathway in Pediatric Medulloblastoma. Cancers 2015, 7, 2110–2123. [Google Scholar] [CrossRef]
- Cavalli, F.M.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef]
- Neumann, J.E.; Swartling, F.J.; Schüller, U. Medulloblastoma: Experimental models and reality. Acta Neuropathol. 2017, 134, 679–689. [Google Scholar] [CrossRef]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef]
- Colafati, G.S.; Voicu, I.P.; Carducci, C.; Miele, E.; Carai, A.; Di Loreto, S.; Marrazzo, A.; Cacchione, A.; Cecinati, V.; Tornesello, A.; et al. MRI features as a helpful tool to predict the molecular subgroups of medulloblastoma: State of the art. Ther. Adv. Neurol. Disord. 2018, 11. [Google Scholar] [CrossRef]
- Blüml, S.; Margol, A.S.; Sposto, R.; Kennedy, R.J.; Robison, N.J.; Vali, M.; Hung, L.T.; Muthugounder, S.; Finlay, J.L.; Erdreich-Epstein, A.; et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro-Oncology 2015, 18, 126–131. [Google Scholar] [CrossRef]
- Blionas, A.; Giakoumettis, D.; Klonou, A.; Neromyliotis, E.; Karydakis, P.; Themistocleous, M.S. Paediatric gliomas: Diagnosis, molecular biology and management. Ann. Transl. Med. 2018, 6, 251. [Google Scholar] [CrossRef]
- Sturm, D.; Pfister, S.; Jones, D.T.W. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J. Clin. Oncol. 2017, 35, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R.; Reifenberger, G.; Frappaz, D.; Pfister, S.M.; Laprie, A.; Santarius, T.; Roth, P.; Tonn, J.C.; Soffietti, R.; Weller, M.; et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro-Oncology 2017, 20, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Cosnarovici, M.M.; Cosnarovici, R.V.; Piciu, D. Updates on the 2016 World Health Organization Classification of Pediatric Tumors of the Central Nervous System—A systematic review. Med. Pharm. Rep. 2021, 94, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Penman, C.L.; Efaulkner, C.; Lowis, S.P.; Kurian, K.M. Current Understanding of BRAF Alterations in Diagnosis, Prognosis, and Therapeutic Targeting in Pediatric Low-Grade Gliomas. Front. Oncol. 2015, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Filbin, M.G. Gliomas in Children. Skull Base 2018, 38, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ferris, S.P.; Hofmann, J.W.; Solomon, D.A.; Perry, A. Characterization of gliomas: From morphology to molecules. Virchows. Arch. 2017, 471, 257–269. [Google Scholar] [CrossRef]
- Bag, A.K.; Chiang, J.; Patay, Z. Radiohistogenomics of pediatric low-grade neuroepithelial tumors. Neuroradiology 2021, 63, 1185–1213. [Google Scholar] [CrossRef]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.-J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncology 2016, 19, 153–161. [Google Scholar] [CrossRef]
- Modzelewska, K.; Boer, E.; Mosbruger, T.L.; Picard, D.; Anderson, D.; Miles, R.R.; Kroll, M.; Oslund, W.; Pysher, T.J.; Schiffman, J.D.; et al. MEK Inhibitors Reverse Growth of Embryonal Brain Tumors Derived from Oligoneural Precursor Cells. Cell Rep. 2016, 17, 1255–1264. [Google Scholar] [CrossRef]
- Diaz, A.K.; Baker, S.J. The Genetic Signatures of Pediatric High-Grade Glioma: No Longer a One-Act Play. Semin. Radiat. Oncol. 2014, 24, 240–247. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Ramaswamy, V.; Bouffet, E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur. J. Cancer 2016, 70, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Partap, S. Pediatric Ependymoma. J. Child Neurol. 2016, 31, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Malayeri, A.A.; El Khouli, R.H.; Zaheer, A.; Jacobs, M.A.; Corona-Villalobos, C.P.; Kamel, I.R.; Macura, K.J. Principles and Applications of Diffusion-weighted Imaging in Cancer Detection, Staging, and Treatment Follow-up. Radio Graph. 2011, 31, 1773–1791. [Google Scholar] [CrossRef] [PubMed]
- Morana, G.; Alves, C.A.; Tortora, D.; Severino, M.; Nozza, P.; Cama, A.; Ravegnani, M.; D’Apolito, G.; Raso, A.; Milanaccio, C.; et al. Added value of diffusion weighted imaging in pediatric central nervous system embryonal tumors surveillance. Oncotarget 2017, 8, 60401–60413. [Google Scholar] [CrossRef]
- Bull, J.G.; Saunders, D.E.; Clark, C.A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 2011, 22, 447–457. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Yoon, H.-K.; Shin, H.-J.; Roh, H.G.; Cho, J.M. MR imaging of glioblastoma in children: Usefulness of diffusion/perfusion-weighted MRI and MR spectroscopy. Pediatr. Radiol. 2003, 33, 836–842. [Google Scholar] [CrossRef]
- Chen, H.; Panigrahy, A.; Dhall, G.; Finlay, J.; Nelson, M.; Blüml, S. Apparent Diffusion and Fractional Anisotropy of Diffuse Intrinsic Brain Stem Gliomas. Am. J. Neuroradiol. 2010, 31, 1879–1885. [Google Scholar] [CrossRef]
- Choudhri, A.F.; Whitehead, M.T.; Siddiqui, A.; Klimo, P.; Boop, A.F. Diffusion characteristics of pediatric pineal tumors. Neuroradiol. J. 2015, 28, 209–216. [Google Scholar] [CrossRef]
- Gimi, B.; Cederberg, K.; Derinkuyu, B.; Gargan, L.; Koral, K.M.; Bowers, D.C.; Koral, K. Utility of Apparent Diffusion Coefficient Ratios in Distinguishing Common Pediatric Cerebellar Tumors. Acad. Radiol. 2012, 19, 794–800. [Google Scholar] [CrossRef]
- Koral, K.; Mathis, D.; Gimi, B.; Gargan, L.; Weprin, B.; Bowers, D.C.; Margraf, L. Common Pediatric Cerebellar Tumors: Correlation between Cell Densities and Apparent Diffusion Coefficient Metrics. Radiology 2013, 268, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Kralik, S.F.; Taha, A.; Kamer, A.P.; Cardinal, J.S.; Seltman, T.A.; Ho, C.Y. Diffusion Imaging for Tumor Grading of Supratentorial Brain Tumors in the First Year of Life. Am. J. Neuroradiol. 2013, 35, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Orman, G.; Bosemani, T.; Higgins, L.; Carson, K.A.; Huisman, T.A.; Poretti, A. Pediatric Cerebellar Tumors: Does ADC Analysis of Solid, Contrast-Enhancing Tumor Components Correlate Better with Tumor Grade than ADC Analysis of the Entire Tumor? J. Neuroimaging 2014, 25, 785–791. [Google Scholar] [CrossRef]
- Poretti, A.; Meoded, A.; Cohen, K.J.; Grotzer, M.A.; Boltshauser, E.; Huisman, T.A. Apparent diffusion coefficient of pediatric cerebellar tumors: A biomarker of tumor grade? Pediatr. Blood Cancer 2013, 60, 2036–2041. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Zarinabad, N.; Rose, H.; Arvanitis, T.; MacPherson, L.; Pinkey, B.; Oates, A.; Hales, P.; Grundy, R.; Auer, D.; et al. Classification of paediatric brain tumours by diffusion weighted imaging and machine learning. Sci. Rep. 2021, 11, 2987. [Google Scholar] [CrossRef]
