Simple Summary
Autotaxin (ATX) has been linked with the pathogenesis of several cancers and especially with breast cancer (BC). BC is one of the most common cancers among women and although significant steps have been made regarding its early detection and treatment options, challenges still remain. This review aims to summarize the current knowledge of the role and regulation of ATX in BC and to shed light on its potential for clinical applications.
Abstract
Autotaxin (ATX), the protein product of Ectonucleotide Pyrophosphatase Phosphodiesterase 2 (ENPP2), is a secreted lysophospholipase D (lysoPLD) responsible for the extracellular production of lysophosphatidic acid (LPA). ATX-LPA pathway signaling participates in several normal biological functions, but it has also been connected to cancer progression, metastasis and inflammatory processes. Significant research has established a role in breast cancer and it has been suggested as a therapeutic target and/or a clinically relevant biomarker. Recently, ENPP2 methylation was described, revealing a potential for clinical exploitation in liquid biopsy. The current review aims to gather the latest findings about aberrant signaling through ATX-LPA in breast cancer and discusses the role of ENPP2 expression and epigenetic modification, giving insights with translational value.
1. Introduction
Autotaxin (ATX) is a secreted glycoprotein that was first isolated by Stracke’s lab in 1992 in human melanoma cells [1]. ATX belongs to the Ectonucleotide Pyrophosphatase/Phosphodiesterase (ENPP) family, encoded by ENPP2 [2]. Expression of ATX is reported in many tissues and biological fluids and it is considered to be responsible for the production of the circulating lysophosphatidic acid (LPA) [3]. It is now well-established that the biological effects of ATX emerge from the production of LPA and ATX-LPA axis signaling [4]. Notably, it was observed that ENPP2+/− mice had reduced LPA in plasma by half in comparison to normal controls [5]. Although ATX is found in human circulation, its action is more local than systemic, due to its short half-life in blood [6].
In health, ATX is related with embryonic development and wound healing. However, growing evidence has linked the ATX-LPA axis to several diseases but also to cancer [6,7,8]. Aberrant ATX-LPA signaling seems to affect tumor progression, metastatic potential and invasiveness [9]. In fact, ENPP2 is considered one of the top 40 genes that promote the metastatic process [10]. Importantly, ATX and LPA have been recognized as potential diagnostic biomarkers and drug targets for cancer and chronic inflammatory diseases [11]. Recently, our studies have shown that at the epigenetic level, methylation could play a key role in ATX regulation and pathogenesis [12,13]. Below, we present a review of the literature regarding the role of ATX and ENNP2 expression in the malignancy that it is more studied, i.e., breast cancer (BC), focusing on its potential implementation as a liquid biopsy biomarker. Finally, we highlight its potential for clinical application as a therapeutic target and prognostic biomarker in other cancer types.
2. ATX Structure and Function
At the gene level, ENPP2 is located on chromosomal region 8q24 and contains 26 introns and 27 exons [14]. It is characterized by alternative splicing of mRNA and so far, five splice variants have been identified, namely α, β, γ and most recently δ and ε [15]. All of the distinct variants are catalytically active with lysoPLD activity, but their isoform specific functions are not yet characterized [16,17]. Each isoform presents differences in stability, while their expression pattern also differs among tissues [14].
At the protein level, ATX is a type II ectonucleotide of 125 kDa [18]. When ATX was first discovered, it was characterized as a cell motility factor, whilst now, it is included in the ENPP family, which consists of seven structurally related ectoenzymes [2,19]. ATX functions as a lysophospholipase D (lysoPLD) that mainly hydrolyzes extracellular lysophosphatidylcholine (LPC) to generate LPA plus choline and is also the only LysoPLD in the ENPP family [2]. LPA is a bioactive phospholipid, which is widely expressed in many different tissues and acts via six G-protein coupled receptors (LPAR1-6) which can activate several different signaling pathways in physiological and pathological conditions [20]. Besides its ability to hydrolyze lysophospholipids, such as LPC into LPA, ATX can also hydrolyze phosphosphingolipids, such as sphingosylphosphorylcholine (SPC) into sphingosine 1-phosphate (S1P), and nucleotides, still showing higher affinity to LPC; it is clear that by generating different products, ATX can lead to different signaling results [20,21,22,23]. In addition, it is important to mention that the actions of ATX are restricted by liver sinusoidal endothelial cells, which rapidly clear ATX from the circulation [24].
Structurally, ATX is a multi-domain protein, first synthesized as a pre-proenzyme that undergoes maturation for a full active enzyme to be produced [25,26]. ATX consists of a central catalytic phosphodiesterase domain (PDE) which interacts on one side with two N-terminal somatomedin-like domain regions (SMB1-2) and on the other side with the catalytically inactive N-terminal nuclease type (NYC) domain [27]. Importantly, the formation of a deep hydrophobic pocket in the catalytic domain of ATX which bounds the substrate LPC, not found in any other phospholipase, seems to be responsible for ATX’s activity as a lysoPD [26,28].
Notably, the structure of ATX allows the ability to bind to the cell surface and directly deliver LPA to cells. In particular, ATX loaded by LPA, can bind to the surface of exosomes and be transported to the target cells, there it can interact with adhesive molecules such as integrins and bind to the cell surface releasing LPA and promoting cell specific signaling [27,29,30]. By binding to cells, ATX can regulate LPA production, enhance signaling responses specific to cells and also protect LPA from degradation [10,31].
3. ATX-LPA Signaling
As already mentioned, most of the circulating LPA is generated by ATX [32,33]. LPA is a bioactive lipid that is produced both extracellularly and intracellularly and has been correlated with a variety of biological functions [34]. Normally, it can be detected in small amounts in all eukaryotic tissues with the highest levels in the brain and it is also detected in most biological fluids, with high concentration in blood plasma, while being one of the major active constituents in serum [11,20].
LPA signals through its LPAR1-6 receptors, being widely expressed in many different tissues and organs, where they regulate a broad range of cellular processes in both physiological and pathological conditions [20]. The LPAR1,2 and 3 genes are included to the subfamily of Endothelial Differentiation Gene (EdG), while LPAR4 and LPAR5 are included to the purinergic subfamily [35]. More recently, the novel GPC receptor P2Y5 was detected, which was named LPAR6 [36]. It has been found that LPA can also bind to non-GPC receptors, although most LPA functions are known from binding with the GPCRs [8,37]. The first in vivo biological role that was attributed to signaling through LPA was the regulation of blood pressure [38]. Since then, many biological responses have been attributed to the ATX-LPA axis such as angiogenesis, re-epithelialization, cell proliferation and migration, cell differentiation, platelet activation and aggregation, neurite remodeling and ion channel activation, enhanced cell survival and induced inflammation by the production of pro-inflammatory cytokines and also mitogenic and chemotactic activities [17,20,26,34]. Those diverse and widespread results of the ATX-LPA signaling are greatly influenced by the heterogeneity of the LPA receptors, the different expression patterns, tissue context, selective activation and also the different pathways involved in signaling [11]. Meanwhile, concentration levels of LPA are regulated primarily by ATX that controls its production rate and then by LPPs which are responsible for its degradation [39].
4. Regulation of ATX Production and Activity
ENPP2 is expressed in many tissues, with high levels in brain and adipose tissue, and is also found in many biological fluids with high concentration in blood and serum [20]. Regulation of ATX production is crucial, as it is linked with several cancer types and inflammatory conditions [10,40], but it is still not yet fully understood how the ATX production is regulated due to the complicated mechanisms and the different stages that are involved in the regulation of ATX (Figure 1).
