Estimation of ALU Repetitive Elements in Plasma as a Cost-Effective Liquid Biopsy Tool for Disease Prognosis in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Plasma Preparation and DNA Extraction
2.3. Quantitative PCR of ALU Repeats
2.4. Next-Gen Sequencing-Primer Synthesis, Library Preparation, and Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Evaluation of ctDNA and the DI Index; ALU 247 Levels Decrease Post-Surgical Intervention and Are Higher in Metastatic Patients
3.3. Implication of Baseline ALU 115, ALU 247, and DI Levels on Prognosis and Association with Tumor Clinico-Pathological Characteristics
3.4. Detection of Mutations from ctDNA by NGS
3.5. Utility of Estimating Post-Surgical ALU 247 Levels for Predicting Prognosis
3.6. Nomogram and Decision Curve Analysis for Prediction of Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The Incidence of Breast Cancer Recurrence 10–32 Years after Primary Diagnosis. JNCI J. Natl. Cancer Inst. 2021, 114, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Arko-Boham, B.; Aryee, N.A.; Blay, R.M.; Owusu, E.D.A.; Tagoe, E.A.; Doris Shackie, E.S.; Debrah, A.B.; Adu-Aryee, N.A. Circulating cell-free DNA integrity as a diagnostic and prognostic marker for breast and prostate cancers. Cancer Genet. 2019, 235–236, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Vishnubhatla, S.; Raina, V.; Sharma, S.; Gogia, A.; Deo, S.S.V.; Mathur, S.; Shukla, N.K. Circulating cell-free DNA and its integrity as a prognostic marker for breast cancer. SpringerPlus 2015, 4, 265. [Google Scholar] [CrossRef] [PubMed]
- Lamminaho, M.; Kujala, J.; Peltonen, H.; Tengström, M.; Kosma, V.-M.; Mannermaa, A. High Cell-Free DNA Integrity Is Associated with Poor Breast Cancer Survival. Cancers 2021, 13, 4679. [Google Scholar] [CrossRef]