- Chamdine, O.; Broniscer, A.; Wu, S.; Gajjar, A.; Qaddoumi, I. Metastatic Low-Grade Gliomas in Children: 20 Years’ Experience at St. Jude Children’s Research Hospital. Pediatr. Blood Cancer 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Shih, R.Y.; Koeller, K.K. Embryonal Tumors of the Central Nervous System: From the Radiologic Pathology Archives. Radio Graph. 2018, 38, 525–541. [Google Scholar] [CrossRef]
- Pierce, T.; Kranz, P.G.; Roth, C.; Leong, D.; Wei, P.; Provenzale, J.M. Use of Apparent Diffusion Coefficient Values for Diagnosis of Pediatric Posterior Fossa Tumors. Neuroradiol. J. 2014, 27, 233–244. [Google Scholar] [CrossRef]
- Jaremko, J.; Jans, L.; Coleman, L.; Ditchfield, M. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. Am. J. Neuroradiol. 2010, 31, 1613–1616. [Google Scholar] [CrossRef]
- Al-Sharydah, A.M.; Al-Arfaj, H.K.; Al-Muhaish, H.S.; Al-Suhaibani, S.S.; Al-Aftan, M.S.; Almedallah, D.K.; Al-Abdulwahhab, A.H.; Al-Hedaithy, A.A.; Al-Jubran, S.A. Can apparent diffusion coefficient values help distinguish between different types of pediatric brain tumors? Eur. J. Radiol. Open 2019, 6, 49–55. [Google Scholar] [CrossRef]
- Koob, M.; Girard, N. Cerebral tumors: Specific features in children. Diagn. Interv. Imaging 2014, 95, 965–983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kazerooni, A.F.; Bagley, S.J.; Akbari, H.; Saxena, S.; Bagheri, S.; Guo, J.; Chawla, S.; Nabavizadeh, A.; Mohan, S.; Bakas, S.; et al. Applications of Radiomics and Radiogenomics in High-Grade Gliomas in the Era of Precision Medicine. Cancers 2021, 13, 5921. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Hassanein, S.; Bisdas, S.; Rees, J.H.; Hyare, H.; Maynard, J.A.; Brandner, S.; Tur, C.; Jäger, H.R.; Yousry, T.A.; et al. Apparent diffusion coefficient for molecular subtyping of non-gadolinium-enhancing WHO grade II/III glioma: Volumetric segmentation versus two-dimensional region of interest analysis. Eur. Radiol. 2018, 28, 3779–3788. [Google Scholar] [CrossRef]
- Wu, C.-C.; Jain, R.; Radmanesh, A.; Poisson, L.; Guo, W.-Y.; Zagzag, D.; Snuderl, M.; Placantonakis, D.; Golfinos, J.; Chi, A. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from The Cancer Genome Atlas. Am. J. Neuroradiol. 2018, 39, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, C.; Vajapeyam, S.; Brown, D.; Kao, P.-C.; Ma, C.; Greenspan, L.; Gupta, N.; Goumnerova, L.; Bandopahayay, P.; Dubois, F.; et al. MR Imaging Correlates for Molecular and Mutational Analyses in Children with Diffuse Intrinsic Pontine Glioma. Am. J. Neuroradiol. 2020, 41, 874–881. [Google Scholar] [CrossRef]
- Ramaglia, A.; Tortora, D.; Mankad, K.; Lequin, M.; Severino, M.; D’Arco, F.; Löbel, U.; Benenati, M.; de Leng, W.W.J.; De Marco, P.; et al. Role of diffusion weighted imaging for differentiating cerebral pilocytic astrocytoma and ganglioglioma BRAF V600E-mutant from wild type. Neuroradiology 2019, 62, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, P.; Jonasson, L.; Maeder, P.; Thiran, J.-P.; Wedeen, V.J.; Meuli, R. Understanding Diffusion MR Imaging Techniques: From Scalar Diffusion-weighted Imaging to Diffusion Tensor Imaging and Beyond. Radio Graph. 2006, 26, S205–S223. [Google Scholar] [CrossRef]
- Salama, G.R.; Heier, L.A.; Patel, P.; Ramakrishna, R.; Magge, R.; Tsiouris, A.J. Diffusion Weighted/Tensor Imaging, Functional MRI and Perfusion Weighted Imaging in Glioblastoma—Foundations and Future. Front. Neurol. 2018, 8, 660. [Google Scholar] [CrossRef]
- Potgieser, A.R.; Wagemakers, M.; van Hulzen, A.L.; de Jong, B.M.; Hoving, E.W.; Groen, R.J. The role of diffusion tensor imaging in brain tumor surgery: A review of the literature. Clin. Neurol. Neurosurg. 2014, 124, 51–58. [Google Scholar] [CrossRef]
- Zhu, F.-P.; Wu, J.-S.; Song, Y.-Y.; Yao, C.-J.; Zhuang, D.-X.; Xu, G.; Tang, W.-J.; Qin, Z.-Y.; Mao, Y.; Zhou, L.-F. Clinical Application of Motor Pathway Mapping Using Diffusion Tensor Imaging Tractography and Intraoperative Direct Subcortical Stimulation in Cerebral Glioma Surgery: A prospective cohort study. Neurosurgery 2012, 71, discussion 1170–1184. [Google Scholar] [CrossRef]
- Wu, J.-S.; Zhou, L.-F.; Tang, W.-J.; Mao, Y.; Hu, J.; Song, Y.-Y.; Hong, X.-N.; Du, G.-H. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007, 61, discussion 935–949. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, Techniques, and Applications of T2*-based MR Imaging and Its Special Applications. Radio Graph. 2009, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Stippich, C.; Blatow, M. Clinical Functional MRI, Presurgical Functional Neuroimaging; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3540244697. [Google Scholar]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef]
- Trinh, V.T.; Fahim, D.K.; Maldaun, M.V.; Shah, K.; McCutcheon, I.E.; Rao, G.; Lang, F.; Weinberg, J.; Sawaya, R.; Suki, D.; et al. Impact of Preoperative Functional Magnetic Resonance Imaging during Awake Craniotomy Procedures for Intraoperative Guidance and Complication Avoidance. Ster. Funct. Neurosurg. 2014, 92, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Krieg, S.M.; Meyer, B.; Ringel, F. Functional preoperative and intraoperative mapping and monitoring: Increasing safety and efficacy in glioma surgery. Neurosurg. Focus 2015, 38, E3. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Sakatani, K.; Katayama, Y.; Murata, Y.; Hoshino, T.; Fukaya, C.; Yamamoto, T. Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors. NeuroImage 2004, 21, 1464–1471. [Google Scholar] [CrossRef]
- Ulmer, J.L.; Hacein-Bey, L.; Mathews, V.P.; Mueller, W.M.; DeYoe, E.A.; Prost, R.W.; Meyer, G.A.; Krouwer, H.G.; Schmainda, K.M. Lesion-induced Pseudo-dominance at Functional Magnetic Resonance Imaging: Implications for Preoperative Assessments. Neurosurgery 2004, 55, discussion 569–581. [Google Scholar] [CrossRef]
- Lee, M.H.; Miller-Thomas, M.M.; Benzinger, T.; Marcus, D.S.; Hacker, C.D.; Leuthardt, E.C.; Shimony, J.S. Clinical Resting-state fMRI in the Preoperative Setting: Are We Ready for Prime Time? Top. Magn. Reson. Imaging 2016, 25, 11–18. [Google Scholar] [CrossRef]
- Hadjiabadi, D.H.; Pung, L.; Zhang, J.; Ward, B.; Lim, W.-T.; Kalavar, M.; Thakor, N.V.; Biswal, B.B.; Pathak, A.P. Brain tumors disrupt the resting-state connectome. NeuroImage Clin. 2018, 18, 279–289. [Google Scholar] [CrossRef]
