Simple Summary
Gliomas remain the most common primary brain tumor in adults. Although they are classified based on the IDH mutation status very little is known considering this alteration in the elderly glioma population. Because IDH-mutated gliomas are associated with better prognosis in the young population, it is essential to characterize its role in elderly and more frail patients to help physicians’ therapeutic decisions. In this study, we demonstrated that elderly IDH-mutated gliomas had very similar characteristics to those found in the younger population but were significantly different from elderly IDH wild-type gliomas. However, patient management in this population appeared to be suboptimal, with less frequent gross total resection and irradiation. We showed that an optimal therapeutic combination of radio-chemotherapy could be safe and feasible for these elderly patients to aid in their management. Finally, we identified specific geriatric prognostic factors such as mobility, neuropsychological disorders, body mass index, and autonomy that can help physicians make future therapeutic decisions for this specific elderly population with a better prognosis.
Abstract
Background: Describe the characteristics, patterns of care, and predictive geriatric factors of elderly patients with IDHm high-grade glioma (HGG) included in the French POLA network. Material and Methods: The characteristics of elderly (≥70 years) patients IDHm HGG were compared to those of younger patients IDHm HGG (<70 years) and of elderly patients IDHwt HGG. Geriatric features were collected. Results: Out of 1433 HGG patients included, 119 (8.3%) were ≥70 years. Among them, 39 presented with IDHm HGG. The main characteristics of elderly IDHm HGG were different from those of elderly IDHwt HGG but similar to those of younger IDHm HGG. In contrast, their therapeutic management was different from those of younger IDHm HGG with less frequent gross total resection and radiotherapy. The median progression-free survival (PFS) and overall survival (OS) were longer for elderly patients IDHm HGG (29.3 months and 62.1 months) than elderly patients IDHwt HGG (8.3 months and 13.3 months) but shorter than those of younger patients IDHm HGG (69.1 months and not reached). Geriatric factors associated with PFS and OS were mobility, neuropsychological disorders, body mass index, and autonomy. Geriatric factors associated with PFS and OS were mobility, neuropsychological disorders, and body mass index, and autonomy. Conclusion: the outcome of IDHm HGG in elderly patients is better than that of IDHwt HGG. Geriatric assessment may be particularly important to optimally manage these patients.
1. Introduction
Gliomas are the most prevalent and aggressive primary brain tumors in adults. They are classified based on the revised World Health Organization (WHO) classification of 2021, they are divided into three histomolecular subgroups based on the presence of isocitrate dehydrogenase 1/2 (IDH1/2) mutations and 1p19q codeletion. Adult diffuse gliomas are divided into IDH-mutated (IDHm) 1p19q codeleted oligodendroglioma, IDH-mutated astrocytoma, and IDH wild-type (IDHwt) glioblastoma [1]. Younger patients are more likely to have IDH mutations, which also provide a better prognosis [2,3]. Currently, recommendations for grade 2 and 3 IDHm gliomas are based on the combination of radiotherapy and chemotherapy by temozolomide [4] or procarbazine, CCNU, and vincristine for patient treatment (PCV) [5,6]. In particular, for patients with anaplastic oligodendroglioma, an ongoing phase III clinical trial questions the use of radiotherapy as first-line treatment in favor of a PCV-only regimen due to their better prognosis (POLCA trial [NCT02444000], performed in the French ANOCEF group and supported by the POLA network). However, to date, there is no prospective study with elderly IDHm patients, and the available retrospective studies are rare with limitations. As a result, the prognostic effect of IDH mutation is unknown in elderly patients, and it is important to characterize the unique traits and therapeutic options for these patients. Due to an increase in life expectancy, recent data suggests an increase in the number of elderly patients with glioma, particularly attributable to IDHwt glioblastomas [7,8,9]. Along with a low Karnofsky Performance Status and the lack of surgical resection, old age is one of the traditional criteria associated with a bad prognosis for glioma patients [10,11]. The prognostic effect of age could be associated not only with the frailties and comorbidities of elderly patients but also with their suboptimal therapeutic management, highlighting the need to specifically improve their oncological treatments [12,13,14,15,16]. There is still a lack of information regarding the prognostic stratification of the elderly population suffering from a primary brain tumor and a dedicated geriatric scoring system for brain tumor patients is missing. Since 2008, a special program in France has been established for more uniform management of de novo adult high-grade gliomas with an oligodendroglial component. The program inter alia aims to provide a pathological centralized evaluation of the cases and molecular analysis linked to a prospective record of clinical and radiological characteristics of patients. Currently, this network has more than 1500 cases including IDHm and IDHwt gliomas. In this context, the present study aims to describe the features, care practices, and survival-predictive geriatric factors of elderly patients with IDHm HGG included in the French POLA network.
2. Patients and Methods
2.1. Study Design and Participants
The POLA network is a dedicated program that has been set up for more homogeneous management of de novo adult high-grade glioma with an oligodendroglial component (Prise en charge des OLigodendrogliomes Anaplasiques (POLA network)). The aim of the program inter alia is to provide a pathological centralized review of the cases and centralized molecular analysis. Totally, this program includes a large population of oligodendroglioma, IDHm astrocytoma and IDHwt glioblastoma sent for pathological review. From September 2008 to November 2017, 1433 patients, who were sent for a central pathological review and included in the French nationwide POLA cohort, were included in the present study.