- Duffy, M.J. Serum Tumor Markers in Breast Cancer: Are They of Clinical Value? Clin. Chem. 2006, 52, 345–351. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Perakis, S.; Speicher, M.R. Emerging concepts in liquid biopsies. BMC Med. 2017, 15, 75. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Sobhani, N.; Generali, D.; Zanconati, F.; Bortul, M.; Scaggiante, B. Cell-free DNA integrity for the monitoring of breast cancer: Future perspectives? World J. Clin. Oncol. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat. Rev. 2019, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.A.; Al-Rahim, A.M.; Suleiman, A.A. ALU repeat as potential molecular marker in the detection and prognosis of different cancer types: A systematic review. Mol. Clin. Oncol. 2022, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Hosseini, J.; Mirfakhraie, R.; Habibi, M.; Azargashb, E.; Pouresmaeili, F. The value of the plasma circulating cell-free DNA concentration and integrity index as a clinical tool for prostate cancer diagnosis: A prospective case-control cohort study in an Iranian population. Cancer Manag. Res. 2019, 11, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Husain, N.; Agarwal, A.; Neyaz, A.; Gupta, S.; Chaturvedi, A.; Lohani, M.; Sonkar, A.A. Diagnostic Value of Circulating Free DNA Integrity and Global Methylation Status in Gall Bladder Carcinoma. Pathol. Oncol. Res. 2019, 25, 925–936. [Google Scholar] [CrossRef]
- Salzano, A.; Israr, M.Z.; Garcia, D.F.; Middleton, L.; D’Assante, R.; Marra, A.M.; Arcopinto, M.; Yazaki, Y.; Bernieh, D.; Cassambai, S.; et al. Circulating cell-free DNA levels are associated with adverse outcomes in heart failure: Testing liquid biopsy in heart failure. Eur. J. Prev. Cardiol. 2020, 28, e28–e31. [Google Scholar] [CrossRef]