- Nenning, K.-H.; Furtner, J.; Kiesel, B.; Schwartz, E.; Roetzer, T.; Fortelny, N.; Bock, C.; Grisold, A.; Marko, M.; Leutmezer, F.; et al. Distributed changes of the functional connectome in patients with glioblastoma. Sci. Rep. 2020, 10, 18312. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, N.; Teng, C.; Li, X. Alternations and Applications of the Structural and Functional Connectome in Gliomas: A Mini-Review. Front. Neurosci. 2022, 16, 856808. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.L.; Bhatia, S.; Carpenter, J.S. Quantitative comparisons on hand motor functional areas determined by resting state and task BOLD fMRI and anatomical MRI for pre-surgical planning of patients with brain tumors. NeuroImage Clin. 2016, 11, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Sair, H.I.; Yahyavi-Firouz-Abadi, N.; Calhoun, V.D.; Airan, R.D.; Agarwal, S.; Intrapiromkul, J.; Choe, A.S.; Gujar, S.K.; Caffo, B.; Lindquist, M.A.; et al. Presurgical brain mapping of the language network in patients with brain tumors using resting-state f MRI: Comparison with task f MRI. Hum. Brain Mapp. 2015, 37, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Rigolo, L.; Norton, I.H.; Huang, R.; Wu, W.; Orringer, D.; Mukundan, S., Jr.; Golby, A.J. Defining language networks from resting-state fMRI for surgical planning-a feasibility study. Hum. Brain Mapp. 2013, 35, 1018–1030. [Google Scholar] [CrossRef]
- Branco, P.; Seixas, D.; Deprez, S.; Kovacs, S.; Peeters, R.; Castro, S.L.; Sunaert, S. Resting-State Functional Magnetic Resonance Imaging for Language Preoperative Planning. Front. Hum. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef]
- Lemée, J.; Berro, D.H.; Bernard, F.; Chinier, E.; Leiber, L.; Menei, P.; Ter Minassian, A. Resting-state functional magnetic resonance imaging versus task-based activity for language mapping and correlation with perioperative cortical mapping. Brain Behav. 2019, 9, e01362. [Google Scholar] [CrossRef]
- Stavinoha, P.L.; Askins, M.A.; Powell, S.K.; Smiley, N.P.; Robert, R.S. Neurocognitive and Psychosocial Outcomes in Pediatric Brain Tumor Survivors. Bioengineering 2018, 5, 73. [Google Scholar] [CrossRef]
- Kerner, D.M.; Nikam, R.; Kandula, V.V.; Averill, L.W. Pearls and Pitfalls in Arterial Spin Labeling Perfusion-Weighted Imaging in Clinical Pediatric Imaging. Semin. Ultrasound CT MRI 2022, 43, 19–30. [Google Scholar] [CrossRef]
- Ata, E.S.; Turgut, M.; Eraslan, C.; Dayanır, Y. Comparison between dynamic susceptibility contrast magnetic resonance imaging and arterial spin labeling techniques in distinguishing malignant from benign brain tumors. Eur. J. Radiol. 2016, 85, 1545–1553. [Google Scholar] [CrossRef]
- Luan, J.; Wu, M.; Wang, X.; Qiao, L.; Guo, G.; Zhang, C. The diagnostic value of quantitative analysis of ASL, DSC-MRI and DKI in the grading of cerebral gliomas: A meta-analysis. Radiat. Oncol. 2020, 15, 204. [Google Scholar] [CrossRef]
- Kitajima, M.; Uetani, H. Arterial Spin Labeling for Pediatric Central Nervous System Diseases: Techniques and Clinical Applications. Magn. Reson. Med. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Radenkovic, S.; Stosic-Opincal, T.; Lavrnic, S.; Gavrilovic, S.; Lazovic-Popovic, B.; Soldatovic, I.; Maksimovic, R. Differentiation between progression and pseudoprogresion by arterial spin labeling MRI in patients with glioblastoma multiforme. J. BUON 2017, 22, 1061–1067. [Google Scholar] [PubMed]
- Manning, P.; Daghighi, S.; Rajaratnam, M.K.; Parthiban, S.; Bahrami, N.; Dale, A.M.; Bolar, D.; Piccioni, D.E.; McDonald, C.R.; Farid, N. Differentiation of progressive disease from pseudoprogression using 3D PCASL and DSC perfusion MRI in patients with glioblastoma. J. Neuro-Oncol. 2020, 147, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Averill, L.W.; Kandula, V.V.R. Utility of Arterial Spin Labeling MRI in Pediatric Neuroimaging: A Pictorial Essay. Curr. Radiol. Rep. 2017, 5, 37. [Google Scholar] [CrossRef]
- Dangouloff-Ros, V.; Deroulers, C.; Foissac, F.; Badoual, M.; Shotar, E.; Grévent, D.; Calmon, R.; Pagès, M.; Grill, J.; Dufour, C.; et al. Arterial Spin Labeling to Predict Brain Tumor Grading in Children: Correlations between Histopathologic Vascular Density and Perfusion MR Imaging. Radiology 2016, 281, 553–566. [Google Scholar] [CrossRef]
- Dangouloff-Ros, V.; Grevent, D.; Pagès, M.; Blauwblomme, T.; Calmon, R.; Elie, C.; Puget, S.; Sainte-Rose, C.; Brunelle, F.; Varlet, P.; et al. Choroid Plexus Neoplasms: Toward a Distinction between Carcinoma and Papilloma Using Arterial Spin-Labeling. Am. J. Neuroradiol. 2015, 36, 1786–1790. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hiwatashi, A.; Togao, O.; Yamashita, K.; Yoshimoto, K.; Mizoguchi, M.; Suzuki, S.O.; Iwaki, T.; Suzuki, Y.; Honda, H. Correlation between arterial spin-labeling perfusion and histopathological vascular density of pediatric intracranial tumors. J. Neuro-Oncol. 2017, 135, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Morana, G.; Tortora, D.; Staglianò, S.; Nozza, P.; Mascelli, S.; Severino, M.; Piatelli, G.; Consales, A.; Lequin, M.; Garrè, M.L.; et al. Pediatric astrocytic tumor grading: Comparison between arterial spin labeling and dynamic susceptibility contrast MRI perfusion. Neuroradiology 2018, 60, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Yeom, K.W.; Mitchell, L.A.; Lober, R.M.; Barnes, P.D.; Vogel, O.H.; Fisher, P.; Edwards, M.S. Arterial Spin-Labeled Perfusion of Pediatric Brain Tumors. Am. J. Neuroradiol. 2013, 35, 395–401. [Google Scholar] [CrossRef]
- Khashbat, D.; Harada, M.; Abe, T.; Ganbold, M.; Iwamoto, S.; Uyama, N.; Irahara, S.; Otomi, Y.; Kageji, T.; Nagahiro, S. Diagnostic Performance of Arterial Spin Labeling for Grading Nonenhancing Astrocytic Tumors. Magn. Reson. Med. Sci. 2018, 17, 277–282. [Google Scholar] [CrossRef]
- Duc, N.M. Three-Dimensional Pseudo-Continuous Arterial Spin Labeling Parameters Distinguish Pediatric Medulloblastoma and Pilocytic Astrocytoma. Front. Pediatr. 2021, 8, 598190. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, S.A.; Assadsangabi, R.; Hajmomenian, M.; Santi, M.; Vossough, A. High accuracy of arterial spin labeling perfusion imaging in differentiation of pilomyxoid from pilocytic astrocytoma. Neuroradiology 2015, 57, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gareton, A.; Tauziède-Espariat, A.; Dangouloff-Ros, V.; Roux, A.; Saffroy, R.; Castel, D.; Kergrohen, T.; Fina, F.; Figarella-Branger, D.; Pagès, M.; et al. The histomolecular criteria established for adult anaplastic pilocytic astrocytoma are not applicable to the pediatric population. Acta Neuropathol. 2019, 139, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Álvarez-Torres, M.D.M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef]
- Ulmer, S.; Backens, M.; Ahlhelm, F.J. Basic Principles and Clinical Applications of Magnetic Resonance Spectroscopy in Neuroradiology. J. Comput. Assist. Tomogr. 2016, 40, 1–13. [Google Scholar] [CrossRef]
- Liserre, R.; Pinelli, L.; Gasparotti, R. MR spectroscopy in pediatric neuroradiology. Transl. Pediatr. 2021, 10, 1169–1200. [Google Scholar] [CrossRef]