For all cases, formalin-fixed, paraffin-embedded (FFPE) tumor tissue was available for pathological and immunohistochemical analyses. Medical, radiological, histological data and treatment patterns were also prospectively collected from medical records.
All patients were analyzed and segregated according to their age and their IDH mutation status. The cut-off age defining the elderly patients was 70 years or older at the time of diagnosis. Firstly, the characteristics and patterns of care of elderly patients IDHm HGG were compared to those of younger patients IDHm HGG and to those of elderly patients IDHwt HGG. Secondly, we focused on the subgroup of elderly patients IDHm HGG and gathered geriatric factors to examine their predictive value on survival and toxicity.
The study was approved by a national ethics committee. Patients prospectively included into the POLA cohort provided their written consent for clinical data collection and genetic analysis according to national and POLA network policies.
2.2. Data Collection
For all patients at diagnosis, demographic profile (age and gender), presenting symptoms, radiological characteristics on MRI, histology, type of surgery, post-operative Karnofsky Performance Status (KPS) score, and adjuvant treatments received were prospectively and collected in real-time.
Treatment received in the first line were “wait and scan policy”, radiotherapy (RT) alone, chemotherapy (CT) alone, radiotherapy in combination with chemotherapy (RT-CT), or palliative care. Radiotherapy (RT) included conventional radiation therapy (60 Gy delivered in 30 fractions) or hypo-fractionated radiation therapy (40 Gy in 15 fractions). First-line chemotherapy (CT) treatments included temozolomide (TMZ), procarbazine, lomustine (CCNU), and vincristine schedule (PCV) or bevacizumab alone or in combination with temozolomide.
Geriatric parameters including geriatric scores were retrospectively collected, in POLA-centers, by geriatricians, neuro-oncologist, surgeons, radiotherapists, and clinical research assistants participating in the POLA Network.
Geriatric frailties were detected by the G8 score. This screening tool consists of 8 items: appetite changes, weight loss, mobility skills, neuropsychological disorders, body mass index, number of medications, self-rated health, and patient’s age. The score ranges from 0 to 17 and the cut-off value for an impaired G8 score was ≤14/17 [17,18]. The neuropsychological item gathers both depression and dementia disorders. Depression and or dementia are classified as severe, or moderate by clinical assessment, without specific scales or scores.
Functional status was determined by using the Activities of Daily Living (ADL) scale (impaired < 6) [19] and the Instrumental Activities of Daily Living (IADL) scale (impaired < 4) [20]. Comorbidities were identified using the age-adjusted Charlson’s comorbidity Index (severe score ≥ 5) [21].
Cognitive impairment was assessed using the Mini-Mental State Examination (MMSE) (impaired < 24) [22]. Biological markers recorded were hemoglobin, neutrophils, lymphocytes, platelets, serum albumin, protein c reactive, and serum creatinine at diagnosis.
Concerning feasibility, it was defined as the completion of 6 courses of chemotherapy without early stopping for disease progression, death, or unacceptable toxicity (adverse event related to chemotherapy leading either too early treatment stopping, to a dose delay lasting more than 14 days or more than 2 dose reductions, to an unplanned hospital admission or to death).
Concerning toxicity, treatment dose reductions (at the start or during treatment), chemotherapy delay for toxicity, and treatment discontinuation for toxicity were reported. Adverse events were scored using the Common Toxicity Criteria scale for adverse events version 4.0 (CTCAE v4.0).
IDH1 and IDH2 mutations: Automated immunohistochemistry (IHC) was performed on 4-µm-thick FFPE sections with an avidin-biotin-peroxidase complex on Benchmark XT (Ventana Medical System Inc., Tucson AZ, USA) using the Ventana Kit including DAB reagent to search for the expression of IDH1 R132H (Dianova, H09). When the results of IDH1 R132H IHC were negative or unreliable, the status of IDH1 and IDH2 mutation was assessed by direct sequencing using the Sanger method and primers, as described previously [23].
Tumor DNA was extracted from frozen tissue, if available, or from FFPE samples using the iPrep ChargeSwitch® Forensic Kit. Qualification and quantification of tumor DNA were performed using a NanoVue spectrophotometer and gel electrophoresis, respectively. The genomic profile and assessment of the 1p/19q codeletion status were determined as described previously [24]. When the quantity of DNA was insufficient to perform SNP or CGH, microsatellite analysis was conducted (LOH) of chromosomes 1p and 19q were assessed via PCR techniques described elsewhere [25].