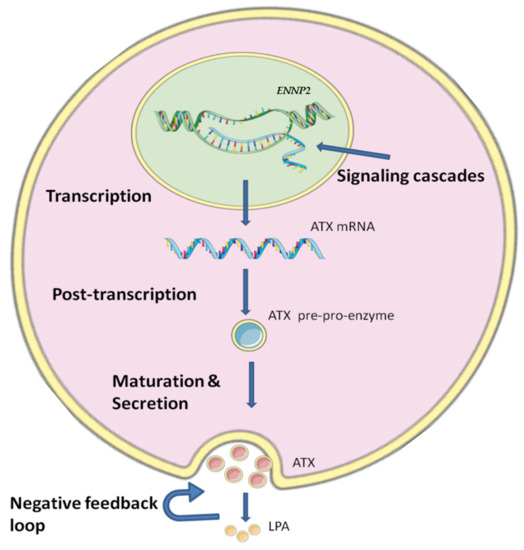
Figure 1.
The regulatory levels of ATX’s expression and production. The expression of ATX is regulated in many ways. The first step of regulation for ENPP2 expression is during transcription by methylation and different transcription factors, growth factors and cytokines which can modulate ENNP2 expression by a diversity of signaling cascades. Then, at post-transcriptional level, a variety of factors act on ENNP2 mRNA affecting its stability and translation. Maturation and secretion also have an important role in regulating ATX, as a negative feedback loop is formed by ATX’s product LPA. The figure was prepared using SMART (smart.servier.com, accessed on 5 July 2022).
At the transcriptional level, it has been reported that ENPP2 expression is regulated by several Transcription Factor (TFs), some participating in carcinogenic cascades. To begin with, ENPP2 has been reported to be activated by the STAT3 leading to increased migration of BC cells [41]. On the other hand, reduced ENPP2 expression is observed after decreased expression of the NFAT1, resulting in a reduction of growth and metastasis of melanoma cells [42]. AP-1 and SP seem to act on the ENPP2 promoter affecting its transcription in human neuroblastoma cell lines [43], whereas v-jun and c-jun also seem to hold a role [44,45]. In addition to TFs, a regulating role at transcriptional level has been attributed to the Hypoxia Inducible Factors (HIFs). It has been shown that in Hepatocellular carcinoma (HCC), HIFs increase ENPP2 mRNA and ATX protein expression, which further supports HCC progression [46]. Signaling pathways have also been linked with ENPP2 expression. In fact, RSPO2, a regulator of the Wnt/b-catenin signaling, has been reported to act directly on ENPP2 and increase its expression in myoblast cells [47]. ENPP2 expression was also induced by type I interferons through activation of the signaling pathways JAK-STAT and PI3K-AKT [48]. Furthermore, after inhibition of the AKT pathway, it was found that it also has a role in secretion of ATX [49].
At the post-transcriptional level, ATX is regulated by RNA-binding proteins HuR and AUF1. HuR acts through stabilizing the ENPP2 mRNA and leads to increased ENPP2 expression, while AUF1 promotes ENPP2 decay and suppress ENPP2 expression; it was suggested that via these mechanisms they can modulate cancer cell migration [50]. In addition, it has been shown that the tumor suppressor microRNA-101-3p could bind to the 3′-UTR of ENPP2 mRNA and inhibit its translation, leading to the reduction of migration, invasion and proliferation [51]. Another study showed that the 3′-UTR of ENPP2 mRNA could also be targeted by the RNA methyltransferase NSUN2 and its methylation seems to promote the mRNA export from the nucleus leading to enhanced translation of ATX [52].
The secretion of ATX is also a regulated process. ATX is synthesized as a pre-pro-enzyme and in order to produce a mature full activated enzyme it undergoes firstly cleavage of a N-terminal signal peptide between amino-acids G27 and G28, and then N-glycosylation at the amino acids N53 and N410, those steps being both required for the secretion of ATX. In addition to that, N-glycosylation of the site Asn-524 has been implicated in ATX’s activity as a lysoPLD and as a chemoattractant [53,54,55]. After secretion, the activity of ATX seems to also be regulated through a negative feedback loop that is created because the ATX products, LPA and S1P, can bind to ATX more strongly than LPC [40,56]. However, it has been suggested that inflammatory cytokines are able to counteract the inhibition of ENPP2 mRNA expression by S1P and LPA [40].
5. The Role of Methylation in the Regulation of ENPP2 Expression
Recently the epigenetic changes of ENPP2 have been investigated and a few studies showed that ENPP2 expression can be regulated by DNA methylation (Table 1). DNA methylation is probably the most studied epigenetic alteration in mammals, as it provides a vital mechanism for stable gene silencing [57,58]. DNA methylation occurs with the addition of a methyl group solely in the 5 position of the cytosine ring and is almost inclusively seen in CpG dinucleotides (CGs) which are called “CpG islands” [57,59].

Table 1.
Methylation status of ENPP2 in cancer in relation to health.
In our recent analysis, we studied the methylation of ENPP2 in multiple healthy tissues via a bioinformatic in silico approach and correlated it with gene and isoform expression. We examined publicly available high-throughput methylation datasets from studies involving the Illumina methylation bead-chip arrays found in Gene Expression Omnibus (GEO), to identify Differentially Methylated CpGs (DMCs) of ENPP2 [12]. We showed a consistent methylation pattern throughout the ENPP2 gene across 17 healthy human tissues. In particular, increased methylation was observed in the gene body CGs and decreased methylation in the promoter and the 1st exon CGs. Given the fact that ENPP2 is abundantly expressed in many tissues and biological fluids [66], we suggest that the decreased methylation in the promoter is associated with the active transcription of the gene in healthy tissues.
6. Role of ATX in Breast Cancer
Since the discovery of ATX as a motility factor, many studies have focused on its role in cancer [1]. ATX expression and subsequent LPA production are elevated or aberrant in both tumor and serum from almost every type of cancer, such as breast [67], thyroid [68], HCC [69], lung [70], renal [71], pancreatic [72], bladder [73], ovarian [3], endometrial [74], prostate [75], glioblastoma [76] and neuroblastoma [77]. Consequently, ATX and LPA have been proposed as diagnostic biomarkers and drug targets, with some inhibitors already in phase I and II of clinical trials [11,23,72].
ATX is strongly related with enhanced proliferation, migration, and survival of cancer cells [9]. The first link of ATX with tumorigenesis came from a study on Ras transformed NIH3T3 cells that overexpressed ATX and enhanced tumor growth, angiogenesis and aggression of cancer cells, while inactive mutant ATX did not [78,79]. Consistent with this, enhanced expression of ATX and production of LPA by Epstein–Barr virus infection promoted growth and survival in Hodgkin lymphoma cells. In addition, specific downregulation of ATX led to decreased LPA levels resulting in reduced cell growth and viability in these cells [80]. Interestingly, transgenic mouse models that expressed either human ATX, LPAR1, LPAR2 or LPAR3 displayed spontaneous development of breast tumors [9], while in human BC MDA-B0-2 cells, overexpression of ATX or LPAR1 resulted in invasion, bone metastasis and destruction [81,82]. Generally, ATX-LPA axis signaling is proposed to highly affect BC-related inflammation and consequently progression [83]. Notably, inhibition of ENPP2 led in the decrease of lung and bone metastasis but did not seem to affect the progression of the breast primary tumor [82]. Inhibition of LPAR1 also had similar results [84].