- Kruglyak, K.M.; Chibuk, J.; McLennan, L.; Nakashe, P.; Hernandez, G.E.; Motalli-Pepio, R.; Fath, D.M.; Tynan, J.A.; Holtvoigt, L.E.; Chorny, I.; et al. Blood-Based Liquid Biopsy for Comprehensive Cancer Genomic Profiling Using Next-Generation Sequencing: An Emerging Paradigm for Non-invasive Cancer Detection and Management in Dogs. Front. Vet. Sci. 2021, 8, 704835. [Google Scholar] [CrossRef]
- Mojtabanezhad Shariatpanahi, A.; Rokni, P.; Shahabi, E.; Varshoee Tabrizi, F.; Kerachian, M.A. Simple and cost-effective laboratory methods to evaluate and validate cell-free DNA isolation. BMC Res. Notes 2018, 11, 757. [Google Scholar] [CrossRef]
- Jin, S.; Zhu, D.; Shao, F.; Chen, S.; Guo, Y.; Li, K.; Wang, Y.; Ding, R.; Gao, L.; Ma, W.; et al. Efficient detection and post-surgical monitoring of colon cancer with a multi-marker DNA methylation liquid biopsy. Proc. Natl. Acad. Sci. USA 2021, 118, e2017421118. [Google Scholar] [CrossRef]
- Umetani, N.; Giuliano, A.E.; Hiramatsu, S.H.; Amersi, F.; Nakagawa, T.; Martino, S.; Hoon, D.S. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J. Clin. Oncol. 2006, 24, 4270–4276. [Google Scholar] [CrossRef]
- Chudasama, D.Y.; Aladag, Z.; Felicien, M.I.; Hall, M.; Beeson, J.; Asadi, N.; Gidron, Y.; Karteris, E.; Anikin, V.B. Prognostic value of the DNA integrity index in patients with malignant lung tumors. Oncotarget 2018, 9, 21281–21288. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:12073907. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, K. wANNOVAR: Annotating genetic variants for personal genomes via the web. J. Med. Genet. 2012, 49, 433–436. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Leszinski, G.; Lehner, J.; Gezer, U.; Holdenrieder, S. Increased DNA integrity in colorectal cancer. In Vivo 2014, 28, 299–303. [Google Scholar]