- Panigrahy, A.; Nelson, M.D.; Blüml, S. Magnetic resonance spectroscopy in pediatric neuroradiology: Clinical and research applications. Pediatr. Radiol. 2009, 40, 3–30. [Google Scholar] [CrossRef]
- Sitter, B.; Sjøbakk, T.E.; Larsson, H.B.W.; Kvistad, K.A. Clinical MR spectroscopy of the brain. Tidsskr. Den. Nor. Legeforening 2019, 139. [Google Scholar] [CrossRef]
- Li, Y.; Lafontaine, M.; Chang, S.; Nelson, S.J. Comparison between Short and Long Echo Time Magnetic Resonance Spectroscopic Imaging at 3T and 7T for Evaluating Brain Metabolites in Patients with Glioma. ACS Chem. Neurosci. 2017, 9, 130–137. [Google Scholar] [CrossRef]
- Bisdas, S.; Chadzynski, G.L.; Braun, C.; Schittenhelm, J.; Skardelly, M.; Hagberg, G.E.; Ethofer, T.; Pohmann, R.; Shajan, G.; Engelmann, J.; et al. MR spectroscopy for in vivo assessment of the oncometabolite 2-hydroxyglutarate and its effects on cellular metabolism in human brain gliomas at 9.4T. J. Magn. Reson. Imaging 2016, 44, 823–833. [Google Scholar] [CrossRef]
- Li, X.; Strasser, B.; Jafari-Khouzani, K.; Thapa, B.; Small, J.; Cahill, D.P.; Dietrich, J.; Batchelor, T.T.; Andronesi, O.C. Super-Resolution Whole-Brain 3D MR Spectroscopic Imaging for Mapping D-2-Hydroxyglutarate and Tumor Metabolism in Isocitrate Dehydrogenase 1–mutated Human Gliomas. Radiology 2020, 294, 589–597. [Google Scholar] [CrossRef]
- Brandão, L.A.; Poussaint, T.Y. Posterior Fossa Tumors. Neuroimaging Clin. N. Am. 2017, 27, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Porto, L.; Kieslich, M.; Franz, K.; Lehrbecher, T.; Pilatus, U.; Hattingen, E. Proton magnetic resonance spectroscopic imaging in pediatric low-grade gliomas. Brain Tumor Pathol. 2010, 27, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yamaki, T.; Harada, K.; Houkin, K. In vivo quantification of the metabolites in normal brain and brain tumors by proton MR spectroscopy using water as an internal standard. Magn. Reson. Imaging 2004, 22, 1017–1024. [Google Scholar] [CrossRef]
- Kovanlikaya, A.; Panigrahy, A.; Krieger, M.D.; Gonzalez-Gomez, I.; Ghugre, N.; McComb, J.G.; Gilles, F.H.; Nelson, M.D.; Blüml, S. Untreated Pediatric Primitive Neuroectodermal Tumor in Vivo: Quantitation of Taurine with MR Spectroscopy. Radiology 2005, 236, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, A.; Martínez-Pérez, I.; Baquero, M.; Campistol, J.; Capdevila, A.; Arús, C.; Pujol, J. Taurine Detection by Proton Magnetic Resonance Spectroscopy in Medulloblastoma: Contribution to Noninvasive Differential Diagnosis with Cerebellar Astrocytoma. Neurosurgery 2004, 55, 824–829. discussion 829. [Google Scholar] [CrossRef]
- Wilke, M.; Eidenschink, A.; Muller-Weihrich, S.; Auer, D.P. MR diffusion imaging and 1H spectroscopy in a child with medulloblastoma. A case report. Acta Radiol. 2001, 42, 39–42. [Google Scholar]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef]
- Tietze, A.; Choi, C.; Mickey, B.; Maher, E.A.; Ulhøi, B.P.; Sangill, R.; Lassen-Ramshad, Y.; Lukacova, S.; Østergaard, L.; von Oettingen, G. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J. Neurosurg. 2018, 128, 391–398. [Google Scholar] [CrossRef]
- Pollack, I.F.; for the Children’s Oncology Group; Hamilton, R.L.; Sobol, R.; Nikiforova, M.N.; Lyons-Weiler, M.A.; La Framboise, W.A.; Burger, P.C.; Brat, D.J.; Rosenblum, M.K.; et al. IDH1 mutations are common in malignant gliomas arising in adolescents: A report from the Children’s Oncology Group. Child’s Nerv. Syst. 2010, 27, 87–94. [Google Scholar] [CrossRef]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Park, J.B.; Ra, Y.-S. Pediatric Glioma at the Optic Pathway and Thalamus. J. Korean Neurosurg. Soc. 2018, 61, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Wilson, M.; MacPherson, L.; Arvanitis, T.N.; Davies, N.P.; Peet, A.C. Clinical protocols for 31P MRS of the brain and their use in evaluating optic pathway gliomas in children. Eur. J. Radiol. 2013, 83, e106–e112. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Wilson, M.; Davies, N.P.; MacPherson, L.; English, M.; Arvanitis, T.N.; Peet, A.C. Diagnosing relapse in children’s brain tumors using metabolite profiles. Neuro-Oncology 2013, 16, 156–164. [Google Scholar] [CrossRef]
- Lazareff, J.A.; Gupta, R.K.; Alger, J. Variation of Post-treatment H-MRSI Choline Signal Intensity in Pediatric Gliomas. J. Neuro-Oncol. 1999, 41, 291–298. [Google Scholar] [CrossRef]
- Warren, K.E.; Frank, J.A.; Black, J.L.; Hill, R.S.; Duyn, J.H.; Aikin, A.A.; Lewis, B.K.; Adamson, P.C.; Balis, F.M. Proton Magnetic Resonance Spectroscopic Imaging in Children With Recurrent Primary Brain Tumors. J. Clin. Oncol. 2000, 18, 1020. [Google Scholar] [CrossRef]
- Tzika, A.A.; Astrakas, L.G.; Zarifi, M.K.; Zurakowski, D.; Poussaint, T.Y.; Goumnerova, L.; Tarbell, N.J.; Black, P.M. Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumors. Cancer 2004, 100, 1246–1256. [Google Scholar] [CrossRef]
- Wald, L.; Nelson, S.J.; Day, M.R.; Noworolski, S.E.; Henry, R.G.; Huhn, S.L.; Chang, S.; Prados, M.D.; Sneed, P.K.; Larson, D.A.; et al. Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J. Neurosurg. 1997, 87, 525–534. [Google Scholar] [CrossRef]
- Kazda, T.; Bulik, M.; Pospisil, P.; Lakomy, R.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. NeuroImage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef]
- Manduca, A.; Bayly, P.J.; Ehman, R.L.; Kolipaka, A.; Royston, T.J.; Sack, I.; Sinkus, R.; Van Beers, B.E. MR elastography: Principles, guidelines, and terminology. Magn. Reson. Med. 2020, 85, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; McGarry, M.D.; Gharibans, A.A.; Weaver, J.B.; Paulsen, K.D.; Wang, H.; Olivero, W.C.; Sutton, B.P.; Georgiadis, J.G. Local mechanical properties of white matter structures in the human brain. NeuroImage 2013, 79, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Muthupillai, R.; Lomas, D.J.; Rossman, P.J.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic Resonance Elastography by Direct Visualization of Propagating Acoustic Strain Waves. Science 1995, 269, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Romano, A.J.; Manduca, A.; Ehman, R.L.; Huston, J., 3rd. Stiffness and Beyond: What MR Elastography Can Tell Us about Brain Structure and Function Under Physiologic and Pathologic Conditions. Top. Magn. Reson. Imaging 2018, 27, 305–318. [Google Scholar] [CrossRef]
- Klatt, D.; Hamhaber, U.; Asbach, P.; Braun, J.; Sack, I. Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: A study of brain and liver viscoelasticity. Phys. Med. Biol. 2007, 52, 7281–7294. [Google Scholar] [CrossRef]
- Bunevicius, A.; Schregel, K.; Sinkus, R.; Golby, A.; Patz, S. REVIEW: MR elastography of brain tumors. NeuroImage Clin. 2019, 25, 102109. [Google Scholar] [CrossRef]