However, ATX mRNA levels in BC tumor biopsies are not good indicators for cancer metastasis and progression [82]. Intriguingly, ATX protein was found to be stored in a-granules of resting human platelets [85]. BC cells co-cultured with platelets showed an increase in LPA and cancer cell proliferation, indicating that interaction between cancer cells and platelets promotes platelet activation. Furthermore, inhibition of integrin αIIbβ3 in cancer cells prevented platelet degranulation and release of ATX leading to reduced cell proliferation [85]. These findings imply that non-tumor ATX in circulation might derive from platelets after interaction with circulating tumor cells, affecting metastasis, a pathway that could present a therapeutic target.
In addition, as mentioned previously, cell-secreted exosomes can bind to ATX, which provides a mechanism for extracellular LPA production and LPA delivery to cell surface receptors, protecting LPA from degradation. Importantly, this mechanism also seems to stimulate LPA signaling in cells and promote exosome-stimulated cell migration [29].
ENPP2 expression can be altered by several inflammatory cytokines and growth factors, frequently increased in cancer [86]. TNFα/NF-kB has been associated with increased ENPP2 expression and activity in human Hep3B and Huh7 liver cancer cells [69]. On the contrary, decreased ATX activity was caused by the cytokines IL-1β, TGF-β and IL-4 in thyroid carcinoma UTC-1736 cells, while IL-6 enhanced ENPP2 expression [68]. In addition, a concentration-dependent effect was observed from the growth factors EGF and bEGF that caused increased mRNA expression of ENPP2 [68]. Finally, it was proposed that induction of ENPP2 expression by VEGF was associated with an aggressive phenotype in ovarian cancer via a positive feedback loop [87]. All these factors are dysregulated in BC and could form pathophysiological loops with ATX, needing further assessment.
ATX-LPA axis signaling was also proposed to cause resistance in chemotherapy and radiotherapy, by protecting cancer cells against cell death caused by therapy [56]. Similar to findings in ovarian cancer cells lines, where expression of ATX was linked to a delay in chemotherapy-induced apoptosis [88], increased levels of ENPP2 mRNA and inflammatory mediators were observed in adipose tissue during γ-radiation, suggesting activation of the inflammatory response cycle, which protected BC cells and reduced the efficiency of radiotherapy [89]. Similarly, inhibition of ATX blocked the protection from apoptosis induced by the chemoattractant Taxol in the BC cell line MCF-7 [90]. Notably, it was also observed that radiation could induce the production of ATX, associating ATX with DNA damage in BC, while dexamethasone can attenuate the radiation-induced increase of ATX [91]. Drug resistance was also observed in Breast Cancer Stem like Cells (CSCs) and was attributed to ATX. In breast CSCs, ATX inhibitors 30 and 3b were combined with the chemotherapeutic drug Paclitaxel (PT), resulting in 25–30% reduced cell viability as compared to PT alone, suggesting that ATX is implicated in drug resistance and its inhibition can resensitize CSCs [92]. Similar observations were also found in ovarian cancer [93]. Additionally, ENPP2 was identified as the second most upregulated gene in breast CSCs after treatment with PT in an in silico analysis, indicating that CSCs could favor LPA-enhanced microenvironment [94].
All these results give strong evidence that pathological signaling through the ATX-LPA axis and aberrant expression of ENPP2 is linked to poor outcome in BC. However, as a word of caution, although increased ATX levels is correlated with poor outcome, it does not automatically reflect LPA levels and signaling, as many factors such as LPC availability and LPA degradation can affect LPA levels [4]. Eventually, the LPA expression and signaling on both the tumor and surrounding stromal cells will determine the results of ATX expression [6]. In fact, it is now established that the direct production of ENPP2 mRNA by cancer cells is significantly low in many cancer types and the majority of ATX is produced from the tumor microenvironment [95,96]. Recent studies in both thyroid and BC revealed a prototypical model for the crosstalk between the tumor microenvironment and ATX expression. Particularly, inflammatory mediators from cancer cells increased LPA and ATX levels in surrounding fibroblasts and adipose tissue that further induce the inflammation and progression of the tumor, creating a feedback cycle [97,98]. In a recent study in BC cell line models, ENPP2 mRNA expression levels decreased in the more aggressive MDA-MB-231 line as compared to the much less aggressive MCF-7 [60]. In addition to that, in human lung-cancer tissue samples, ENPP2 mRNA was founbred significantly down-regulated, in both in silico and experimental analysis. However, the ATX protein expression in tissues and activity in serum were increased compared to control samples. Identical findings were found in two lung cancer mouse models. Those results were proposed to arise from integrin binding of ATX to cancer cells that protected it from degradation [99]. Taken together, these results suggest that several mechanisms are implicated in mRNA/protein ENPP2 expression and LPA signaling in tumors during cancer pathogenesis, building a complex and possibly tissue-specific process.
7. ENPP2 Methylation and Cancer
Abnormal methylation detected in tissue or liquid biopsy has been associated with cancer development and progression [100,101,102,103,104,105]. Several studies address the regulation of ATX expression at its gene methylation level in cancer and may enlighten different aspects of its involvement in the pathogenetic process in BC (Table 1). A relevant work showed that ENNP2 was highly methylated in BC tissues, also presenting low mRNA expression levels compared to adjacent tissues [60]. Furthermore, in silico studies based on TCGA datasets reported high methylation of ENPP2 in BC and characterized it as a methylation-driven gene in cancer [61,62]. In our recent in silico analysis of ENPP2 methylation in BC tissues, it was found that the promoter CGs were hypermethylated in comparison to normal tissues and mRNA expression was downregulated and inversely correlated with the methylation. Hypermethylation of ENPP2 was also associated with the progression of BC [13]. Together, these findings suggest that ENPP2 is highly methylated in BC, correlated with aggressiveness and low expression levels. Interestingly, Wang et al. demonstrated that the promoter region of ENPP2 was hypermethylated in tissue samples from BC. Furthermore, in an analysis of circulating cell free DNA (ccfDNA), ENPP2 was also found hypermethylated but failed to find any significant difference between methylation levels of healthy and BC patients [63]. In contrast to that, in our recent work, methylation of ENPP2 1st exon in ccfDNA was found significant hypermethylated in BC patients as compared to healthy ccfDNA and it was also correlated with the cancer load [13], suggesting a role of ENPP2 methylation as a BC prognostic biomarker. These controversial results could be methodologically explained due to the different regions of ENPP2 gene that were examined in the patient samples. In addition, the difference in sample size might explain the failing to demonstrate statistical significance in differential methylation. In silico analysis of ENPP2 conducted in different cancer types such as Pancreatic cancer (PC), LC, Colorectal cancer (CC), melanoma and HCC revealed a specific methylation pattern, as ENPP2 was hypermethylated in promoter and hypomethylated in gene body, accordingly. Most importantly, decreased expression levels were associated with increased promoter methylation. Additionally, in the same study, methylation was related to poor prognostic parameters [12]. In another in silico analysis, ENPP2 was found hypermethylated in Lung Adenocarcinoma (LUAD) and Squamous Cell Carcinoma (SCC) tumors and this was consistent with lower mRNA expression, highly associated with worse progression in LUAD [64]. Consistent with that, a study investigating epigenetic changes reported ENPP2 promoter hypermethylation in metastatic Uveal Melanoma (UM) that was associated with downregulation in mRNA expression [65]. The above studies demonstrate that hypermethylation of ENPP2 promoter and 1st Exon in cancer is correlated with reduced ATX expression, presenting an epigenetic expression regulation level, and suggests a potential of ENPP2 methylation as a prognostic biomarker, awaiting further validation.
As mentioned earlier, although elevated ENPP2 levels have been observed in cancer patients, most of the produced ENPP2 is proposed to originate from the tumor surrounding environment rather than directly from the cancer cells themselves [95,96]. This could provide an explanation for the increased methylation and consecutively decreased expression that is mainly observed in cancer cells.