- El-Gayar, D.; El-Abd, N.; Hassan, N.; Ali, R. Increased Free Circulating DNA Integrity Index as a Serum Biomarker in Patients with Colorectal Carcinoma. Asian Pac. J. Cancer Prev. 2016, 17, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ma, J.; Ru, D.; Wu, N.; Zhang, Y.; Li, H.; Liu, X.; Li, J.; Zhang, H.; Xu, Y.; et al. Plasma DNA Integrity as a Prognostic Biomarker for Colorectal Cancer Chemotherapy. J. Oncol. 2021, 2021, 5569783. [Google Scholar] [CrossRef]
- Soliman, S.E.; Alhanafy, A.M.; Habib, M.S.E.; Hagag, M.; Ibrahem, R.A.L. Serum circulating cell free DNA as potential diagnostic and prognostic biomarker in non small cell lung cancer. Biochem. Biophys. Rep. 2018, 15, 45–51. [Google Scholar] [CrossRef]
- Madhavan, D.; Wallwiener, M.; Bents, K.; Zucknick, M.; Nees, J.; Schott, S.; Cuk, K.; Riethdorf, S.; Trumpp, A.; Pantel, K.; et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res. Treat. 2014, 146, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Fleming, C.; O’Leary, D.P.; Hassan, F.; Kelly, L.; O’Sullivan, M.J.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Association of Circulating Tumor DNA with Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2026921. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Teama, S.; Fawzy, A.; El Deftar, M. Plasma DNA integrity index as a potential molecular diagnostic marker for breast cancer. Tumor Biol. 2016, 37, 7565–7572. [Google Scholar] [CrossRef] [PubMed]