- Itamura, K.; Chang, K.-E.; Lucas, J.; Donoho, D.A.; Giannotta, S.; Zada, G. Prospective clinical validation of a meningioma consistency grading scheme: Association with surgical outcomes and extent of tumor resection. J. Neurosurg. 2019, 131, 1356–1360. [Google Scholar] [CrossRef]
- Reiss-Zimmermann, M.; Streitberger, K.-J.; Sack, I.; Braun, J.; Arlt, F.; Fritzsch, D.; Hoffmann, K.-T. High Resolution Imaging of Viscoelastic Properties of Intracranial Tumours by Multi-Frequency Magnetic Resonance Elastography. Clin. Neuroradiol. 2014, 25, 371–378. [Google Scholar] [CrossRef]
- Streitberger, K.-J.; Reiss-Zimmermann, M.; Freimann, F.B.; Bayerl, S.; Guo, J.; Arlt, F.; Wuerfel, J.; Braun, J.; Hoffmann, K.-T.; Sack, I. High-Resolution Mechanical Imaging of Glioblastoma by Multifrequency Magnetic Resonance Elastography. PLoS ONE 2014, 9, e110588. [Google Scholar] [CrossRef]
- Pepin, K.; McGee, K.; Arani, A.; Lake, D.; Glaser, K.; Manduca, A.; Parney, I.; Ehman, R.; Huston, J. MR Elastography Analysis of Glioma Stiffness andIDH1-Mutation Status. Am. J. Neuroradiol. 2017, 39, 31–36. [Google Scholar] [CrossRef]
- Sakai, N.; Takehara, Y.; Yamashita, S.; Ohishi, N.; Kawaji, H.; Sameshima, T.; Baba, S.; Sakahara, H.; Namba, H. Shear Stiffness of 4 Common Intracranial Tumors Measured Using MR Elastography: Comparison with Intraoperative Consistency Grading. Am. J. Neuroradiol. 2016, 37, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.; Fattahi, N.; Van Gompel, J.J.; Arani, A.; Ehman, R.; Huston, J., 3rd. Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary 2016, 19, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Huston, J.; Glaser, K.J.; Manduca, A.; Meyer, F.B.; Lanzino, G.; Morris, J.M.; Felmlee, J.P.; Ehman, R.L. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J. Neurosurg. 2013, 118, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, J.; Peng, Y. Amide Proton Transfer–Weighted MR Imaging of Pediatric Central Nervous System Diseases. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 631–641. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Park, K.J.; Choi, C.G.; Kim, S.J. Histogram Analysis of Amide Proton Transfer Imaging to Identify Contrast-enhancing Low-Grade Brain Tumor That Mimics High-Grade Tumor: Increased Accuracy of MR Perfusion. Radiology 2015, 277, 151–161. [Google Scholar] [CrossRef]
- Yu, H.; Lou, H.; Zou, T.; Wang, X.; Jiang, S.; Huang, Z.; Du, Y.; Jiang, C.; Ma, L.; Zhu, J.; et al. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur. Radiol. 2017, 27, 4516–4524. [Google Scholar] [CrossRef]
- Joo, B.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kang, S.-G.; Kim, S.H.; Zhou, J.; Lee, S.-K. Amide proton transfer imaging might predict survival and IDH mutation status in high-grade glioma. Eur. Radiol. 2019, 29, 6643–6652. [Google Scholar] [CrossRef]
- Sartoretti, E.; Sartoretti, T.; Wyss, M.; Reischauer, C.; van Smoorenburg, L.; Binkert, C.A.; Sartoretti-Schefer, S.; Mannil, M. Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci. Rep. 2021, 11, 5506. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, H.; Wang, X.; Lu, S.; Li, Y.; Feng, L.; Zhang, Y.; Heo, H.-Y.; Lee, D.-H.; Zhou, J.; et al. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur. Radiol. 2015, 26, 64–71. [Google Scholar] [CrossRef]
- Suh, C.H.; Park, J.E.; Jung, S.C.; Choi, C.G.; Kim, S.J.; Kim, H.S. Amide proton transfer-weighted MRI in distinguishing high- and low-grade gliomas: A systematic review and meta-analysis. Neuroradiology 2019, 61, 525–534. [Google Scholar] [CrossRef]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef]
- Pérez-Beteta, J.; Molina-García, D.; Martínez-González, A.; Henares-Molina, A.; Amo-Salas, M.; Luque, B.; Arregui, E.; Calvo, M.; Borrás, J.M.; Martino, J.; et al. Morphological MRI-based features provide pretreatment survival prediction in glioblastoma. Eur. Radiol. 2018, 29, 1968–1977. [Google Scholar] [CrossRef]
- Pérez-Beteta, J.; Molina-García, D.; Martínez-González, A.; Henares-Molina, A.; Amo-Salas, M.; Luque, B.; Arregui, E.; Calvo, M.; Borrás, J.M.; Martino, J.; et al. Correction to: Morphological MRI-based features provide pretreatment survival prediction in glioblastoma. Eur. Radiol. 2018, 29, 2729. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Beteta, J.; Molina-García, D.; Villena, M.; Rodríguez, M.; Velásquez, C.; Martino, J.; Meléndez-Asensio, B.; De Lope, R.; Morcillo, R.; Sepúlveda, J.; et al. Morphologic Features on MR Imaging Classify Multifocal Glioblastomas in Different Prognostic Groups. Am. J. Neuroradiol. 2019, 40, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Kriesen, T.; Glass, A.; Schneider, B.; Piek, J. Volumetric quantification of glioblastoma: Experiences with different measurement techniques and impact on survival. J. Neuro-Oncol. 2017, 135, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Beteta, J.; Molina-García, D.; Ortiz-Alhambra, J.A.; Fernández-Romero, A.; Luque, B.; Arregui, E.; Calvo, M.; Borrás, J.M.; Meléndez, B.; De Lope, R.; et al. Tumor Surface Regularity at MR Imaging Predicts Survival and Response to Surgery in Patients with Glioblastoma. Radiology 2018, 288, 218–225. [Google Scholar] [CrossRef]
- Nicolasjilwan, M.; Hu, Y.; Yan, C.; Meerzaman, D.; Holder, C.A.; Gutman, D.; Jain, R.; Colen, R.; Rubin, D.L.; Zinn, P.O.; et al. Addition of MR imaging features and genetic biomarkers strengthens glioblastoma survival prediction in TCGA patients. J. Neuroradiol. 2014, 42, 212–221. [Google Scholar] [CrossRef]
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2021, 10, 570465. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, L.-F.; Zhang, X.; Hu, Y.-C.; Han, Y.; Liu, Z.-C.; Nan, H.-Y.; Sun, Q.; Sun, Y.-Z.; Yang, Y.; et al. Radiomics strategy for glioma grading using texture features from multiparametric MRI. J. Magn. Reson. Imaging 2018, 48, 1518–1528. [Google Scholar] [CrossRef]
- Wagner, M.; Hainc, N.; Khalvati, F.; Namdar, K.; Figueiredo, L.; Sheng, M.; Laughlin, S.; Shroff, M.; Bouffet, E.; Tabori, U.; et al. Radiomics of Pediatric Low-Grade Gliomas: Toward a Pretherapeutic Differentiation of BRAF-Mutated and BRAF-Fused Tumors. Am. J. Neuroradiol. 2021, 42, 759–765. [Google Scholar] [CrossRef]
- Tam, L.T.; Yeom, K.W.; Wright, J.N.; Jaju, A.; Radmanesh, A.; Han, M.; Toescu, S.; Maleki, M.; Chen, E.; Campion, A.; et al. MRI-based radiomics for prognosis of pediatric diffuse intrinsic pontine glioma: An international study. Neuro-Oncol. Adv. 2021, 3, vdab042. [Google Scholar] [CrossRef] [PubMed]