8. ENPP2 Methylation in BC Liquid Biopsy
Assessing methylation in liquid biopsy biomaterial such as ccfDNA can dynamically reflect methylation events of the tumor, as supported by in vitro studies showing that the methylation profile of ccfDNA released by BC cell lines in culture is identical to their genomic DNA [106]. ccfDNA has, therefore, emerged as a valuable source of clinically relevant information and it is currently exploited in multiple applications for detecting epigenetic biomarkers for prognosis, diagnosis and disease monitoring in BC [102,107]. In parallel to studies in BC tissues, ENPP2 hypermethylation of promoter associated CGs was also demonstrated in silico. Thus, datasets of ccfDNAs from BC patients showed higher methylation in relation to ccfDNAs from healthy individuals [13]. We further evaluated ENPP2 methylation in ccfDNA of BC patients in order to examine its clinical value as a biomarker. Analysis of the 1st Exon cg02534163 by a targeted qMSP assay showed that ENPP2 hypermethylation was detected more often in ccfDNA of BrCa patients than in healthy individuals [13]. However, in a previous study addressing ENPP2 methylation in ccfDNA from 22 healthy and 45 Taiwanese BC patients, no significant differences were reported [63], although ENPP2 methylation showed a two-fold increase in BC in relation to adjacent normal tissue. Methodological differences or even population genetic variations might explain these different findings. Importantly, in our study, ccfDNA methylation levels of ENPP2 were also elevated in the neoadjuvant and metastatic groups of patients in relation to adjuvant and control group of patients. This result could be due to the fact that in the neoadjuvant and metastatic groups, patients still have a significant tumor burden. Our bioinformatic analysis also showed that ENPP2 methylation was increased in metastasis in relation to primary cancers. In addition to that, according to our experimental analysis, patients having two or more metastatic foci presented more increased ENPP2 methylation levels than those patients having a distant metastasis in one focus [13]. Cumulative experimental results are in accordance with those from bioinformatic analysis showing hypermethylation of ENPP2 in both BC tissue and ccfDNA and a correlation with cancer aggressiveness and metastasis, suggesting its potential as a novel circulating biomarker in BC.
Circulating microRNAs are also promising biomarkers, as they often present a distinct expression profile in cancers [108]. An interesting case is miR489-3p, as it was found significantly increased in the serum of mouse models with ATX-induced tumors, and in an in silico analysis of human cancers’ serum [109]. In general, miR-489-3p has a tumor suppressing role and acts by suppressing mitogen-activated protein kinase (MEK1), a protein implicated in tumor development and progression [110]. However, it was observed that expression of ATX could alter the tumor suppressive function of miR-489-3p in tumor cells and enhance MEK1 activity and consecutively tumor appearance [109].
9. The ATX-LPA Axis as a Therapeutic Target in BC
Due to the established implication of ATX in cancer, blocking the ATX-LPA axis signaling could present an important target of therapeutic intervention. Most importantly, ATX is considered a strongly druggable target as the action of its PDE domain is easily inhibited, and ATX also acts extracellularly; therefore, it has gained a lot of attention from both academic and industrial settings with several ATX inhibitors being in clinical or pre-clinical studies for drug development [26]. In principle, inhibitors of ATX function could be directed towards its synthesis, maturation and/or its catalytic activity [25]. Initially, many lipid analogs were designed based on the fact that LPA can inhibit the LysoPLD activity of ATX [110]. One of the most successful was the inhibitor BMP-22, an ATX inhibitor, which resulted in reduced lung metastasis in melanoma; while it was also shown that it can reduce bone metastasis in BC [85,111]. However, the bioavailability and efficiency of lipid analogs is limited in vivo [110]. After the discovery of ATX structure, potent non-lipid inhibitors have been designed with great success. Indeed, the competitive inhibitor of ATX, GLPG1690, was originally designed for the IPF treatment reaching phase III clinical trial [112]. Unfortunately, the clinical trials (NCT03711162, NCT03733444), were recently discontinued due to the benefit-risk profile. GLPG1690 was also studied in BC resulting in decreased proliferation of cancer cells, while it promoted apoptosis induced by radiotherapy when combined together [113,114]. Another promising ATX inhibitor is ONO-8430506, which inhibited breast tumor growth and subsequent blocked metastasis in liver and lung. ONO-8430506 has also resulted in reduced tumor growth in thyroid cancer [97,115]. In addition, the LPA receptor antagonist BrP-LPA was studied for the treatment of BC, leading to reduced cancer cell migration and tumor regression [116]. Other ATX inhibitors such as IOA-289 and PF8380, or BMS-986020, a LPAR1 antagonist, were developed and showed promising results in preclinical and clinical trials in other conditions [117,118,119,120]; however, they have not yet been tested in BC. Given the importance of ATX-LPA signaling, expanding research of these novel molecules in BC could lead to new therapeutic alternatives.
10. Conclusions
ENPP2 is responsible for the production of circulating LPA, and it is well established that the actions of ATX emerge from ATX-LPA axis signaling through LPAR1-6. Aberrant levels of ATX have been found in BC, where its production seems downregulated and hypermethylated in tumor cells and overexpressed in surrounding tissue. It is quite clear by recent findings that methylation of ENPP2 at the promoter region or 1st exon holds a significant role in the regulation of its expression, and it has been proposed that ENPP2 methylation can serve as a biomarker for BC diagnosis and prognosis with the potential to be implemented in liquid biopsy, i.e., detected in ccfDNA, baring additional clinical value. In addition, ATX activity has a significant role in BC progression, invasion and metastasis, opening the way for new treatment strategies based on ENPP2 inhibition.