2.3. Statistical Analysis
Data are presented as a median, range, mean and standard error of the mean (se). For correlation analysis, the chi-square test (or Fisher’s exact test) was used to compare qualitative variables. Continuous variables were compared using the Mann-Whitney U test. Progression-free survival (PFS) was defined as the time from the date of surgery to recurrence or death from any cause, censored at the date of the last contact. Overall survival (OS) was defined as the time from the date of surgery to death from any cause, censored at the date of the last contact. The Kaplan-Meier method was used to estimate survival distributions. Log-rank tests were used for univariate comparisons. Cox proportional hazards models were used for multivariate analyses and for estimating hazard ratios in survival regression models. The sensitivity and specificity of the brain geriatric score were analyzed using receiver operating characteristic (ROC) curve analysis after dichotomization of patient survival (<48 months vs. ≥48 months). All statistical tests were two-sided, and the threshold for statistical significance was p = 0.05. Analyses were conducted using PASW Statistics version 22 (IBM SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Elderly Patients IDHm HGG
3.1.1. Clinical Characteristics and Patterns of Care
Depending on the age and the presence of IDH mutation, patients were divided into four groups: 39 elderly patients IDHm HGG; 80 elderly patients IDHwt HGG; 933 non-elderly patients IDHm HGG and 381 non-elderly patients IDHwt HGG (Supplementary Figure S1).
The clinical, radiological, histological, and treatment characteristics of the elderly patients IDHm HGG are summarized in Table 1. The median age at diagnosis was 74 years (range 70.2–87.1 years). At diagnosis, half of the elderly patients IDHm HGG presented with epilepsy and a quarter of them with cognitive disorders. Median post-operative Karnofsky Performance Status (KPS) was 80 (range 50–100). Epilepsy was present at diagnosis in 15 patients (44%) and was the only symptom in 9/15 patients (60%). Seizures were polymorphous and independent from onco-geriatric factors and patient survival. Regarding neuroimaging, the majority of patients presented with contrast enhancement (78.8%). Diagnoses consisted of 1p/19q co-deleted anaplastic oligodendroglioma in 72% of cases and IDHm grade III or IV astrocytomas in the remaining 28%. Median Ki67 expression was 15% (range: 0–40). Steroids were prescribed during the post-operative period for half of the patients (48.5%). Regarding the treatment approaches, two-thirds of patients had biopsy alone and adjuvant treatments consisted of chemotherapy alone (Table 1 and Table S1).

Table 1.
Baseline characteristics and treatment patterns in elderly patients IDHm, elderly patients IDHwt and younger patients IDHm high-grade glioma.
3.1.2. Geriatric Characteristics
Out of the 39 IDHm HGG elderly patients, geriatric data were available for 34 of them (Table 2). IDHm elderly patients HGG had heavy comorbidities and medications as age-adjusted Charlson’s index was ≥5 for 23 patients (72%). Seven patients (28%) presented with neuropsychological disorders, assessed by G8 score. Mobility was preserved for 16 patients (66.7%). Weight loss was experienced by 12 elderly patients (48%) and 7 (26%) met criteria of malnutrition at diagnosis. Elderly patients suffered from autonomy loss as showed by ADL score < 6 for 6 patients (33.3%) and IADL score < 4 for 8 patients (47%). The estimated G8 score was ≤14/17 for 16 patients (64%). Neuropsychological disorders assessed by G8 score, loss of mobility, number of comorbidities, malnutrition, loss of autonomy assessed by ADL score, and impaired G8 score were more frequent in astrocytoma than oligodendroglioma elderly patients (Supplementary Table S1).

Table 2.
Baseline geriatric parameters in elderly patients IDH-mutated high-grade glioma (N = 34).
3.1.3. Feasibility and Safety of Adjuvant Treatment Chemotherapy and Radiotherapy
Regarding the treatment feasibility, 72% of patients treated by TMZ received at least six cycles while 56% of patients treated by PCV completed six cycles (Supplementary Table S2). In terms of chemotherapy treatment safety (Supplementary Table S2), at treatment initiation, seven patients (41%) in the TMZ group and five patients (62%) in the PCV group had a baseline dose reduction due to their age. In the TMZ group only, the dose was increased for four patients for a second time. During treatment, three patients (19%) had a TMZ dose reduction and seven (87%) had a PCV dose reduction. Treatment interruption due to adverse events occurred fortwo patients (12%) treated by TMZ and six patients (75%) treated by PCV (Supplementary Table S2). In total, grade 3 or 4 adverse events occurred for three patients treated by TMZ and two patients treated by PCV patients (Supplementary Table S3).
Regarding radiotherapy treatment safety, only one treatment interruption was reported due to toxicity (grade 2 asthenia) and only one patient had an early serious adverse event (post-radiation encephalopathy two months after the end of RT).
Regarding predictive factors of treatment toxicity, a low post-operative KPS and a poor self-reported state of health were correlated with a higher grade 3–4 toxicity probability. The loss of mobility was predictive of treatment interruption due to toxicity (p = 0.047) (Supplementary Table S4).
3.2. Characteristics and Patterns of Care Group Comparisons and Outcomes
3.2.1. Comparison between Elderly Patients IDHm HGG (n = 39) and IDHwt HGG (n = 80)
Clinical, radiological, and histological presentations of elderly patients IDHm HGG significantly differed from those of elderly patients IDHwt HGG (Table 1). Elderly patients IDHm HGG presented with less frequent mnesic disorders (11.4% vs. 37%, p = 0.054), radiological necrosis (31.8% vs. 61.2%, p = 0.039), contrast enhancement (78.8% vs. 95.8%, p = 0.01) and had a lower proliferative index (p = 0.005).