- Chukwueke, U.N.; Wen, P.Y. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019, 8, CNS28. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Fangusaro, J.; Witt, O.; Driever, P.H.; Bag, A.K.; de Blank, P.; Kadom, N.; Kilburn, L.; Lober, R.M.; Robison, N.J.; Fisher, M.J.; et al. Response assessment in paediatric low-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e305–e316. [Google Scholar] [CrossRef]
- Erker, C.; Tamrazi, B.; Poussaint, T.Y.; Mueller, S.; Mata-Mbemba, D.; Franceschi, E.; Brandes, A.A.; Rao, A.; Haworth, K.B.; Wen, P.Y.; et al. Response assessment in paediatric high-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e317–e329. [Google Scholar] [CrossRef]
- Cooney, T.M.; Cohen, K.J.; Guimaraes, C.V.; Dhall, G.; Leach, J.; Massimino, M.; Erbetta, A.; Chiapparini, L.; Malbari, F.; Kramer, K.; et al. Response assessment in diffuse intrinsic pontine glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e330–e336. [Google Scholar] [CrossRef]
- Phelps, E.M. PET: The merging of biology and imaging into molecular imaging. J. Nucl. Med. 2000, 41, e330–e336. [Google Scholar]
- Jones, T. The imaging science of positron emission tomography. Eur. J. Pediatr. 1996, 23, 807–813. [Google Scholar] [CrossRef]
- Cistaro, A.; Albano, D.; Alongi, P.; Laudicella, R.; Pizzuto, D.; Formica, G.; Romagnolo, C.; Stracuzzi, F.; Frantellizzi, V.; Piccardo, A.; et al. The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors. Curr. Oncol. 2021, 28, 2481–2495. [Google Scholar] [CrossRef]
- Uslu, L.; Donig, J.; Link, M.; Rosenberg, J.; Quon, A.; Daldrup-Link, H.E. Value of 18F-FDG PET and PET/CT for Evaluation of Pediatric Malignancies. J. Nucl. Med. 2015, 56, 274–286. [Google Scholar] [CrossRef]
- Gururangan, S.; Hwang, E.; Herndon, J.E.; Fuchs, H.; George, T.; Coleman, R.E. [18F]Fluorodeoxyglucose-Positron Emission Tomography in Patients with Medulloblastoma. Neurosurgery 2004, 55, 1280–1289, discussion 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Clinical Applications of PET in Brain Tumors. J. Nucl. Med. 2007, 48, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Juhász, C.; Dwivedi, S.; Kamson, D.O.; Michelhaugh, S.K.; Mittal, S. Comparison of Amino Acid Positron Emission Tomographic Radiotracers for Molecular Imaging of Primary and Metastatic Brain Tumors. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Grosse, F.; Wedel, F.; Thomale, U.-W.; Steffen, I.; Koch, A.; Brenner, W.; Plotkin, M.; Driever, P.H. Benefit of Static FET PET in Pretreated Pediatric Brain Tumor Patients with Equivocal Conventional MRI Results. Klin. Padiatr. 2021, 233, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dunkl, V.; Cleff, C.; Stoffels, G.; Judov, N.; Sarikaya-Seiwert, S.; Law, I.; Bøgeskov, L.; Nysom, K.; Andersen, S.B.; Steiger, H.-J.; et al. The Usefulness of Dynamic O-(2-18F-Fluoroethyl)-l-Tyrosine PET in the Clinical Evaluation of Brain Tumors in Children and Adolescents. J. Nucl. Med. 2014, 56, 88–92. [Google Scholar] [CrossRef]
- Misch, M.; Guggemos, A.; Driever, P.H.; Koch, A.; Grosse, F.; Steffen, I.G.; Plotkin, M.; Thomale, U.-W. 18F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Child’s Nerv. Syst. 2014, 31, 261–267. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Milanaccio, C.; Puntoni, M.; Nozza, P.; Cama, A.; Zefiro, D.; Cabria, M.; Rossi, A.; Garrè, M.L. Value of 18F-3,4-Dihydroxyphenylalanine PET/MR Image Fusion in Pediatric Supratentorial Infiltrative Astrocytomas: A Prospective Pilot Study. J. Nucl. Med. 2014, 55, 718–723. [Google Scholar] [CrossRef][Green Version]
- Morana, G.; Piccardo, A.; Puntoni, M.; Nozza, P.; Cama, A.; Raso, A.; Mascelli, S.; Massollo, M.; Milanaccio, C.; Garrè, M.L.; et al. Diagnostic and prognostic value of18F-DOPA PET and1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: A comparative study. Neuro-Oncology 2015, 17, 1637–1647. [Google Scholar] [CrossRef][Green Version]
- Morana, G.; Puntoni, M.; Garrè, M.L.; Massollo, M.; Lopci, E.; Naseri, M.; Severino, M.; Tortora, D.; Rossi, A.; Piccardo, A. Ability of 18F-DOPA PET/CT and fused 18F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur. J. Pediatr. 2016, 43, 1664–1672. [Google Scholar] [CrossRef]
- Gauvain, K.; Ponisio, M.R.; Barone, A.; Grimaldi, M.; Parent, E.; Leeds, H.; Goyal, M.; Rubin, J.; McConathy, J. 18F-FDOPA PET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neuro-Oncol. Pract. 2017, 5, 28–36. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Tortora, D.; Puntoni, M.; Severino, M.; Nozza, P.; Ravegnani, M.; Consales, A.; Mascelli, S.; Raso, A.; et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F–DOPA PET. Eur. J. Pediatr. 2017, 44, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, A.; Etzl, M.; Bandy, D.; Carpenteri, D.; Gieseking, A.; Dvorchik, I.; Kaplan, A. Use of Positron Emission Tomography in the Evaluation of Diffuse Intrinsic Brainstem Gliomas in Children. J. Pediatr. Hematol. 2011, 33, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Laser, B.S.; Merchant, T.E.; Indelicato, D.J.; Hua, C.-H.; Shulkin, B.L.; Snyder, S.E. Evaluation of children with craniopharyngioma using carbon-11 methionine PET prior to proton therapy. Neuro-Oncology 2013, 15, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lindemann, F.; Weckesser, M.; Hesselmann, V.; Ligges, S.; Wölfer, J.; Jeibmann, A.; Zinnhardt, B.; Viel, T.; Schäfers, M.; et al. Multimodal Imaging of Patients With Gliomas Confirms 11C-MET PET as a Complementary Marker to MRI for Noninvasive Tumor Grading and Intraindividual Follow-Up After Therapy. Mol. Imaging 2017, 16, 1536012116687651. [Google Scholar] [CrossRef] [PubMed]
- Phi, J.H.; Paeng, J.C.; Lee, H.S.; Wang, K.-C.; Cho, B.-K.; Lee, J.-Y.; Park, S.-H.; Lee, J.; Lee, D.S.; Kim, S.-K. Evaluation of Focal Cortical Dysplasia and Mixed Neuronal and Glial Tumors in Pediatric Epilepsy Patients Using 18F-FDG and 11C-Methionine PET. J. Nucl. Med. 2010, 51, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rheims, S.; Rubí, S.; Bouvard, S.; Bernard, E.; Streichenberger, N.; Guenot, M.; Le Bars, D.; Hammers, A.; Ryvlin, P. Accuracy of distinguishing between dysembryoplastic neuroepithelial tumors and other epileptogenic brain neoplasms with [11C]methionine PET. Neuro-Oncology 2014, 16, 1417–1426. [Google Scholar] [CrossRef]
- Pirotte, B.; Acerbi, F.; Lubansu, A.; Goldman, S.; Brotchi, J.; Levivier, M. PET imaging in the surgical management of pediatric brain tumors. Child’s Nerv. Syst. 2007, 23, 739–751. [Google Scholar] [CrossRef]
- Kumar, A.; Asano, E.; Chugani, H.T. α-[11C]-methyl-L-tryptophan PET for tracer localization of epileptogenic brain regions: Clinical studies. Biomark. Med. 2011, 5, 577–584. [Google Scholar] [CrossRef]