- Elhelaly, R.; Effat, N.; Hegazy, M.A.E.; Abdelwahab, K.; Hamdy, O.; Abo Hashem, E.M.; Elzehery, R.R. Circulating Cell Free DNA and DNA Integrity Index as Discriminating Tools between Breast Cancer and Benign Breast Disease. Asian Pac. J. Cancer Prev. 2022, 23, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Wang, J.H.; Cullinane, C.; Ita, M.; Corrigan, M.; O’Leary, D.P.; Redmond, H.P. Assessment of cell-free DNA (cfDNA) concentrations in the perioperative period can predict risk of recurrence in patients with non-metastatic breast cancer. Surg. Oncol. 2022, 42, 101753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Holland-Letz, T.; Wallwiener, M.; Surowy, H.; Cuk, K.; Schott, S.; Trumpp, A.; Pantel, K.; Sohn, C.; Schneeweiss, A.; et al. Circulating free DNA integrity and concentration as independent prognostic markers in metastatic breast cancer. Breast Cancer Res. Treat. 2018, 169, 69–82. [Google Scholar] [CrossRef]
- Hussein, N.A.; Mohamed, S.N.; Ahmed, M.A. Plasma ALU-247, ALU-115, and cfDNA Integrity as Diagnostic and Prognostic Biomarkers for Breast Cancer. Appl. Biochem. Biotechnol. 2019, 187, 1028–1045. [Google Scholar] [CrossRef]
- Fawzy, A.; Sweify, K.M.; El-Fayoumy, H.M.; Nofal, N. Quantitative analysis of plasma cell-free DNA and its DNA integrity in patients with metastatic prostate cancer using ALU sequence. J. Egypt. Natl. Cancer Inst. 2016, 28, 235–242. [Google Scholar] [CrossRef]
- Vizza, E.; Corrado, G.; De Angeli, M.; Carosi, M.; Mancini, E.; Baiocco, E.; Chiofalo, B.; Patrizi, L.; Zampa, A.; Piaggio, G.; et al. Serum DNA integrity index as a potential molecular biomarker in endometrial cancer. J. Exp. Clin. Cancer Res. 2018, 37, 16. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Weiss, G.J.; Beck, J.; Braun, D.P.; Bornemann-Kolatzki, K.; Barilla, H.; Cubello, R.; Quan, W., Jr.; Sangal, A.; Khemka, V.; Waypa, J.; et al. Tumor Cell-Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin. Cancer Res. 2017, 23, 5074–5081. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Chlon, L.; Pharoah, P.D.; Markowetz, F.; Caldas, C. Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study. PLoS Med. 2016, 13, e1002194. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tong, Y.; Guo, X.; Feng, L.; Jiang, Z.; Ying, S.; Jia, J.; Fang, Y.; Yu, M.; Xia, H.; et al. Diagnostic Value of Concentration of Circulating Cell-Free DNA in Breast Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
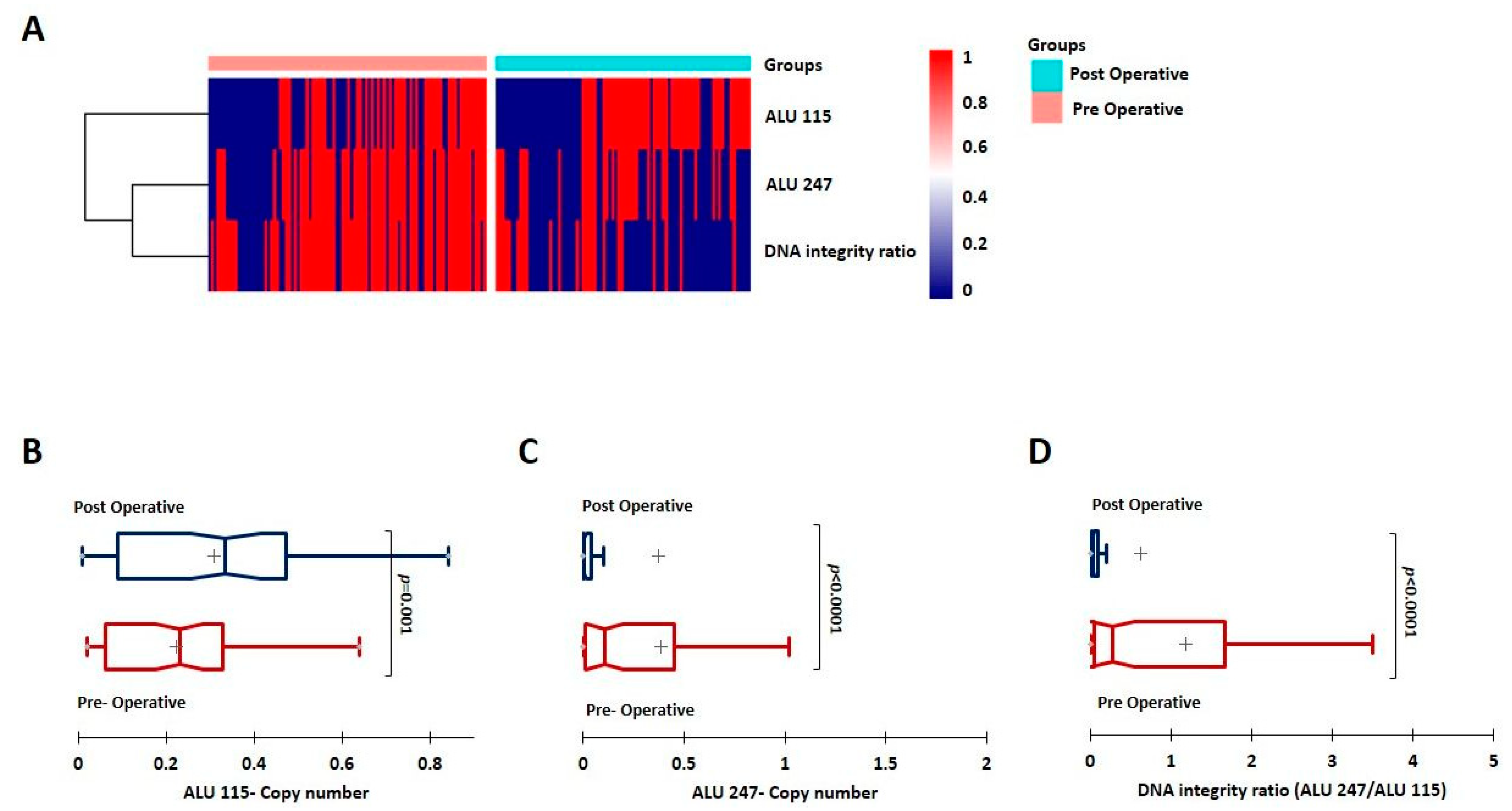
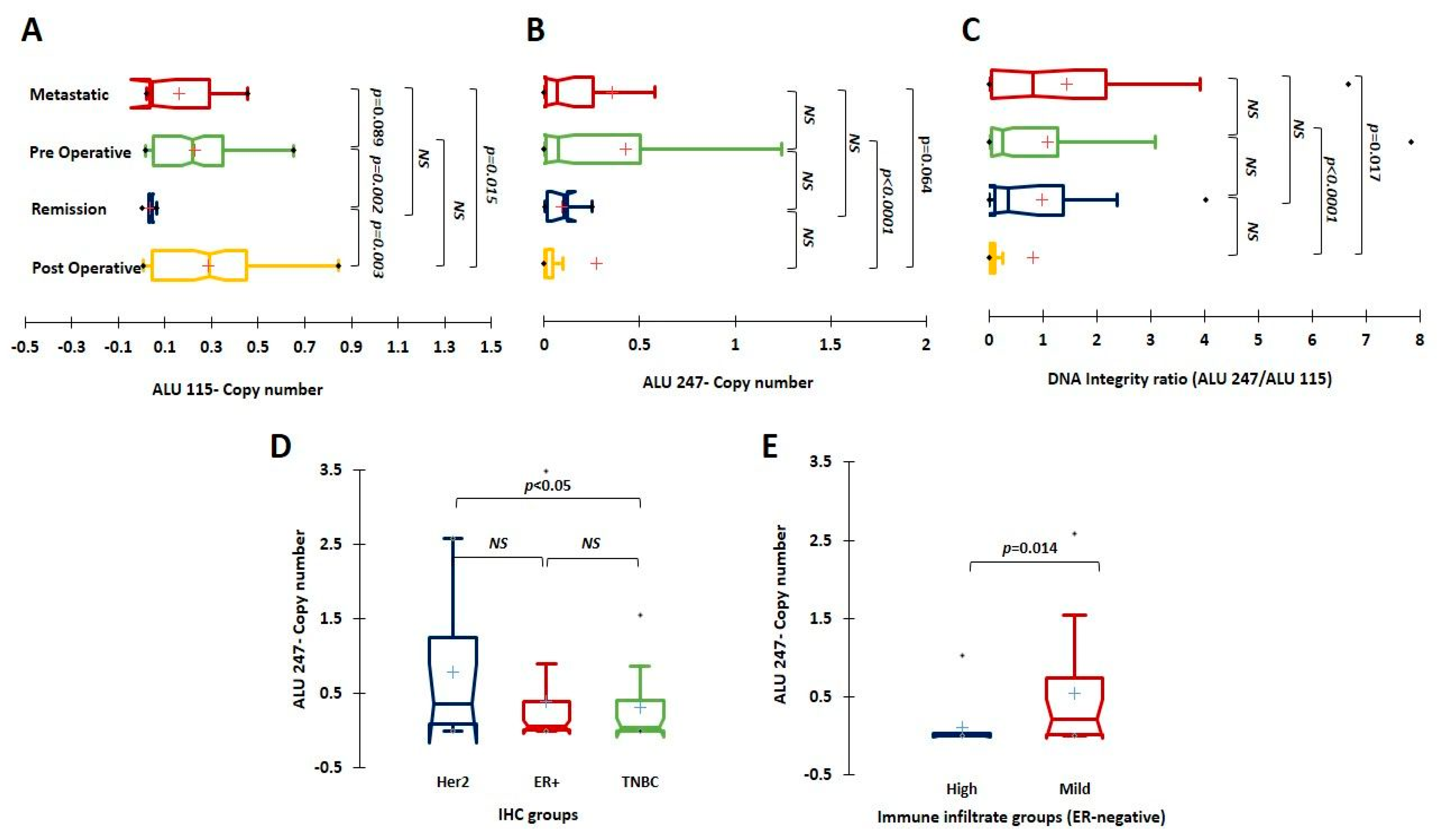
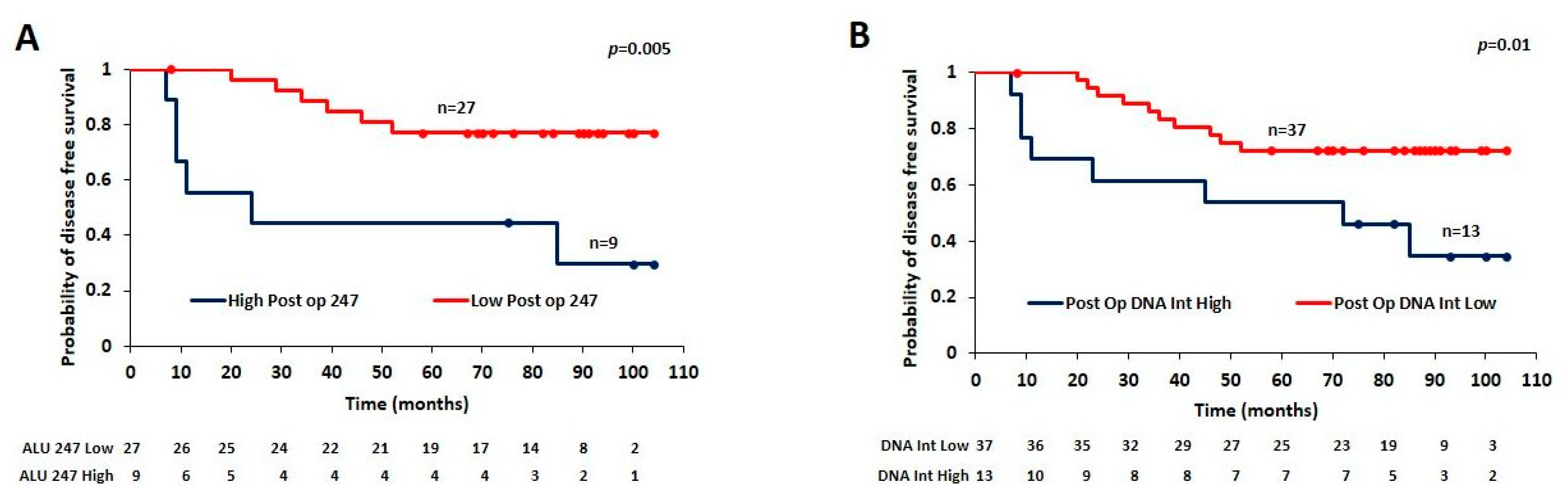
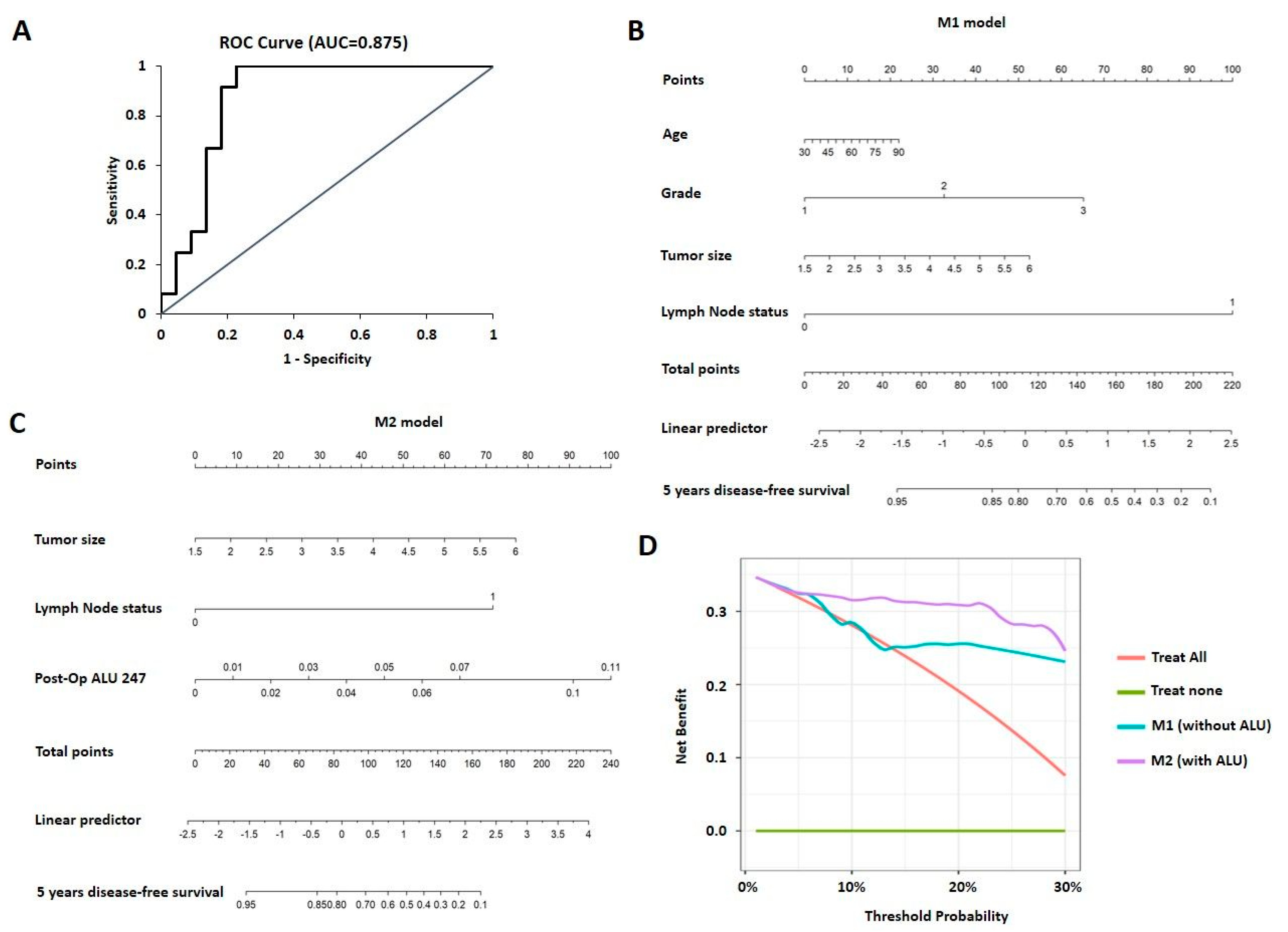
| Sl No. | Parameter (n) | Category | Rel. Frequency/Category (%) | qPCR ALU115 Mean | p Value (Mann–Whitney) | qPCR ALU247 Mean | p Value (Mann–Whitney) | qPCR DNA Integrity Ratio Mean | p Value (Mann–Whitney) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Age (129) | <50 | 29 | 0.227 | 0.837 | 0.611 | 0.360 | 1.485 | 0.464 |
| >50 | 71 | 0.233 | 0.364 | 0.966 | |||||
| 2 | Age at Menarche (118) | Early | 14 | 0.197 | 0.259 | 0.402 | 0.453 | 1.029 | 0.462 |
| Late | 86 | 0.252 | 0.490 | 1.175 | |||||
| 3 | Menopausal status (129) | Pre- | 26 | 0.225 | 0.912 | 0.554 | 0.520 | 1.242 | 0.864 |
| Post- | 74 | 0.233 | 0.398 | 1.071 | |||||
| 4 | Breast side (129) | Left | 55 | 0.227 | 0.846 | 0.488 | 0.679 | 1.304 | 0.436 |
| Right | 43 | 0.234 | 0.350 | 0.861 | |||||
| 5 | Diabetic (109) | Yes | 33 | 0.240 | 0.585 | 0.295 | 0.087 | 0.733 | 0.053 |
| No | 67 | 0.253 | 0.597 | 1.477 | |||||
| 7 | Hypertension (114) | Yes | 48 | 0.250 | 0.986 | 0.433 | 0.313 | 1.097 | 0.372 |
| No | 52 | 0.241 | 0.518 | 1.376 | |||||