3.2.2. Comparison between Elderly (n = 39) and Non-Elderly (n = 919) Patients IDHm HGG
No difference regarding the clinical, radiological, and histological presentations of elderly and non-elderly patients IDHm HGG were observed. In contrast, their managements were significantly different (Table 1). Compared to their younger counterpart, elderly patients IDHm HGG less frequently underwent gross total or subtotal resection (24.3% vs. 50.3%, p = 0.002) and radiotherapy (48.7% vs. 80%, p < 0.001).
3.2.3. Outcomes
The median progression-free survival (PFS) and overall survival (OS) were longer for elderly patients IDHm HGG (29.3 months (95% CI 22.1–36.4 months) and 62.1 months (95% CI 13.2–111.1 months) respectively) than elderly patients IDHwt HGG (8.3 months (95% CI 6.0–10.6 months) and 13.3 months (95% CI 10.6–16.0 months) respectively, p < 0.001) but shorter than those of younger IDHm patients HGG (69.1 months (95% CI 59.0–79.1 months) and not reached respectively, p < 0.001). Finally, median PFS and OS for elderly patients IDHm HGG were longer than those of younger patients IDHwt HGG. More precisely, in elderly patients, IDHm HGG, PFS, and OS in the oligodendroglioma subgroup were longer than those of astrocytoma (Figure 1). When focusing on patients receiving radiotherapy, we still observed different outcomes between these four subgroups and especially between elderly IDHm vs. IDHwt HGG patients.
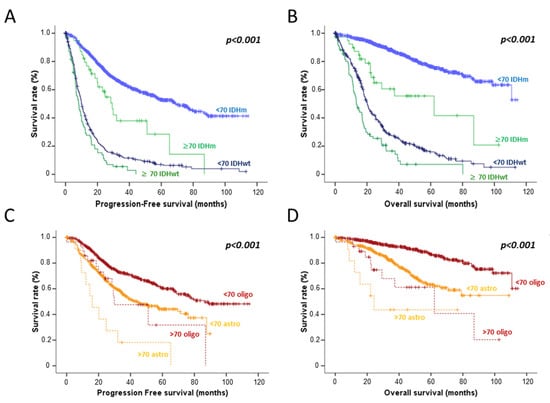
Figure 1.
Progression free-survival (A) and overall survival (B) according to the age (< or ≥70 years) and IDH mutation status. Progression free-survival (C) and overall survival (D) according to the age and the histological subtype of IDH mutated HGG patients.
3.3. Prognostic Factors in Elderly Patients IDHm HGG
Median PFS was 29.3 months (95% CI 22.1–36.4 months). In univariate analyses (Table 3), median PFS was shorter for elderly patients IDHm HGG presenting with a loss of mobility (11.0 months (95% CI 3.75–18.32) vs. 53.7 months (95% CI 28.82–78.68), p = 0.003); severe neuropsychological disorders according to G8 score (10.2 months (95% CI 7.70–12.79) vs. 50.0 months (95% CI 19.40–80.69 months), p = 0.003); clinical criteria of denutrition (11.0 months (95% CI 9.00–13.07) vs. 50.0 months (95% CI 21.29–78.80), p < 0.001); and a loss of autonomy according to ADL score [9.25 months (95% CI 6.51–11.99) vs. 65.19 months (95% CI 31.24–99.13), p < 0.001 ). Low post-operative KPS, low hemoglobin serum level, low lymphocyte count, and grade IV glioma were also associated with poor PFS (p = 0.012 and p = 0.004, respectively). Multivariate analysis adjusted by 1p19q codeletion confirmed that loss of mobility (HR = 4.7 (1.30–17.21), p = 0.018), presence of severe neuropsychological disorders (HR = 4.3 (1.56–11.92), p = 0.005)), clinical denutrition (HR = 5.26 (1.83–15.04), p = 0.002), loss of autonomy according to the ADL score (HR = 14.97 (2.73–81.95), p = 0.002 for ADL score), lymphopenia (HR = 4.8 (1.27–18.08), p = 0.02) and lower KPS (HR = 3.77 (1.20–11.80), p = 0.023) were significantly associated with shorter PFS.

Table 3.
Univariate and multivariate analyses of prognostic factors in elderly patients IDH mutated high-grade glioma (N = 34). PFS: Progression-free survival; OS: Overall survival; HR: Hazard ratio; BMI: Body Mass Index; ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; KPS: Karnofsky Performance Scale.
Median OS was 62.1 months (95% CI 13.2–111.1 months). In univariate analyses (Figure 2), median OS was shorter for elderly patients IDHm HGG presenting with a loss of mobility (15.50 months (95% CI 0.156–30.85) vs. not reached, p < 0.001), severe neuropsychological disorders (22.16 months (95% CI 0–50.0) vs. not reached, p = 0.036), clinical criteria of denutrition (22.16 months (95% CI 5.1–39.0) vs. 87 months (95% CI 37.12–136.89), p = 0.002) and a loss of autonomy according to ADL score (11.04 months (95% CI 0–27.07) vs. not reached, p < 0.001). A low post-operative KPS, grade IV rating, and the absence of 1p19q codeletion were also associated with a poor OS (p = 0.002, p = 0.001, p = 0.043, respectively).