Author Contributions
Conceptualization, A.D., M.P. and E.C.; investigation A.D. and M.P.; writing—original draft preparation, A.D. and M.P.; writing—review and editing, A.D., M.P., V.A. and E.C.; supervision, V.A. and E.C.; project administration, E.C.; funding acquisition, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I), 1st Call for H.F.R.I. Research Projects to Support Faculty Members & Researchers and Procure High-Value Research Equipment, grant no 1955.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stracke, M.L.; Krutzsch, H.C.; Unsworth, E.J.; Arestad, A.; Cioce, V.; Schiffmann, E.; Liotta, L.A. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 1992, 267, 2524–2529. [Google Scholar] [CrossRef]
- Yuelling, L.M.; Fuss, B. Autotaxin (ATX): A multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2008, 1781, 525–530. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Li, M.; Yin, N.; Zhang, J. The Expression Regulation and Biological Function of Autotaxin. Cells 2021, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, L.A.; Moolenaar, W.H. Regulation and biological activities of the autotaxin—LPA axis. Prog. Lipid Res. 2007, 46, 145–160. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradère, J.P.; Pettit, T.R.; Wakelam, M.J.O.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a Secreted Lysophospholipase D, Is Essential for Blood Vessel Formation during Development. J. Mol. Cell Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.S.; Moolenaar, W.H. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis 2011, 30, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Magkrioti, C.; Galaris, A.; Kanellopoulou, P.; Stylianaki, E.-A.; Kaffe, E.; Aidinis, V. Autotaxin and chronic inflammatory diseases. J. Autoimmun. 2019, 104, 102327. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Rives, S.A.; González-Arenas, A. Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediat. Inflamm. 2017, 2017, 9173090. [Google Scholar] [CrossRef]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of Autotaxin and Lysophosphatidic Acid Receptors Increases Mammary Tumorigenesis, Invasion, and Metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef]
- Peyruchaud, O.; Saier, L.; Leblanc, R. Autotaxin Implication in Cancer Metastasis and Autoimunne Disorders: Functional Implication of Binding Autotaxin to the Cell Surface. Cancers 2019, 12, 105. [Google Scholar] [CrossRef]
- Quan, M.; Cui, J.-j.; Feng, X.; Huang, Q. The critical role and potential target of the autotaxin/lysophosphatidate axis in pancreatic cancer. Tumor Biol. 2017, 39, 1010428317694544. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Fanidis, D.; Aidinis, V.; Chatzaki, E. ENPP2 Methylation in Health and Cancer. Int. J. Mol. Sci. 2021, 22, 11958. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Drosouni, A.; Fanidis, D.; Karaglani, M.; Balgkouranidou, I.; Xenidis, N.; Aidinis, V.; Chatzaki, E. ENPP2 Promoter Methylation Correlates with Decreased Gene Expression in Breast Cancer: Implementation as a Liquid Biopsy Biomarker. Int. J. Mol. Sci. 2022, 23, 3717. [Google Scholar] [CrossRef]
- Giganti, A.; Rodriguez, M.; Fould, B.; Moulharat, N.; Cogé, F.; Chomarat, P.; Galizzi, J.-P.; Valet, P.; Saulnier-Blache, J.-S.; Boutin, J.A.; et al. Murine and Human Autotaxin α, β, and γ Isoforms: Gene Organization, Tissue Distribution, And Biochemical Characterization*. J. Biol. Chem. 2008, 283, 7776–7789. [Google Scholar] [CrossRef]
- Herr, D.R.; Chew, W.S.; Satish, R.L.; Ong, W.-Y. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol. Neurobiol. 2020, 57, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.; van Wijk, X.M.; van Meeteren, L.A.; van Zeijl, L.; van de Westerlo, E.M.; Hausmann, J.; Fish, A.; Perrakis, A.; van Kuppevelt, T.H.; Moolenaar, W.H. The polybasic insertion in autotaxin α confers specific binding to heparin and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2013, 288, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Benesch, M.G.K.; Brindley, D.N. Role of the autotaxin–lysophosphatidate axis in the development of resistance to cancer therapy. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158716. [Google Scholar] [CrossRef] [PubMed]
- Clair, T.; Lee, H.Y.; Liotta, L.A.; Stracke, M.L. Autotaxin Is an Exoenzyme Possessing 5′-Nucleotide Phosphodiesterase/ATP Pyrophosphatase and ATPase Activities*. J. Biol. Chem. 1997, 272, 996–1001. [Google Scholar] [CrossRef]
- Borza, R.; Salgado-Polo, F.; Moolenaar, W.H.; Perrakis, A. Structure and function of the ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) family: Tidying up diversity. J. Biol. Chem. 2022, 298, 101526. [Google Scholar] [CrossRef]
- Barbayianni, E.; Kaffe, E.; Aidinis, V.; Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 2015, 58, 76–96. [Google Scholar] [CrossRef]
- Umezu-Goto, M.; Kishi, Y.; Taira, A.; Hama, K.; Dohmae, N.; Takio, K.; Yamori, T.; Mills, G.B.; Inoue, K.; Aoki, J.; et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002, 158, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Clair, T.; Aoki, J.; Koh, E.; Bandle, R.W.; Nam, S.W.; Ptaszynska, M.M.; Mills, G.B.; Schiffmann, E.; Liotta, L.A.; Stracke, M.L. Autotaxin Hydrolyzes Sphingosylphosphorylcholine to Produce the Regulator of Migration, Sphingosine-1-Phosphate. Cancer Res. 2003, 63, 5446–5453. [Google Scholar] [PubMed]
- Nakanaga, K.; Hama, K.; Aoki, J. Autotaxin—An LPA producing enzyme with diverse functions. J. Biochem. 2010, 148, 13–24. [Google Scholar] [CrossRef]
- Silvia Jansen, M.A.; Vekemans, K.; Vanbilloen, H.; Verbruggen, A.; Bollen, M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009, 284, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Stefan, C.; Creemers, J.W.M.; Waelkens, E.; Van Eynde, A.; Stalmans, W.; Bollen, M. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J. Cell Sci. 2005, 118, 3081–3089. [Google Scholar] [CrossRef]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef]
- Fulkerson, Z.; Wu, T.; Sunkara, M.; Kooi, C.V.; Morris, A.J.; Smyth, S.S. Binding of Autotaxin to Integrins Localizes Lysophosphatidic Acid Production to Platelets and Mammalian Cells*. J. Biol. Chem. 2011, 286, 34654–34663. [Google Scholar] [CrossRef]
- Magkrioti, C.; Kaffe, E.; Stylianaki, E.-A.; Sidahmet, C.; Melagraki, G.; Afantitis, A.; Matralis, A.N.; Aidinis, V. Structure-Based Discovery of Novel Chemical Classes of Autotaxin Inhibitors. Int. J. Mol. Sci. 2020, 21, 7002. [Google Scholar] [CrossRef]
- Jethwa, S.A.; Leah, E.J.; Zhang, Q.; Bright, N.A.; Oxley, D.; Bootman, M.D.; Rudge, S.A.; Wakelam, M.J. Exosomes bind to autotaxin and act as a physiological delivery mechanism to stimulate LPA receptor signalling in cells. J. Cell Sci. 2016, 129, 3948–3957. [Google Scholar] [CrossRef]
- Wu, T.; Vander Kooi, C.; Shah, P.; Charnigo, R.; Huang, C.; Smyth, S.S.; Morris, A.J. Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration. FASEB J. 2014, 28, 861–870. [Google Scholar] [CrossRef]