| 8 | Tumor grade (128) | 1 | 19 | 0.228 | 1 v/s 2–0.641 | 0.155 | 1 v/s 2–0.704 | 0.464 | 1 v/s 2–0.494 |
| 2 | 55 | 0.244 | 1 v/s 3–0.788 | 0.561 | 1 v/s 3–0.952 | 1.396 | 1 v/s 3–0.886 | ||
| 3 | 21 | 0.195 | 2 v/s 3–0.353 | 0.300 | 2 v/s 3–0.623 | 0.873 | 2 v/s 3–0.577 | ||
| 9 | Tumor size (127) | <3cm | 40 | 0.185 | 0.048 | 0.437 | 0.409 | 1.094 | 0.639 |
| >3cm | 60 | 0.250 | 0.414 | 1.102 | |||||
| 10 | Lymph node status (125) | Positive | 42 | 0.249 | 0.407 | 0.394 | 0.749 | 0.964 | 0.348 |
| Negative | 58 | 0.221 | 0.471 | 1.202 | |||||
| 11 | Lymphovascular invasion (108) | Present | 40 | 0.244 | 0.597 | 0.376 | 0.844 | 0.953 | 0.526 |
| Absent | 60 | 0.230 | 0.489 | 1.273 | |||||
| 12 | Tumor stage (117) | 1 v/s 0–0.953 | 1 v/s 0–0.953 | 1 v/s 0–0.961 | |||||
| 0 | 3 | 0.170 | 2 v/s 0–0.344 | 0.048 | 2 v/s 0–0.210 | 0.287 | 2 v/s 0–0.513 | ||
| 1 | 15 | 0.161 | 3 v/s 0–0.166 | 0.435 | 3 v/s 0–0.439 | 1.126 | 3 v/s 0–0.903 | ||
| 2 | 50 | 0.238 | 2 v/s 1–0.121 | 0.515 | 2 v/s 1–0.141 | 1.340 | 2 v/s 1–0.304 | ||
| 3 | 32 | 0.291 | 3 v/s 1–0.025 | 0.433 | 3 v/s 1–0.328 | 1.038 | 3 v/s 1–0.644 | ||
| 3 v/s 2–0.209 | 3 v/s 2–0.828 | 3 v/s 2–0.387 | |||||||
| 13 | Ki-67 index (91) | <15 | 40 | 0.279 | 0.044 | 0.531 | 0.218 | 1.290 | 0.890 |
| >15 | 60 | 0.188 | 0.408 | 1.153 | |||||
| 14 | Immune infiltrate (120) | Mild | 43 | 0.252 | 0.309 | 0.572 | 0.263 | 1.456 | 0.098 |
| High | 43 | 0.218 | 0.374 | 0.980 |
| All; n = 36 | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.006 (0.965–1.05) | 0.76 | 1.013 (0.972–1.056) | 0.537 |
| T-size | 1.416 (0.911–2.203) | 0.12 | 1.604 (0.990–2.601) | 0.05 |
| Lymph Node status | ||||
| Negative | Reference | |||
| Positive | 9.441 (2.026–44.003) | 0.004 | 8.073 (1.611–40.449) | 0.01 |