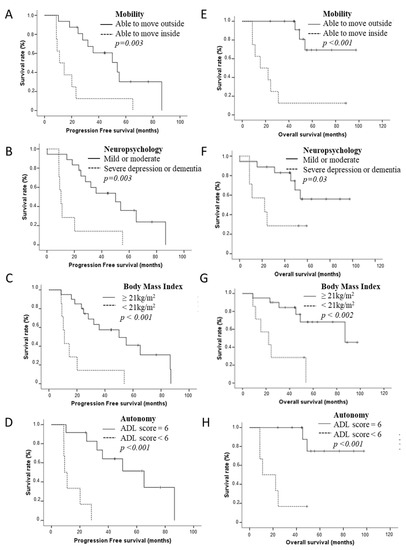
Figure 2.
Progression-free survival (A–D) according to geriatric parameters in elderly patients IDHm HGG: motricity (A), neuropsychological disorders (B), Body Mass Index (BMI) (C), and autonomy (D). Overall survival (E–H) according to geriatric parameters in elderly patients IDHm HGG: mobility (E), neuropsychological disorders (F), BMI (G), and autonomy (H).
Multivariate analysis adjusted by 1p19q codeletion confirmed that loss of mobility ([HR = 9.93 × (1.82 − 53.98), p = 0.008), presence of severe neuropsychological disorders (HR = 3.51 × (1.02 − 12.14), p = 0.047), clinical criteria of denutrition (HR = 5.85 × (1.66 − 20.61), p = 0.006), loss of autonomy according to the ADL score (HR = 21.56 × (2.11 − 219.86), p = 0.01) and lower KPS (HR = 5.3 × (1.5 − 17.95), p = 0.007) were correlated with shorter OS. In contrast, the G8 score was not correlated either with PFS or OS.
Finally, focusing on older patients with IDHm 1p19q codeleted oligodendroglioma, patients treated by RT-PCV demonstrated longer PFS (p = 0.08) and OS (p = 0.037) than the patients treated by RT-TMZ (Supplementary Figure S2).
3.4. Towards a Brain Tumor Geriatric Score
Because the classical G8 score was not able to predict elderly patients’ survival, we designed a new geriatric scoring system based on geriatric factors significantly correlated with patient survival in our multivariate analysis. This brain geriatric score (BGS) included the following items: mobility, neuropsychological disorders, and body mass index as evaluated by the G8 score and the ADL score as reported by Katz et al. (Figure 3). After dichotomization of patient survival (<48 months vs. ≥48 months), the sensitivity and the specificity of the BGS to predict long-term survival were 100% and 83% in our cohort with an AUC of 0.948 (Figure 3). Using a cut-off of 10, the BGS score was significantly correlated to PFS and OS (p < 0.001 both, Figure 3). Median PFS was 50.1 months (95% CI 17.6–82.4) for patients with a BGS ≥ 10 vs. 9.2 months (95% CI 8.1–10.3) for patients with a BGS < 10 (p < 0.001). Median OS was not reached for patients with a BGS ≥ 10 vs. 11.0 months (95% CI 6.1—15.9) for patients with a BGS < 10 (p < 0.001) (Figure 3).
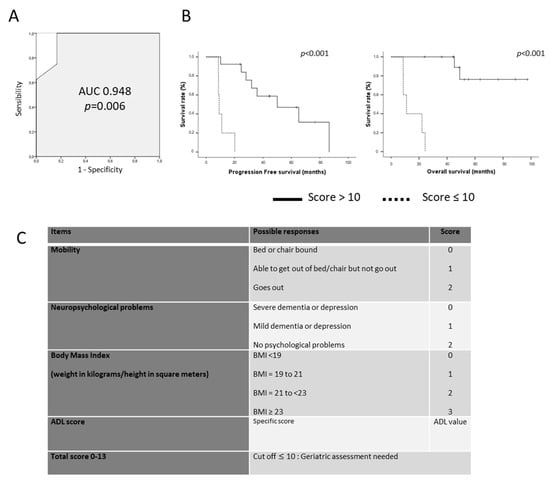
Figure 3.
(A). ROC (Receiver Operating Characteristics) curve of the Brain Geriatric Score according to long-term survivors. (B). Progression-Free Survival (left) and Overall Survival (right) of patients according to the BGS using a cut-off of 10. (C). Brain Tumor Geriatric Score detail.
4. Discussion
In the present study, we showed that, albeit infrequent, IDHm HGG occurred in a small number of elderly patients. Compared to elderly patients IDHwt HGG, elderly patients IDHm HGG presented with distinct clinical, radiological, and histological characteristics and a better outcome. In contrast, their baseline characteristics were similar to those of younger patients IDHm HGG despite a worse outcome. We subsequently showed that adjuvant treatment feasibility and toxicity were advisable, with limited grade 3–4 toxicity occurrence. Finally, we analyzed the prognostic value of classical geriatric factors and identified four of them as able to segregate two groups of patients with completely different outcomes.