- Hausmann, J.; Kamtekar, S.; Christodoulou, E.; Day, J.E.; Wu, T.; Fulkerson, Z.; Albers, H.M.H.G.; van Meeteren, L.A.; Houben, A.J.S.; van Zeijl, L.; et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 2011, 18, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.H.G.; Dong, A.; van Meeteren, L.A.; Egan, D.A.; Sunkara, M.; van Tilburg, E.W.; Schuurman, K.; van Tellingen, O.; Morris, A.J.; Smyth, S.S.; et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.C.L.d.S.; Amaral, R.F.d.; Fonseca, A.C.C.d.; Garcia, C.; Mendes, F.d.A.; Freitas, C.; dos Santos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- Okudaira, S.; Yukiura, H.; Aoki, J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 2010, 92, 698–706. [Google Scholar] [CrossRef]
- Frisca, F.; Sabbadini, R.A.; Goldshmit, Y.; Pébay, A. Chapter five—Biological Effects of Lysophosphatidic Acid in the Nervous System. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 296, pp. 273–322. [Google Scholar]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef]
- Smyth, S.S.; Cheng, H.-Y.; Miriyala, S.; Panchatcharam, M.; Morris, A.J. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta 2008, 1781, 563–570. [Google Scholar] [CrossRef]
- Brindley, D.N.; Raouf, A. Chapter 3—Autotaxin is an important component of the tumor microenvironment and a major modulator of therapy responses for breast cancer. In Biological Mechanisms and the Advancing Approaches to Overcoming Cancer Drug Resistance; Freywald, A., Vizeacoumar, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 12, pp. 47–63. [Google Scholar]
- Benesch, M.G.K.; Zhao, Y.Y.; Curtis, J.M.; McMullen, T.W.; Brindley, D.N. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J. Lipid Res. 2015, 56, 1134–1144. [Google Scholar] [CrossRef]
- Azare, J.; Doane, A.; Leslie, K.; Chang, Q.; Berishaj, M.; Nnoli, J.; Mark, K.; Al-Ahmadie, H.; Gerald, W.; Hassimi, M.; et al. Stat3 mediates expression of autotaxin in breast cancer. PLoS ONE 2011, 6, e27851. [Google Scholar] [CrossRef]
- Braeuer, R.R.; Zigler, M.; Kamiya, T.; Dobroff, A.S.; Huang, L.; Choi, W.; McConkey, D.J.; Shoshan, E.; Mobley, A.K.; Song, R.; et al. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res. 2012, 72, 5757–5766. [Google Scholar] [CrossRef]
- Farina, A.R.; Cappabianca, L.; Ruggeri, P.; Di Ianni, N.; Ragone, M.; Merolle, S.; Sano, K.; Stracke, M.L.; Horowitz, J.M.; Gulino, A.; et al. Constitutive autotaxin transcription by Nmyc-amplified and non-amplified neuroblastoma cells is regulated by a novel AP-1 and SP-mediated mechanism and abrogated by curcumin. FEBS Lett. 2012, 586, 3681–3691. [Google Scholar] [CrossRef]
- Black, E.J.; Clair, T.; Delrow, J.; Neiman, P.; Gillespie, D.A.F. Microarray analysis identifies Autotaxin, a tumour cell motility and angiogenic factor with lysophospholipase D activity, as a specific target of cell transformation by v-Jun. Oncogene 2004, 23, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Sioletic, S.; Czaplinski, J.; Hu, L.; Fletcher, J.A.; Fletcher, C.D.; Wagner, A.J.; Loda, M.; Demetri, G.D.; Sicinska, E.T.; Snyder, E.L. c-Jun promotes cell migration and drives expression of the motility factor ENPP2 in soft tissue sarcomas. J. Pathol. 2014, 234, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.J.; Humphreys, I.S.; Rudge, S.A.; Wilson, G.K.; Bhattacharya, B.; Ciaccia, M.; Hu, K.; Zhang, Q.; Mailly, L.; Reynolds, G.M.; et al. Autotaxin-lysophosphatidic acid receptor signalling regulates hepatitis C virus replication. J. Hepatol. 2017, 66, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Jin, Y.R.; Tan, L.; Kosciuk, T.; Lee, J.S.; Yoon, J.K. Regulation of the follistatin gene by RSPO-LGR4 signaling via activation of the WNT/β-catenin pathway in skeletal myogenesis. Mol. Cell Biol. 2014, 34, 752–764. [Google Scholar] [CrossRef]
- Song, J.; Guan, M.; Zhao, Z.; Zhang, J. Type I Interferons Function as Autocrine and Paracrine Factors to Induce Autotaxin in Response to TLR Activation. PLoS ONE 2015, 10, e0136629. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, B.; Xiong, C.; Zhang, X.; Zhang, X.; Zhang, J. Selective export of autotaxin from the endoplasmic reticulum. J. Biol. Chem. 2017, 292, 7011–7022. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Lyu, L.; Li, X.; Yao, S.; Zhang, J. Autotaxin Expression Is Regulated at the Post-transcriptional Level by the RNA-binding Proteins HuR and AUF1. J. Biol. Chem. 2016, 291, 25823–25836. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, L.; Zhang, X.; Zhang, J. Autotaxin is a novel target of microRNA-101-3p. FEBS Open Bio 2019, 9, 707–716. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Zhang, J.; Zhang, X. NSun2 promotes cell migration through methylating autotaxin mRNA. J. Biol. Chem. 2020, 295, 18134–18147. [Google Scholar] [CrossRef]
- Pradère, J.P.; Tarnus, E.; Grès, S.; Valet, P.; Saulnier-Blache, J.S. Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 93–102. [Google Scholar] [CrossRef]
- Koyama, M.; Nishimasu, H.; Ishitani, R.; Nureki, O. Molecular Dynamics Simulation of Autotaxin: Roles of the Nuclease-like Domain and the Glycan Modification. J. Phys. Chem. B 2012, 116, 11798–11808. [Google Scholar] [CrossRef]
- Jansen, S.; Callewaert, N.; Dewerte, I.; Andries, M.; Ceulemans, H.; Bollen, M. An essential oligomannosidic glycan chain in the catalytic domain of autotaxin, a secreted lysophospholipase-D. J. Biol. Chem. 2007, 282, 11084–11091. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Bekele, R.; Capatos, D.; Venkatraman, G.; Sariahmetoglu, M.; Brindley, D.N. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie 2011, 93, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. 2-DNA Methylation and Cancer. In Advances in Genetics; Herceg, Z., Ushijima, T., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 27–56. [Google Scholar]
- Liu, X.; Peng, Y.; Wang, J. Integrative analysis of DNA methylation and gene expression profiles identified potential breast cancer-specific diagnostic markers. Biosci. Rep. 2020, 40, BSR20201053. [Google Scholar] [CrossRef]
- Ivan, J.; Patricia, G.; Agustriawan, D. In silico study of cancer stage-specific DNA methylation pattern in White breast cancer patients based on TCGA dataset. Comput. Biol. Chem. 2021, 92, 107498. [Google Scholar] [CrossRef]
- Zhong, X.; Zhong, G. Prognostic biomarker identification and tumor classification in breast cancer patients by methylation and transcriptome analysis. FEBS Open Bio 2021, 11, 2139–2151. [Google Scholar] [CrossRef]
- Wang, S.-C.; Liao, L.-M.; Ansar, M.; Lin, S.-Y.; Hsu, W.-W.; Su, C.-M.; Chung, Y.-M.; Liu, C.-C.; Hung, C.-S.; Lin, R.-K. Automatic Detection of the Circulating Cell-Free Methylated DNA Pattern of GCM2, ITPRIPL1 and CCDC181 for Detection of Early Breast Cancer and Surgical Treatment Response. Cancers 2021, 13, 1375. [Google Scholar] [CrossRef]