| Grade | ||||
| I and II | Reference | |||
| III | 0.808 (0.175–3.735) | 0.78 | 1.347 (0.248–7.300) | 0.73 |
| Post-Operative ALU 247 | 1.302 (1.074–1.578) | 0.007 | 1.3 (1.047–1.613) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, M.G.; Ramesh, R.S.; Naidu, C.M.; Mavatkar, A.D.; V. P., S.; Ramamurthy, V.; Somashekaraiah, V.M.; C. E., A.; Raghunathan, K.; Panigrahi, A.; et al. Estimation of ALU Repetitive Elements in Plasma as a Cost-Effective Liquid Biopsy Tool for Disease Prognosis in Breast Cancer. Cancers 2023, 15, 1054. https://doi.org/10.3390/cancers15041054
Nair MG, Ramesh RS, Naidu CM, Mavatkar AD, V. P. S, Ramamurthy V, Somashekaraiah VM, C. E. A, Raghunathan K, Panigrahi A, et al. Estimation of ALU Repetitive Elements in Plasma as a Cost-Effective Liquid Biopsy Tool for Disease Prognosis in Breast Cancer. Cancers. 2023; 15(4):1054. https://doi.org/10.3390/cancers15041054
Chicago/Turabian StyleNair, Madhumathy G., Rakesh S. Ramesh, Chandrakala M. Naidu, Apoorva D. Mavatkar, Snijesh V. P., Vishakha Ramamurthy, Vidya M. Somashekaraiah, Anupama C. E., Kiruthiga Raghunathan, Anuradha Panigrahi, and et al. 2023. "Estimation of ALU Repetitive Elements in Plasma as a Cost-Effective Liquid Biopsy Tool for Disease Prognosis in Breast Cancer" Cancers 15, no. 4: 1054. https://doi.org/10.3390/cancers15041054
APA StyleNair, M. G., Ramesh, R. S., Naidu, C. M., Mavatkar, A. D., V. P., S., Ramamurthy, V., Somashekaraiah, V. M., C. E., A., Raghunathan, K., Panigrahi, A., Das, M., Dhar, S. K., & Prabhu, J. S. (2023). Estimation of ALU Repetitive Elements in Plasma as a Cost-Effective Liquid Biopsy Tool for Disease Prognosis in Breast Cancer. Cancers, 15(4), 1054. https://doi.org/10.3390/cancers15041054