To our knowledge, the extensive characterization of elderly IDHm HGG patients was never previously reported with the precision of the present study allowed by the POLA network. Andrews and colleagues [7] evaluated the IDH mutation occurrence in a retrospective cohort of 224 patients aged 55 years or older with diffuse gliomas. In this cohort, 42 patients presented with IDH1 R132H mutations, and 29 patients (13%) presented with minor IDH mutations underlining their occurrence after the cut-off defined by the WHO 2016 classification. Based on our results and the favorable outcome of elderly patients IDHm HGG, the misestimation of this molecular profile could negatively impact their management and survival.
In the present study, we reported that elderly patients presented with the poorer outcome than their younger counterparts despite the absence of additional pejorative factors, including a worse KPS at diagnosis and a larger tumor size. This difference in survival between younger and older IDHm patients cannot be reduced to a simple “age” effect: it seems to be partly explained by a difference in treatment management. In our study, 62% of elderly patients IDHm HGG underwent only an initial biopsy (compared to 19% in patients <70 years) and less than half of them were treated by radiotherapy (compared to 80% in patients <70 years). Regarding the surgery approach, several studies suggest that maximal resection in an elderly patient can be safe and is associated with better outcomes [26,27,28]. These studies only included GBM patients but it seems reasonable to extend their conclusion to IDHm glioma patients with longer life expectancy. Additionally, we reported very encouraging results of feasibility and safety for adjuvant treatment. In oligodendroglioma patients presenting with longer life expectancy, the feasibility of the PCV regimen encourages proposing this treatment alone when possible, according to the younger patients’ guidelines [5]. In contrast, the use of radiotherapy in this population at higher risk of neurotoxicity could be delayed. For IDHm astrocytoma elderly patients, the combination of chemotherapy and irradiation seems feasible and promising. No previous data concerning IDHm elderly patients were available, but the feasibility and efficacy of radiotherapy for GBM elderly patients were first demonstrated by Keime et al. [29]. There were no immediate severe adverse events related to RT in their study. Importantly, quality of life and cognitive assessment did not differ between treatment arms. More recently, a phase III clinical trial [30] reported the feasibility and the efficacy of the combination of short-course radiotherapy (40 Gy, 15 fractions) and temozolomide in elderly patients. A total of 562 older patients (>70 years) were treated by RT-TMZ or RT alone. Median OS was higher in the experimental arm and grade ≥ 3 lymphopenia (27.3%), thrombocytopenia (11.1%), and neutropenia (8.3%) were the more frequent adverse events. The association of radiotherapy and chemotherapy appears to be an interesting option for elderly IDHm astrocytoma HGG patients, keeping in mind that the risk of delayed neurocognitive disorders after irradiation should be evaluated according to the life expectancy of each patient. In this context, a predictive scoring system to identify patients with long life expectancy would be useful for better patient management. Frailty assessment of oncological patients was previously reported in different large tumor-type cohorts and in specific sub-population [15,16]. In 2011, the G8 score was developed to screen frailty in elderly cancer patients. This tool was designed to detect the vulnerable patients who would be offered comprehensive geriatric assessment, with a sensitivity of 87% and a specificity of 58% [18]. Among its main limitations, we can note a low specificity and the absence of patients with a primary brain tumor in its validation prospective cohort. More specifically in neuro-oncology patients, geriatric factors evaluation was previously reported in retrospective cohorts of GBM patients but never in IDHm glioma patients. Ackerl et al. [31], reported a retrospective cohort of 70 patients underlining the wide outcome variability in this population and the importance of geriatric assessment-based therapy management. In their study including 34 elderly patients with newly diagnosed GBM, Giaccherini et al. [32] generated a prognostic score based on the combination of KPS, type of surgery, and Frailty Index. Scheinder et al. [33], demonstrated that modified Frailty Index (≥0.27), comorbidity burden (CCI > 2), and nutritional status (BMI < 30) were significantly associated with poor OS in geriatric patients (≥65 years) with GBM. Lombardi et al. [34], enrolled 113 elderly GBM patients (≥65 years) and reported that comprehensive geriatric assessment was an independent significant predictor of mortality. In a retrospective study, Lorimer et al. [35] demonstrated that geriatric features such as cognition and autonomy impairment were negatively associated with survival in elderly GBM patients. Finally, Deluche et al. [36], reported the first retrospective study demonstrating the feasibility and the prognostic value of the G8 score in 89 elderly patients with GBM. In our study dedicated to elderly IDHm HGG patients, the G8 score and the CCI did not correlate with survival at any cut-off value, leading to the generation of a new prognostic factors combination allowing the identification of long-term survivor patients for whom treatment management should be optimized. This score needs now to be validated in an independent larger series. If confirmed, it could be an interesting tool for neuro-oncologist before the initiation of treatment in this population.
The main limitation of our study is the limited number of elderly IDHm HGG patients, the retrospective record of specific geriatric features, the use of MMSE for neurocognitive evaluation, and the lack of validation of our findings in an independent cohort. However, to our knowledge, this study represents the largest and the most characterized cohort of elderly IDHm HGG in literature. Now, the next steps will be to prospectively validate our results in a dedicated multicentric study, facilitated by the French POLA network.