- Chugani, H.T.; Luat, A.F.; Kumar, A.; Govindan, R.; Pawlik, K.; Asano, E. -[11C]-Methyl-L-tryptophan-PET in 191 patients with tuberous sclerosis complex. Neurology 2013, 81, 674–680. [Google Scholar] [CrossRef]
- Juhasz, C.; Chugani, D.C.; Padhye, U.N.; Muzik, O.; Shah, A.; Asano, E.; Mangner, T.J.; Chakraborty, P.K.; Sood, S.; Chugani, H.T. Evaluation with alpha-[11C]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia 2004, 45, 124–130. [Google Scholar] [CrossRef]
- Booij, L.; Benkelfat, C.; Leyton, M.; Vitaro, F.; Gravel, P.; Lévesque, M.L.; Arseneault, L.; Diksic, M.; Tremblay, R.E. Perinatal effects on in vivo measures of human brain serotonin synthesis in adulthood: A 27-year longitudinal study. Eur. Neuropsychopharmacol. 2012, 22, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Chandana, S.R.; Behen, M.E.; Juhász, C.; Muzik, O.; Rothermel, R.D.; Mangner, T.J.; Chakraborty, P.K.; Chugani, H.T.; Chugani, D.C. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int. J. Dev. Neurosci. 2005, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Juhasz, C.; Bhambhani, K.; Wu, D.; Chugani, D.C.; Chugani, H.T. Assessment of Progression and Treatment Response of Optic Pathway Glioma with Positron Emission Tomography using α-[11C]Methyl-l-Tryptophan. Mol. Imaging Biol. 2007, 9, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Luat, A.F.; Behen, M.E.; Juhász, C.; Sood, S.; Chugani, H.T. Secondary Tics or Tourettism Associated With a Brain Tumor. Pediatr. Neurol. 2009, 41, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.; Juhász, C.; Muzik, O.; Kupsky, W.J.; Barger, G.; Chugani, H.T.; Mittal, S.; Sood, S.; Chakraborty, P.K.; Chugani, D.C. Imaging Correlates of Differential Expression of Indoleamine 2,3-Dioxygenase in Human Brain Tumors. Mol. Imaging Biol. 2009, 11, 460–466. [Google Scholar] [CrossRef]
- Juhász, C.; Chugani, D.C.; Muzik, O.; Wu, D.; Sloan, E.A.; Barger, G.; Watson, C.; Shah, A.K.; Sood, S.; Ergun, E.L.; et al. In Vivo Uptake and Metabolism of α-[11C]Methyl-l-Tryptophan in Human Brain Tumors. J. Cereb. Blood Flow Metab. 2005, 26, 345–357. [Google Scholar] [CrossRef]
- Alkonyi, B.; Mittal, S.; Zitron, I.; Chugani, D.C.; Kupsky, W.J.; Muzik, O.; Chugani, H.T.; Sood, S.; Juhász, C. Increased tryptophan transport in epileptogenic dysembryoplastic neuroepithelial tumors. J. Neuro-Oncol. 2011, 107, 365–372. [Google Scholar] [CrossRef]
- Juhász, C.; Muzik, O.; Chugani, D.C.; Chugani, H.T.; Sood, S.; Chakraborty, P.K.; Barger, G.R.; Mittal, S. Differential kinetics of α-[11C]methyl-l-tryptophan on PET in low-grade brain tumors. J. Neuro-Oncol. 2010, 102, 409–415. [Google Scholar] [CrossRef]
- Bosnyák, E.; Kamson, D.O.; Guastella, A.R.; Varadarajan, K.; Robinette, N.L.; Kupsky, W.J.; Muzik, O.; Michelhaugh, S.K.; Mittal, S.; Juhász, C. Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro-Oncology 2015, 17, 1284–1292. [Google Scholar] [CrossRef]
- Evans, J.D.; Jethwa, K.R.; Ost, P.; Williams, S.; Kwon, E.D.; Lowe, V.J.; Davis, B.J. Prostate cancer–specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract. Radiat. Oncol. 2018, 8, 28–39. [Google Scholar] [CrossRef]
- Fraioli, F.; Shankar, A.; Hargrave, D.; Hyare, H.; Gaze, M.; Groves, A.M.; Alongi, P.; Stoneham, S.; Michopoulou, S.; Syed, R.; et al. 18F-Fluoroethylcholine (18F-Cho) PET/MRI Functional Parameters in Pediatric Astrocytic Brain Tumors. Clin. Nucl. Med. 2015, 40, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Tsouana, E.; Stoneham, S.; Fersht, N.; Kitchen, N.; Gaze, M.; Bomanji, J.; Fraioli, F.; Hargrave, D.; Shankar, A. Evaluation of treatment response using integrated 18F-labeled choline positron emission tomography/magnetic resonance imaging in adolescents with intracranial non-germinomatous germ cell tumours. Pediatr. Blood Cancer 2015, 62, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, S.T.; Parida, G.K.; Damle, N.A.; Arora, S.; Reddy, S.; Chakraborty, D.; Prabhu, M.; Tripathi, M.; Bal, C. 68Ga-DOTANOC PET/CT in Medulloblastoma. Clin. Nucl. Med. 2018, 43, e145–e146. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, S.E.V.; Sewing, A.C.P.; Van Lingen, A.; Hoekstra, O.S.; Wesseling, P.; Meel, M.H.; Van Vuurden, D.G.; Kaspers, G.J.; Hulleman, E.; Bugiani, M. Multiregional Tumor Drug-Uptake Imaging by PET and Microvascular Morphology in End-Stage Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2017, 59, 612–615. [Google Scholar] [CrossRef]
- Kong, F.-L.; Yang, D.J. Amino Acid transporter-targeted radiotracers for molecular imaging in oncology. Curr. Med. Chem. 2012, 19, 3271–3281. [Google Scholar] [CrossRef]
- Cimini, A.; Ricci, M.; Chiaravalloti, A.; Filippi, L.; Schillaci, O. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int. J. Mol. Sci. 2020, 21, 3849. [Google Scholar] [CrossRef]
- Juhász, C.; Bosnyák, E. PET and SPECT studies in children with hemispheric low-grade gliomas. Child’s Nerv. Syst. 2016, 32, 1823–1832. [Google Scholar] [CrossRef]
- Lu, F.-M.; Yuan, Z. PET/SPECT molecular imaging in clinical neuroscience: Recent advances in the investigation of CNS diseases. Quant. Imaging Med. Surg. 2015, 5, 433–447. [Google Scholar] [CrossRef]
- O’Tuama, A.L.; Treves, S.T.; Larar, J.N.; Packard, A.B.; Kwan, A.J.; Barnes, P.D.; Scott, R.M.; Black, P.M.; Madsen, J.R.; Goumnerova, L.C. Thallium-201 versus technetium-99m-MIBI SPECT in evaluation of childhood brain tumors: A within-subject comparison. J. Nucl. Med. 1993, 34, 1045–1051. [Google Scholar]
- Rollins, N.K.; Lowry, P.A.; Shapiro, K.N. Comparison of Gadolinium-Enhanced MR and Thallium-201 Single Photon Emission Computed Tomography in Pediatric Brain Tumors. Pediatr. Neurosurg. 1995, 22, 8–14. [Google Scholar] [CrossRef]
- Kirton, A.; Kloiber, R.; Rigel, J.; Wolff, J. Evaluation of pediatric CNS malignancies with (99m)Tc-methoxyisobutylisonitrile SPECT. J. Nucl. Med. 2002, 43, 1438–1443. [Google Scholar]
- Tamura, M.; Kohga, H.; Ono, N.; Zama, A.; Shibasaki, T.; Horikoshi, S.; Kurihara, H.; Ohye, C. Calcified astrocytoma of the amygdalo-hippocampal region in children. Child’s Nerv. Syst. 1995, 11, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Weckesser, M.; Matheja, P.; Rickert, C.H.; Sträter, R.; Palkovic, S.; Löttgen, J.; Kurlemann, G.; Paulus, W.; Wassmann, H.; Schober, O. High uptake of L-3-[123I]iodo-α-methyl tyrosine in pilocytic astrocytomas. Eur. J. Pediatr. 2001, 28, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Nadel, H.R. SPECT/CT in pediatric patient management. Eur. J. Pediatr. 2014, 41, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ludmir, E.B.; Grosshans, D.R.; Woodhouse, K.D. Radiotherapy Advances in Pediatric Neuro-Oncology. Bioengineering 2018, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Oliver, M.; Leszczynski, K.; Lee, Y.; Karam, I.; Sahgal, A. Tissue segmentation-based electron density mapping for MR-only radiotherapy treatment planning of brain using conventional T1-weighted MR images. J. Appl. Clin. Med. Phys. 2019, 20, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Wang, H.; Albrecht, S.; Ozsahin, M.; Tkachuk, E.; Rouzaud, M.; Nouet, P.; Dipasquale, G. Open Low-field Magnetic Resonance Imaging for Target Definition, Dose Calculations and Set-up Verification during Three-dimensional CRT for Glioblastoma Multiforme. Clin. Oncol. 2008, 20, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, P.; Liney, G.P.; Holloway, L.; Walker, A.; Barton, M.; Delaney, G.P.; Vinod, S.; Tomé, W. The Potential for an Enhanced Role for MRI in Radiation-Therapy Treatment Planning. Technol. Cancer Res. Treat. 2013, 12, 429–446. [Google Scholar] [CrossRef]