- Nema, R.; Shrivastava, A.; Kumar, A. Prognostic role of lipid phosphate phosphatases in non-smoker, lung adenocarcinoma patients. Comput. Biol. Med. 2021, 129, 104141. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.N.; Boers, R.; Vaarwater, J.; Boers, J.; Brands, T.; Mensink, H.; Verdijk, R.M.; van Ijcken, W.F.J.; Gribnau, J.; de Klein, A.; et al. Genome-wide aberrant methylation in primary metastatic UM and their matched metastases. Sci. Rep. 2022, 12, 42. [Google Scholar] [CrossRef]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lee, J.; Park, C.G.; Kim, S.; Hong, S.; Chung, H.C.; Min, S.K.; Han, J.W.; Lee, H.W.; Lee, H.Y. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin. Exp. Metastasis 2002, 19, 603–608. [Google Scholar] [CrossRef]
- Kehlen, A.; Englert, N.; Seifert, A.; Klonisch, T.; Dralle, H.; Langner, J.; Hoang-Vu, C. Expression, regulation and function of autotaxin in thyroid carcinomas. Int. J. Cancer 2004, 109, 833–838. [Google Scholar] [CrossRef]
- Wu, J.-M.; Xu, Y.; Skill, N.J.; Sheng, H.; Zhao, Z.; Yu, M.; Saxena, R.; Maluccio, M.A. Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma. Mol. Cancer 2010, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mou, L.; Liu, N.; Tsao, M.S. Autotaxin expression in non-small-cell lung cancer. Am. J. Respir. Cell Mol. Biol. 1999, 21, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Stassar, M.J.J.G.; Devitt, G.; Brosius, M.; Rinnab, L.; Prang, J.; Schradin, T.; Simon, J.; Petersen, S.; Kopp-Schneider, A.; Zöller, M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br. J. Cancer 2001, 85, 1372–1382. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Li, H.; Xu, W.; Guo, X. Evaluation of serum ATX and LPA as potential diagnostic biomarkers in patients with pancreatic cancer. BMC Gastroenterol. 2021, 21, 58. [Google Scholar] [CrossRef]
- Xu, A.; Ahsanul Kabir Khan, M.; Chen, F.; Zhong, Z.; Chen, H.C.; Song, Y. Overexpression of autotaxin is associated with human renal cell carcinoma and bladder carcinoma and their progression. Med. Oncol. 2016, 33, 131. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y.; Zhang, Q.; Li, X.; Zhou, J.; Wang, J.; Wei, L. ATX-LPA axis facilitates estrogen-induced endometrial cancer cell proliferation via MAPK/ERK signaling pathway. Mol. Med. Rep. 2018, 17, 4245–4252. [Google Scholar] [CrossRef] [PubMed]
- Nouh, M.A.; Wu, X.X.; Okazoe, H.; Tsunemori, H.; Haba, R.; Abou-Zeid, A.M.; Saleem, M.D.; Inui, M.; Sugimoto, M.; Aoki, J.; et al. Expression of autotaxin and acylglycerol kinase in prostate cancer: Association with cancer development and progression. Cancer Sci. 2009, 100, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Mariani, L.; Weis, J.; Woyke, T.; Berens, T.J.; McDonough, W.; Sloan, A.; Coons, S.W.; Berens, M.E. Gene Expression Profile of Glioblastoma Multiforme Invasive Phenotype Points to New Therapeutic Targets. Neoplasia 2005, 7, 7–16. [Google Scholar] [CrossRef]
- Kawagoe, H.; Stracke, M.L.; Nakamura, H.; Sano, K. Expression and Transcriptional Regulation of the PD-Iα/Autotaxin Gene in Neuroblastoma1. Cancer Res. 1997, 57, 2516–2521. [Google Scholar]
- Nam, S.W.; Clair, T.; Kim, Y.-S.; McMarlin, A.; Schiffmann, E.; Liotta, L.A.; Stracke, M.L. Autotaxin (NPP-2), a Metastasis-enhancing Motogen, Is an Angiogenic Factor. Cancer Res. 2001, 61, 6938–6944. [Google Scholar] [PubMed]
- Nam, S.W.; Clair, T.; Campo, C.K.; Lee, H.Y.; Liotta, L.A.; Stracke, M.L. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene 2000, 19, 241–247. [Google Scholar] [CrossRef]
- Baumforth, K.R.N.; Flavell, J.R.; Reynolds, G.M.; Davies, G.; Pettit, T.R.; Wei, W.; Morgan, S.; Stankovic, T.; Kishi, Y.; Arai, H.; et al. Induction of autotaxin by the Epstein-Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood 2005, 106, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Serre, C.-M.; Grès, S.; Saulnier-Blache, J.S.; Bordet, J.-C.; Guglielmi, J.; Clézardin, P.; Peyruchaud, O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Investig. 2004, 114, 1714–1725. [Google Scholar] [CrossRef]
- David, M.; Wannecq, E.; Descotes, F.; Jansen, S.; Deux, B.; Ribeiro, J.; Serre, C.-M.; Grès, S.; Bendriss-Vermare, N.; Bollen, M.; et al. Cancer Cell Expression of Autotaxin Controls Bone Metastasis Formation in Mouse through Lysophosphatidic Acid-Dependent Activation of Osteoclasts. PLoS ONE 2010, 5, e9741. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Expression of Autotaxin–Lysophosphatidate Signaling-Related Proteins in Breast Cancer with Adipose Stroma. Int. J. Mol. Sci. 2019, 20, 2102. [Google Scholar] [CrossRef]
- Boucharaba, A.; Serre, C.-M.; Guglielmi, J.; Bordet, J.-C.; Clézardin, P.; Peyruchaud, O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc. Natl. Acad. Sci. USA 2006, 103, 9643–9648. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Lee, S.-C.; David, M.; Bordet, J.-C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clézardin, P.; et al. Interaction of platelet-derived autotaxin with tumor integrin αVβ3 controls metastasis of breast cancer cells to bone. Blood 2014, 124, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; He, G.; Matsuzawa, A.; Yu, G.-Y.; Maeda, S.; Hardiman, G.; Karin, M. Hepatocyte Necrosis Induced by Oxidative Stress and IL-1α Release Mediate Carcinogen-Induced Compensatory Proliferation and Liver Tumorigenesis. Cancer Cell 2008, 14, 156–165. [Google Scholar] [CrossRef]
- Ptaszynska, M.M.; Pendrak, M.L.; Bandle, R.W.; Stracke, M.L.; Roberts, D.D. Positive feedback between vascular endothelial growth factor-A and autotaxin in ovarian cancer cells. Mol Cancer Res 2008, 6, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Vidot, S.; Witham, J.; Agarwal, R.; Greenhough, S.; Bamrah, H.S.; Tigyi, G.J.; Kaye, S.B.; Richardson, A. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell Signal. 2010, 22, 926–935. [Google Scholar] [CrossRef]
- Meng, G.; Tang, X.; Yang, Z.; Benesch, M.G.K.; Marshall, A.; Murray, D.; Hemmings, D.G.; Wuest, F.; McMullen, T.P.W.; Brindley, D.N. Implications for breast cancer treatment from increased autotaxin production in adipose tissue after radiotherapy. FASEB J. 2017, 31, 4064–4077. [Google Scholar] [CrossRef]
- Samadi, N.; Gaetano, C.; Goping, I.S.; Brindley, D.N. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene 2009, 28, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wuest, M.; Tang, X.; Dufour, J.; McMullen, T.P.W.; Wuest, F.; Murray, D.; Brindley, D.N. Dexamethasone Attenuates X-Ray-Induced Activation of the Autotaxin-Lysophosphatidate-Inflammatory Cycle in Breast Tissue and Subsequent Breast Fibrosis. Cancers 2020, 12, 999. [Google Scholar] [CrossRef]
- Banerjee, S.; Norman, D.D.; Lee, S.C.; Parrill, A.L.; Pham, T.C.T.; Baker, D.L.; Tigyi, G.J.; Miller, D.D. Highly Potent Non-Carboxylic Acid Autotaxin Inhibitors Reduce Melanoma Metastasis and Chemotherapeutic Resistance of Breast Cancer Stem Cells. J. Med. Chem. 2017, 60, 1309–1324. [Google Scholar] [CrossRef]