5. Conclusions
To our knowledge, this is the first study characterizing elderly IDHm HGG patients. Although rare and heterogeneous, IDHm HGG shows better outcomes in elderly patients than that of IDHwt HGG. Geriatric assessment may be particularly important to optimally manage these patients. These results need now to be prospectively confirmed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225509/s1, Figure S1: Patient repartition according to the age (< or ≥ 70 years) and the IDH mutation status in POLA cohort; Figure S2: Progression free-survival (left) and overall survival (right) according to treatment groups in elderly patients with anaplastic oligodendroglioma. RT: radiotherapy; PCV: procarbazine, CCNU, Vincristine; TMZ: temozolomide; Table S1: Baseline characteristics and treatment patterns in elderly patients IDHm HGG (Geriatric cohort, N = 35) according to histological sub-type; Table S2: Treatment feasibility and toxicity in elderly patients according to chemotherapy adjuvant protocol (N = 29). TMZ: temozolomide; PCV: procarbazine, CCNU, vincristine; Table S3: Treatment toxicity in elderly patients according to chemotherapy adjuvant protocol (N = 29); Table S4: Predictive factors of treatment toxicity in elderly IDH mutated high-grade glioma cohort (N = 34).
Author Contributions
Conceptualization, J.-S.G., E.T.; data curation, C.M., J.-S.G., F.D., C.D. (Caroline Dehais), E.C.-J.M., C.D. (Christine Desenclos), A.P., R.S., L.B., C.G., M.J.M.F., M.B., J.-S.F., E.V., O.L., G.N., A.F.C., A.L.D.S., C.B., D.F.-B., O.C., E.T.; formal analysis, C.M., J.-S.G., E.T.; methodology, E.T.; supervision, J.-S.G., E.T.; writing—original draft, C.M., E.T.; writing—review and editing (All authors). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The POLA consent form was approved by the CPP OUEST II ANGERS on 19 April 2018. The authorization number was DR-2010-173. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of CPP Ouest II (protocol code DR-2010-17, on the 19 April 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The medical data are not publicly available due to ethical reasons.
Acknowledgments
We thank the POLA Network (INCa), the ARTC-Sud patients’s association (Association pour le Recherche sur les Tumeurs Cérébrales) and the AP-HM Tumor Bank (authorization number: CRB BB-0033-00097) for providing tissue samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-B.; Yue, W.; Xie, C.; Zhang, R.-Y.; Hu, S.-S.; Wang, Z. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: A meta-analysis. Tumor Biol. 2013, 34, 3555–3559. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; Baumert, B.; Erridge, S.C.; Vogelbaum, M.A.; Nowak, A.K.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Baurain, J.F.; Mason, W.P.; et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: A phase 3, randomised, open-label intergroup study. Lancet 2017, 390, 1645–1653. [Google Scholar] [CrossRef]
- Van Den Bent, M.J.; Carpentier, A.F.; Brandes, A.A.; Sanson, M.; Taphoorn, M.J.B.; Bernsen, H.J.J.A.; Frenay, M.; Tijssen, C.C.; Grisold, W.; Sipos, L.; et al. Adjuvant Procarbazine, Lomustine, and Vincristine Improves Progression-Free Survival but Not Overall Survival in Newly Diagnosed Anaplastic Oligodendrogliomas and Oligoastrocytomas: A Randomized European Organisation for Research and Treatment of Cancer Phase III Trial. J. Clin. Oncol. 2006, 24, 2715–2722. [Google Scholar] [CrossRef]
- Shaw, E.G.; Wang, M.; Coons, S.W.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; Mehta, M. Randomized Trial of Radiation Therapy Plus Procarbazine, Lomustine, and Vincristine Chemotherapy for Supratentorial Adult Low-Grade Glioma: Initial Results of RTOG 9802. J. Clin. Oncol. 2012, 30, 3065–3070. [Google Scholar] [CrossRef]
- Andrews, C.; Prayson, R.A. IDH mutations in older patients with diffuse astrocytic gliomas. Ann. Diagn. Pathol. 2020, 49, 151653. [Google Scholar] [CrossRef]
- Bauchet, L.; Ostrom, Q.T. Epidemiology and Molecular Epidemiology. Neurosurg. Clin. N. Am. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Fang, J.; Lin, D.; Deng, X.; Li, D.; Sheng, H.; Lin, J.; Zhang, N.; Yin, B. Epidemiological trends, relative survival, and prognosis risk factors of WHO Grade III gliomas: A population-based study. Cancer Med. 2019, 8, 3286–3295. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.J.; van den Bent, M.; Tonn, J.-C.; Pentheroudakis, G.; on behalf of the ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii93–iii101. [Google Scholar] [CrossRef]
- Weller, M.; dan der Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Harrison, R.A.; de Groot, J.F. Treatment of Glioblastoma in the Elderly. Drugs Aging 2018, 35, 707–718. [Google Scholar] [CrossRef]