- Rumboldt, Z.; Huda, W.; All, J. Review of Portable CT with Assessment of a Dedicated Head CT Scanner. Am. J. Neuroradiol. 2009, 30, 1630–1636. [Google Scholar] [CrossRef]
- Anders, J.; Lips, K. MR to go. J. Magn. Reson. 2019, 306, 118–123. [Google Scholar] [CrossRef]
- Wald, L.L.; Ms, P.C.M.; Witzel, T.; Stockmann, J.P.; Cooley, C.Z. Low-cost and portable MRI. J. Magn. Reson. Imaging 2019, 52, 686–696. [Google Scholar] [CrossRef] [PubMed]
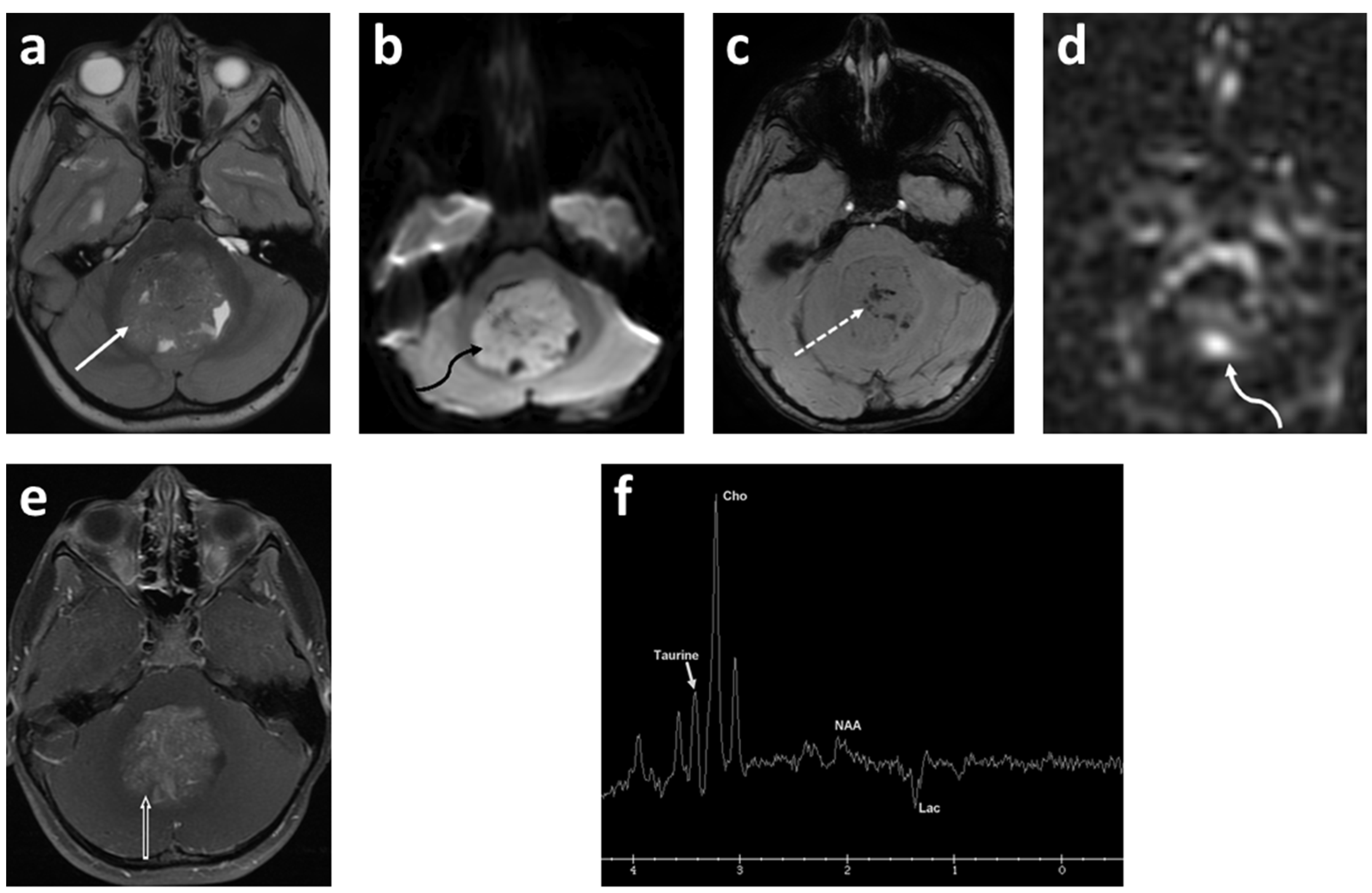
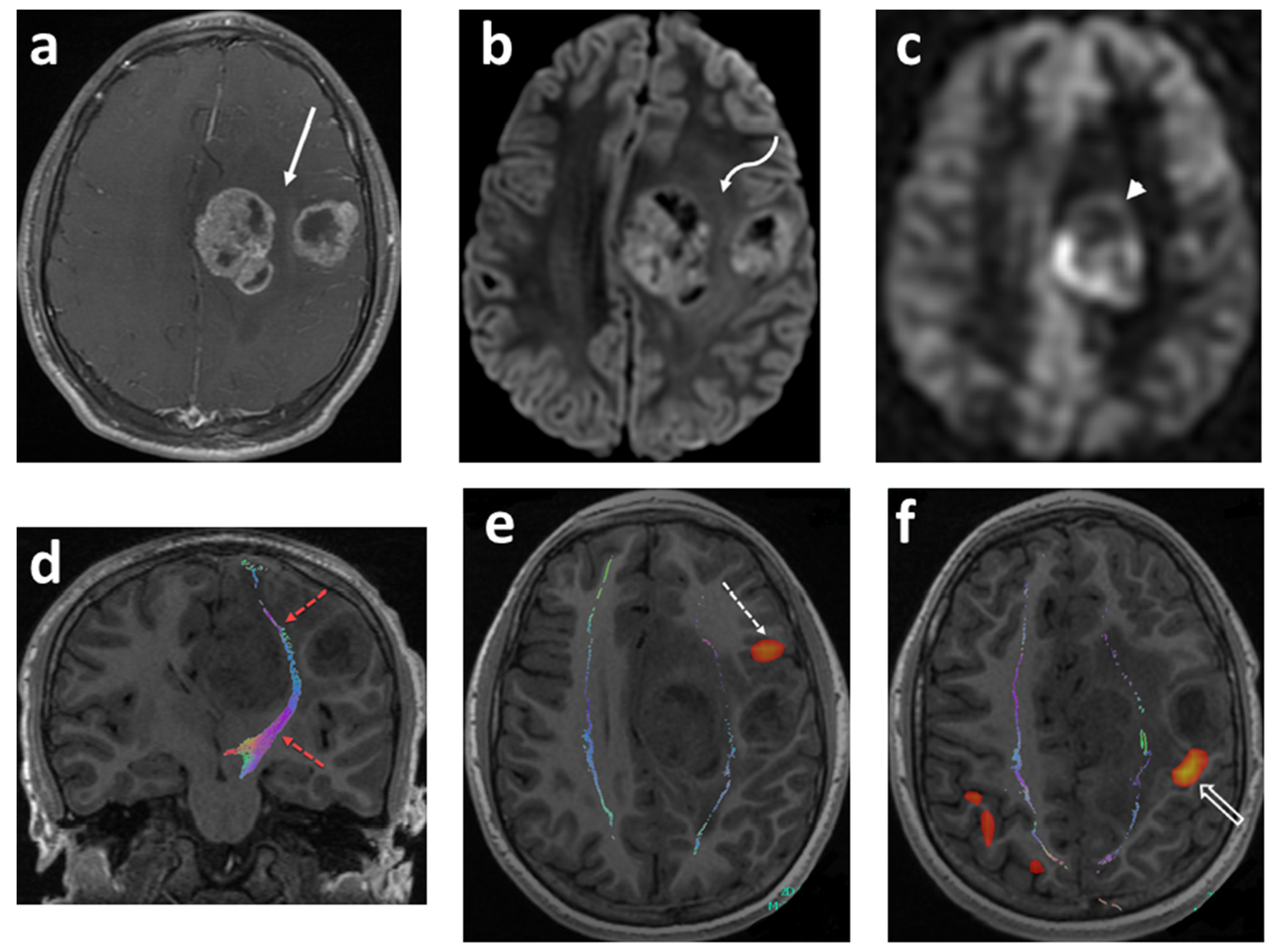

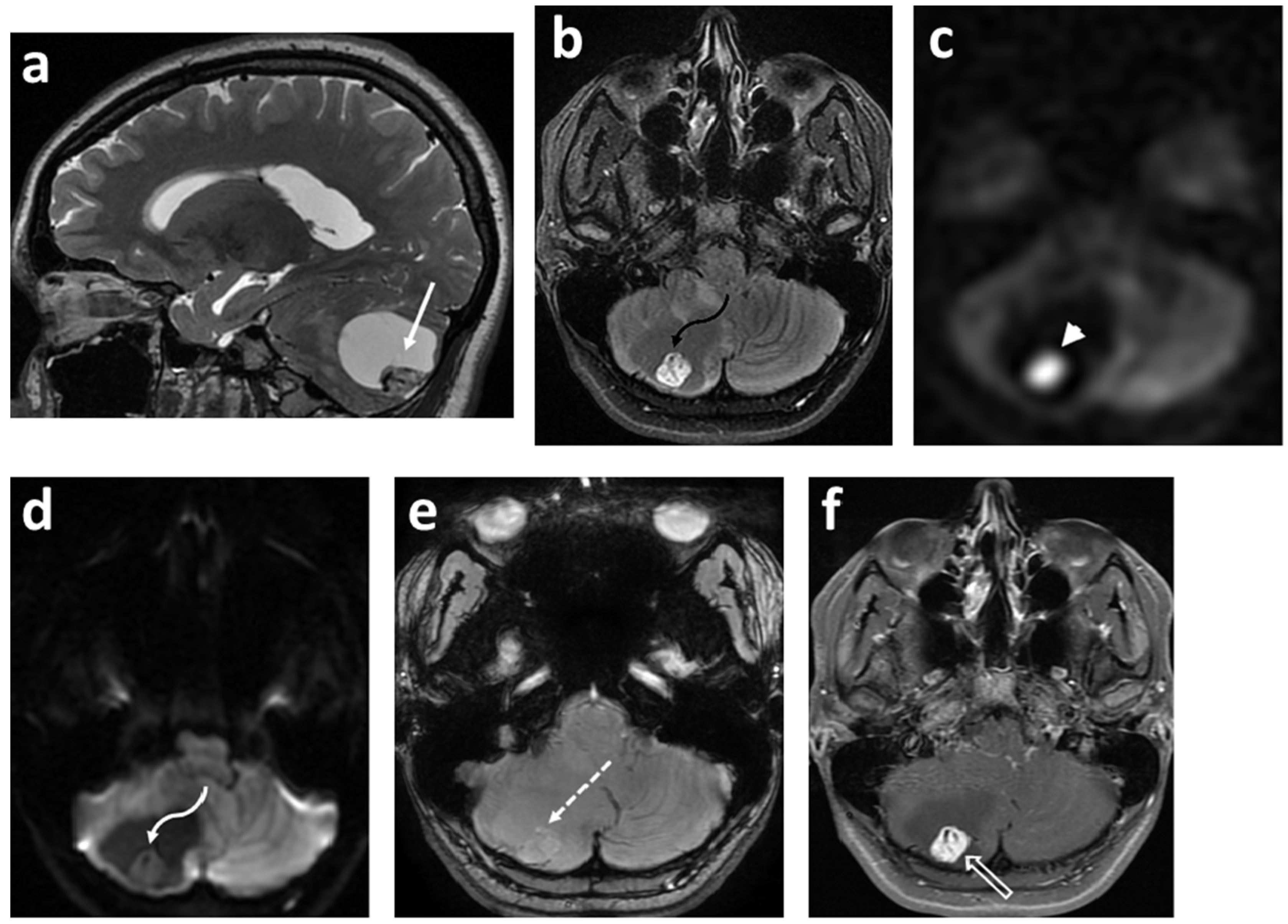
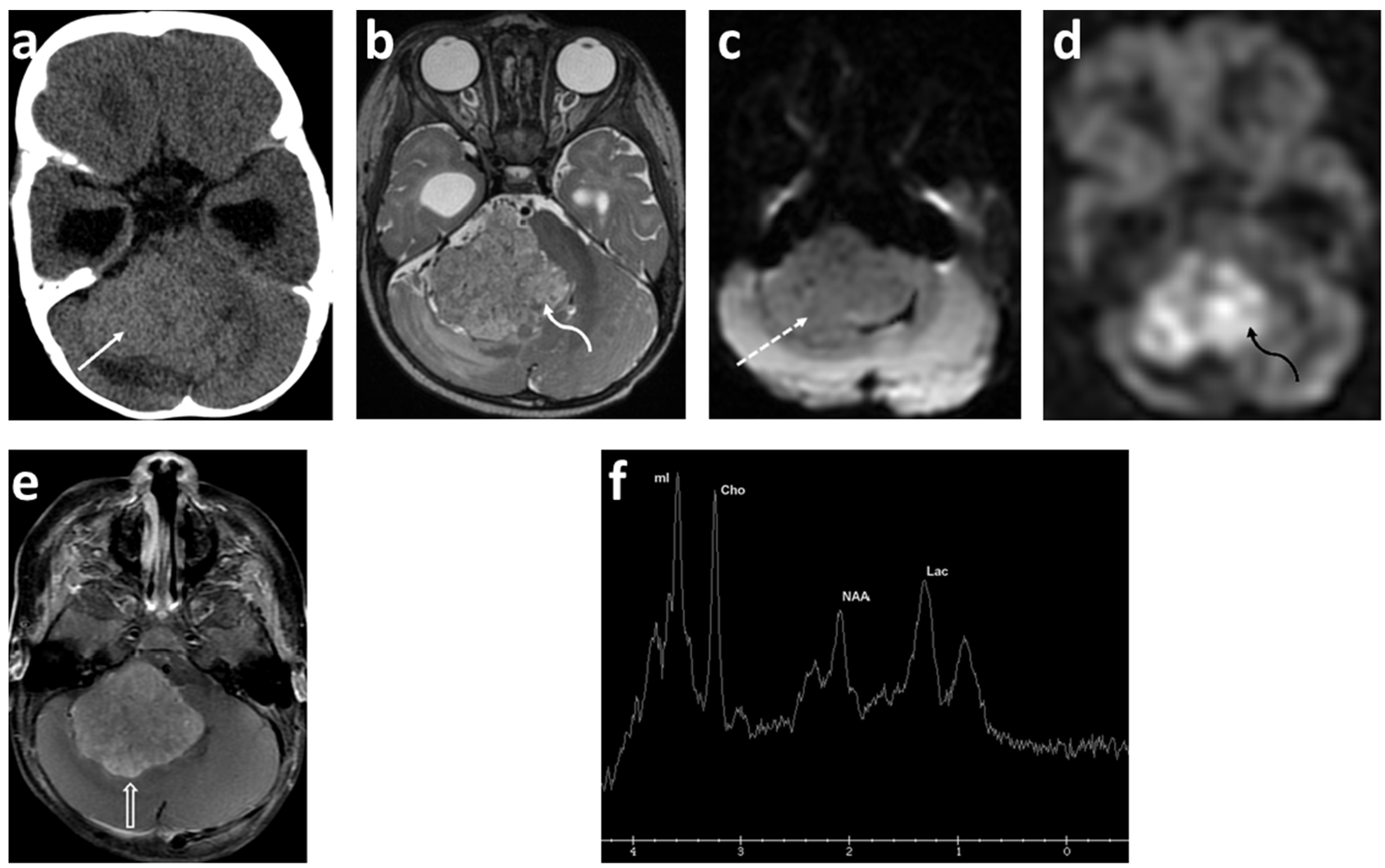
| Family | Tumor Type | Additional Subtyping Based on Molecular Alterations | Frequent Molecular Alterations (*) |
|---|---|---|---|
| CNS embryonal tumors | |||
| Medulloblastomas | WNT-activated | CTNNB1; often in conjunction with monosomy chromosome 6, DDX3X, SMARCA4, and TP53 mutations | |
| SHH-activated (wildtype TP53) | SHH-1, SHH-2, SHH-4 | PTCH1, SUFU, SMO | |
| SHH-activated with mutant TP53 | SHH-3 | TP53, MYCN amplification, GLI2 amplification | |
| Non-WNT/non-SHH (Group 3 and Group 4) | Subtypes 1-8 | MYC or MYC/MYCN amplification, GFI/GFI1B alterations, OTX2, CDK6 or SNCAP1 amplifications; isochromosome 17; PRDM6, KBDBD4 mutations | |
| Atypical teratoid/ rhabdoid tumors | ATRT-TYR, ATRT-SHH, ATRT-MYC | SMARCB1, SHH, NOTCH, loss of 22q, tyrosinase overexpression, MYC activation, HOXC | |
| Gliomas, glioneuronal and neuronal tumors | |||
| Diffuse high-grade gliomas | Diffuse midline glioma, H3 K27-altered | H3.3 K27-mutant; H3.1 or H3.2 K27-mutant; H3 wildtype with EZHIP overexpression; EGFR (and H3 K27) mutant | Histone 3 mutations, TP53, PPM1D, PDGFRA, PIK3CA, PIK3R1, PTEN mutations, EZHIP overexpression, EGFR mutations |
| Diffuse hemispheric glioma, H3 G34-mutant | Histone 3 mutation, TP53, ATRX mutations | ||
| Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype | RTK1; RTK2; MYCN | Enriched for PDGFRA, EGFR or MYCN amplification | |
| Infant-type hemispheric glioma | NTRK-altered; ROS1-altered; ALK-altered; MET-altered | NTRK1/2/3 fusion; ROS1 fusion; ALK fusion; MET amplification/fusion | |
| Diffuse low-grade gliomas | Diffuse astrocytoma | MYB-altered; MYBL1-altered | MYB fusion or MYBL fusion commonly with PCDHGA1, MMP16 and MAML2 |
| Angiocentric glioma | MYB alterations, commonly fused with QKI | ||
| Polymorphous low-grade neuroepithelial tumor | MAPK pathway–BRAF pV600E, fusions with FGFR2 or FGFR3 | ||
| Diffuse low-grade glioma, MAPK pathway-altered | FGFR1 tyrosine kinase domain-duplicated; FGFR1 mutant; BRAF pV600E-mutant | MAPK pathway–FGFR1; BRAF pV600E | |
| Astrocytic gliomas | Pilocytic astrocytoma | Pilomyxoid astrocytoma; pilocytic astrocytoma with histological features of anaplasia | KIA1549:BRAF fusion; NF1; BRAF p.V600E; FGFR1 mutation/fusion; KRAS; RAF1 or NTRK fusion |
| High-grade astrocytoma with piloid features | NF1; FGFR; BRAF:KIAA1549 fusion; often with homozygous deletion of CDKN2A/B | ||
| Pleomorphic xanthoastrocytoma | BRAF pV600E typically with homozygous deletion of CDKN2A/B | ||
| Subependymal giant cell astrocytoma | |||
| Astroblastoma | MN1 fusion with BEND2 or CXXC5 | ||
| Glioneuronal/ neuronal tumors | Ganglioglioma | Most commonly BRAF p.V600E mutation, other MAPK pathway alterations | |
| Desmoplastic infantile ganglioglioma/astrocytoma | BRAF or RAF1 fusions or mutations | ||
| Dysembryoplastic neuroepithelial tumor | FGFR1 mutation, fusion or intragenic duplication | ||
| Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters | Monosomy of chromosome 14 | ||
| Diffuse leptomeningeal glioneuronal tumor | With 1qgain; methylation class 1; methylation class 2 | KIAA1549:BRAF fusion or other MAPK alteration, combined with 1p deletion | |
| Multinodular and vacuolating neuronal tumor | MAPK pathway | ||
| Ependymomas | Supratentorial ependymomas | ZFTA fusion-positive; YAP fusion-positive; additional molecular subgroups awaiting to be defined | ZFTA fusion most commonly with RELA; YAP1 fusion most commonly with MAMLD1 |
| Posterior fossa ependymomas | Group A (PFA); group B (PFB)-retained H3K27 trimethylation; additional molecular subgroups awaiting to be defined | Loss of H3K27 trimethylation, EZHIP overexpression | |
| Imaging Characteristics | Wnt | SHH | Group 3 | Group 4 |
|---|---|---|---|---|
| Location | Cerebellar peduncle/cerebellopontine angle | Cerebellar hemispheres | Midline/fourth ventricle | Midline/fourth ventricle |
| Post-contrast enhancement | Variable | Present, intense | Present | Variable, can be non-enhancing |
| Drop metastasis | Rare | Rare | Frequent | Frequent |
| MRS | - | Prominent choline and lipids, low creatine, no or small taurine peak | Readily detectable taurine and creatine levels | Readily detectable taurine and creatine levels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikam, R.M.; Yue, X.; Kaur, G.; Kandula, V.; Khair, A.; Kecskemethy, H.H.; Averill, L.W.; Langhans, S.A. Advanced Neuroimaging Approaches to Pediatric Brain Tumors. Cancers 2022, 14, 3401. https://doi.org/10.3390/cancers14143401
Nikam RM, Yue X, Kaur G, Kandula V, Khair A, Kecskemethy HH, Averill LW, Langhans SA. Advanced Neuroimaging Approaches to Pediatric Brain Tumors. Cancers. 2022; 14(14):3401. https://doi.org/10.3390/cancers14143401
Chicago/Turabian StyleNikam, Rahul M., Xuyi Yue, Gurcharanjeet Kaur, Vinay Kandula, Abdulhafeez Khair, Heidi H. Kecskemethy, Lauren W. Averill, and Sigrid A. Langhans. 2022. "Advanced Neuroimaging Approaches to Pediatric Brain Tumors" Cancers 14, no. 14: 3401. https://doi.org/10.3390/cancers14143401
APA StyleNikam, R. M., Yue, X., Kaur, G., Kandula, V., Khair, A., Kecskemethy, H. H., Averill, L. W., & Langhans, S. A. (2022). Advanced Neuroimaging Approaches to Pediatric Brain Tumors. Cancers, 14(14), 3401. https://doi.org/10.3390/cancers14143401