- Seo, E.J.; Kwon, Y.W.; Jang, I.H.; Kim, D.K.; Lee, S.I.; Choi, E.J.; Kim, K.-H.; Suh, D.-S.; Lee, J.H.; Choi, K.U.; et al. Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem Cells 2016, 34, 551–564. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yu, Y.; He, Y.; Chen, Q.; Liu, H. Serum ATX as a novel biomarker for breast cancer. Medicine 2019, 98, e14973. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Brindley, D.N. Autotaxin and Breast Cancer: Towards Overcoming Treatment Barriers and Sequelae. Cancers 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Ko, Y.M.; Tang, X.; Dewald, J.; Lopez-Campistrous, A.; Zhao, Y.Y.; Lai, R.; Curtis, J.M.; Brindley, D.N.; McMullen, T.P.W. Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. J. Endocr.-Relat. Cancer 2015, 22, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Dewald, J.; Dong, W.-F.; Mackey, J.R.; Hemmings, D.G.; McMullen, T.P.W.; Brindley, D.N. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J. 2015, 29, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Magkrioti, C.; Oikonomou, N.; Kaffe, E.; Mouratis, M.-A.; Xylourgidis, N.; Barbayianni, I.; Megadoukas, P.; Harokopos, V.; Valavanis, C.; Chun, J.; et al. The Autotaxin—Lysophosphatidic Acid Axis Promotes Lung Carcinogenesis. Cancer Res. 2018, 78, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Cheretaki, A.; Karaglani, M.; Balgkouranidou, I.; Biziota, E.; Amarantidis, K.; Xenidis, N.; Kakolyris, S.; Baritaki, S.; Chatzaki, E. Methylation Status of Corticotropin-Releasing Factor (CRF) Receptor Genes in Colorectal Cancer. J. Clin. Med. 2021, 10, 2680. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Lambropoulou, M.; Balgkouranidou, I.; Nena, E.; Karaglani, M.; Nicolaidou, C.; Asimaki, A.; Konstantinidis, T.; Constantinidis, T.C.; Kolios, G.; et al. Gene promoter methylation and protein expression of BRMS1 in uterine cervix in relation to high-risk human papilloma virus infection and cancer. Tumor Biol. 2017, 39, 1010428317697557. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Esteller, M.; Chatzaki, E. Circulating Cell-Free DNA in Breast Cancer: Searching for Hidden Information towards Precision Medicine. Cancers 2021, 13, 728. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Karaglani, M.; Balgkouranidou, I.; Biziota, E.; Koukaki, T.; Karamitrousis, E.; Nena, E.; Tsamardinos, I.; Kolios, G.; Lianidou, E.; et al. Circulating cell-free DNA in breast cancer: Size profiling, levels, and methylation patterns lead to prognostic and predictive classifiers. Oncogene 2019, 38, 3387–3401. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, H.; Li, H.; Li, X.; Yang, S. DNA methylation as an early diagnostic marker of cancer (Review). Biomed. Rep. 2014, 2, 326–330. [Google Scholar] [CrossRef]
- Wajed, S.A.; Laird, P.W.; DeMeester, T.R. DNA methylation: An alternative pathway to cancer. Ann. Surg. 2001, 234, 10–20. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Karaglani, M.; Balgkouranidou, I.; Pantazi, C.; Kolios, G.; Kakolyris, S.; Chatzaki, E. Circulating cell-free DNA release in vitro: Kinetics, size profiling, and cancer-related gene methylation. J. Cell. Physiol. 2019, 234, 14079–14089. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Zick, A.; Grinshpun, A.; Carmon, E.; Maoz, M.; Ochana, B.L.; Abraham, O.; Arieli, O.; Germansky, L.; Meir, K.; et al. Circulating breast-derived DNA allows universal detection and monitoring of localized breast cancer. Ann. Oncol. 2020, 31, 395–403. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Kuppa, S.S.; Jia, W.; Liu, S.; Nguyen, H.; Smyth, S.S.; Mills, G.B.; Dobbin, K.K.; Hardman, W.J.; Murph, M.M. Autotaxin exacerbates tumor progression by enhancing MEK1 and overriding the function of miR-489-3p. Cancer Lett. 2018, 432, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, Y.; Xu, X.-D.; Tian, Y.; Shang, H. Design and Development of Autotaxin Inhibitors. Pharmaceuticals 2021, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.; Patil, R.; Liu, J.; Wang, Y.; Lee, S.C.; Fujiwara, Y.; Fells, J.; Bolen, A.L.; Emmons-Thompson, K.; Yates, C.R.; et al. Benzyl and Naphthalene Methylphosphonic Acid Inhibitors of Autotaxin with Anti-invasive and Anti-metastatic Activity. ChemMedChem 2011, 6, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Desroy, N.; Housseman, C.; Bock, X.; Joncour, A.; Bienvenu, N.; Cherel, L.; Labeguere, V.; Rondet, E.; Peixoto, C.; Grassot, J.-M.; et al. Discovery of 2-[[2-Ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl]piperazin-1-yl]-8-methylimidazo[1,2-a]pyridin-3-yl]methylamino]-4-(4-fluorophenyl)thiazole-5-carbonitrile (GLPG1690), a First-in-Class Autotaxin Inhibitor Undergoing Clinical Evaluation for the Treatment of Idiopathic Pulmonary Fibrosis. J. Med. Chem. 2017, 60, 3580–3590. [Google Scholar] [CrossRef]
- Tang, X.; Wuest, M.; Benesch, M.G.K.; Dufour, J.; Zhao, Y.; Curtis, J.M.; Monjardet, A.; Heckmann, B.; Murray, D.; Wuest, F.; et al. Inhibition of Autotaxin with GLPG1690 Increases the Efficacy of Radiotherapy and Chemotherapy in a Mouse Model of Breast Cancer. Mol. Cancer Ther. 2020, 19, 63–74. [Google Scholar] [CrossRef]
- Maher, T.M.; Kreuter, M.; Lederer, D.J.; Brown, K.K.; Wuyts, W.; Verbruggen, N.; Stutvoet, S.; Fieuw, A.; Ford, P.; Abi-Saab, W.; et al. Rationale, design and objectives of two phase III, randomised, placebo-controlled studies of GLPG1690, a novel autotaxin inhibitor, in idiopathic pulmonary fibrosis (ISABELA 1 and 2). BMJ Open Respir. Res. 2019, 6, e000422. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Maeda, T.; Ohhata, A.; Zhao, Y.Y.; Kok, B.P.C.; Dewald, J.; Hitt, M.; Curtis, J.M.; McMullen, T.P.W.; et al. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 2014, 28, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, X.; Gajewiak, J.; Tsukahara, R.; Fujiwara, Y.; Liu, J.; Fells, J.I.; Perygin, D.; Parrill, A.L.; Tigyi, G.; et al. Dual Activity Lysophosphatidic Acid Receptor Pan-Antagonist/Autotaxin Inhibitor Reduces Breast Cancer Cell Migration In vitro and Causes Tumor Regression In vivo. Cancer Res. 2009, 69, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Milleri, S.; Deken, M.; Medhi, R.; Carruthers, A.; Tagliavini, A.; Nizzardo, A.; Pergher, M.; Niewola-Staszkowska, K.; Cheasty, A.; Fraser, A.; et al. 131P Translating a novel autotaxin inhibitor from preclinical proof of concept in pancreatic cancer to a biomarker response in human subjects. Ann. Oncol. 2021, 32, S1434. [Google Scholar] [CrossRef]
- Niewola-Staszkowska, K.; Lahn, M.M.; Cheasty, A.; Shah, P.; Farrow, S.; van der Veen, L.; Johnson, Z. IOA-289, a Novel, Clinical Stage Autotaxin Inhibitor for the Treatment of Fibrotic Lung Disease. In American Journal of Respiratory And Critical Care Medicine; American Thoracic Society: New York, NY, USA, 2021; p. A1332. [Google Scholar]
- Bhave, S.; Dadey, D.; Karvas, R.; Ferraro, D.; Kotipatruni, R.; Jaboin, J.; Hallahan, A.; DeWees, T.; Linkous, A.; Hallahan, D.; et al. Autotaxin Inhibition with PF-8380 Enhances the Radiosensitivity of Human and Murine Glioblastoma Cell Lines. Front. Oncol. 2013, 3, 236. [Google Scholar] [CrossRef]
- Decato, B.E.; Leeming, D.J.; Sand, J.M.B.; Fischer, A.; Du, S.; Palmer, S.M.; Karsdal, M.; Luo, Y.; Minnich, A. LPA1 antagonist BMS-986020 changes collagen dynamics and exerts antifibrotic effects in vitro and in patients with idiopathic pulmonary fibrosis. Respir. Res. 2022, 23, 61. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).