- Braun, K.; Ahluwalia, M.S. Treatment of Glioblastoma in Older Adults. Curr. Oncol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Delattre, J.-Y. Management of Elderly Patients with Gliomas. Oncologist 2014, 19, 1258–1267. [Google Scholar] [CrossRef]
- Hanna, C.; Lawrie, T.A.; Rogozińska, E.; Kernohan, A.; Jefferies, S.; Bulbeck, H.; Ali, U.M.; Robinson, T.; Grant, R. Treatment of newly diagnosed glioblastoma in the elderly: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 2020, CD013261. [Google Scholar] [CrossRef]
- Jordan, J.T.; Gerstner, E.R.; Batchelor, T.T.; Cahill, D.P.; Plotkin, S.R. Glioblastoma care in the elderly. Cancer 2016, 122, 189–197. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Curé, H.; Rousselot, H.; Albrand, G.; Servent, V.; Jean, O.S.; Van Praagh, I.; et al. Screening for Vulnerability in Older Cancer Patients: The ONCODAGE Prospective Multicenter Cohort Study. PLoS ONE 2014, 9, e115060. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The index of Adl: A standardized measure of biological and phychological funcation. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Houillier, C.; Wang, X.; Kaloshi, G.; Mokhtari, K.; Guillevin, R.; Laffaire, J.; Paris, S.; Boisselier, B.; Idbaih, A.; Laigle-Donadey, F.; et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 2010, 75, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Figarella-Branger, D.; Mokhtari, K.; Dehais, C.; Jouvet, A.; Uro-Coste, E.; Colin, C.; Carpentier, C.; Forest, F.; Maurage, C.-A.; Vignaud, J.-M.; et al. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neurooncology 2014, 16, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Kaloshi, G.; Benouaich-Amiel, A.; Diakite, F.; Taillibert, S.; Lejeune, J.; Laigle-Donadey, F.; Renard, M.-A.; Iraqi, W.; Idbaih, A.; Paris, S.; et al. Temozolomide for low-grade gliomas: Predictive impact of 1p/19q loss on response and outcome. Neurology 2007, 68, 1831–1836. [Google Scholar] [CrossRef]
- Ewelt, C.; Goeppert, M.; Rapp, M.; Steiger, H.-J.; Stummer, W.; Sabel, M. Glioblastoma multiforme of the elderly: The prognostic effect of resection on survival. J. Neuro-Oncology 2011, 103, 611–618. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Garzon-Muvdi, T.; Parker, S.; Weingart, J.D.; Olivi, A.; Bennett, R.; Brem, H.; Quiñones-Hinojosa, A. Supratentorial Glioblastoma Multiforme: The Role of Surgical Resection Versus Biopsy among Older Patients. Ann. Surg. Oncol. 2011, 18, 239–245. [Google Scholar] [CrossRef]
- Almenawer, S.A.; Badhiwala, J.H.; Alhazzani, W.; Greenspoon, J.; Farrokhyar, F.; Yarascavitch, B.; Algird, A.; Kachur, E.; Cenic, A.; Sharieff, W.; et al. Biopsy vs. partial vs. gross total resection in older patients with high-grade glioma: A systematic review and meta-analysis. Neuro-Oncology 2015, 17, 868–881. [Google Scholar] [CrossRef]
- Keime-Guibert, F.; Chinot, O.; Taillandier, L.; Cartalat-Carel, S.; Frenay, M.; Kantor, G.; Guillamo, J.-S.; Jadaud, E.; Colin, P.; Bondiau, P.-Y.; et al. Radiotherapy for Glioblastoma in the Elderly. N. Engl. J. Med. 2007, 356, 1527–1535. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Ackerl, M.; Flechl, B.; Dieckmann, K.; Preusser, M.; Widhalm, G.; Sax, C.; Marosi, C. Outcome evaluation in glioblastoma patients older than 65 years: Importance of individual assessment of treatment tolerance. Clin. Neuropathol. 2014, 33, 399–406. [Google Scholar] [CrossRef]
- Giaccherini, L.; Galaverni, M.; Renna, I.; Timon, G.; Galeandro, M.; Pisanello, A.; Russo, M.; Botti, A.; Iotti, C.; Ciammella, P. Role of multidimensional assessment of frailty in predicting outcomes in older patients with glioblastoma treated with adjuvant concurrent chemo-radiation. J. Geriatr. Oncol. 2019, 10, 770–778. [Google Scholar] [CrossRef]
- Schneider, M.; Potthoff, A.-L.; Scharnböck, E.; Heimann, M.; Schäfer, N.; Weller, J.; Schaub, C.; Jacobs, A.H.; Güresir, E.; Herrlinger, U.; et al. Newly diagnosed glioblastoma in geriatric (65+) patients: Impact of patients frailty, comorbidity burden and obesity on overall survival. J. Neuro-Oncol. 2020, 149, 421–427. [Google Scholar] [CrossRef]
- Lombardi, G.; Bergo, E.; Caccese, M.; Padovan, M.; Bellu, L.; Brunello, A.; Zagonel, V. Validation of the Comprehensive Geriatric Assessment as a Predictor of Mortality in Elderly Glioblastoma Patients. Cancers 2019, 11, 1509. [Google Scholar] [CrossRef]
- Lorimer, C.F.; Saran, F.; Chalmers, A.J.; Brock, J. Glioblastoma in the elderly—How do we choose who to treat? J. Geriatr. Oncol. 2016, 7, 453–456. [Google Scholar] [CrossRef]
- Deluche, E.; Leobon, S.; Lamarche, F.; Tubiana-Mathieu, N. First validation of the G-8 geriatric screening tool in older patients with glioblastoma. J. Geriatr. Oncol. 2019, 10, 159–163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).