Higher Risk of Recurrence in Patients Treated for Head and Neck Cancer with Low BMI and Elevated Levels of C-Reactive Protein
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection, Demographics, and Disease-Specific Data
2.3. Statistical Analysis
3. Results
3.1. Patient Data
3.2. Patterns of Treatment Failure
3.3. Association of CRP, BMI, Hb, Trc, and Lkc with Recurrence
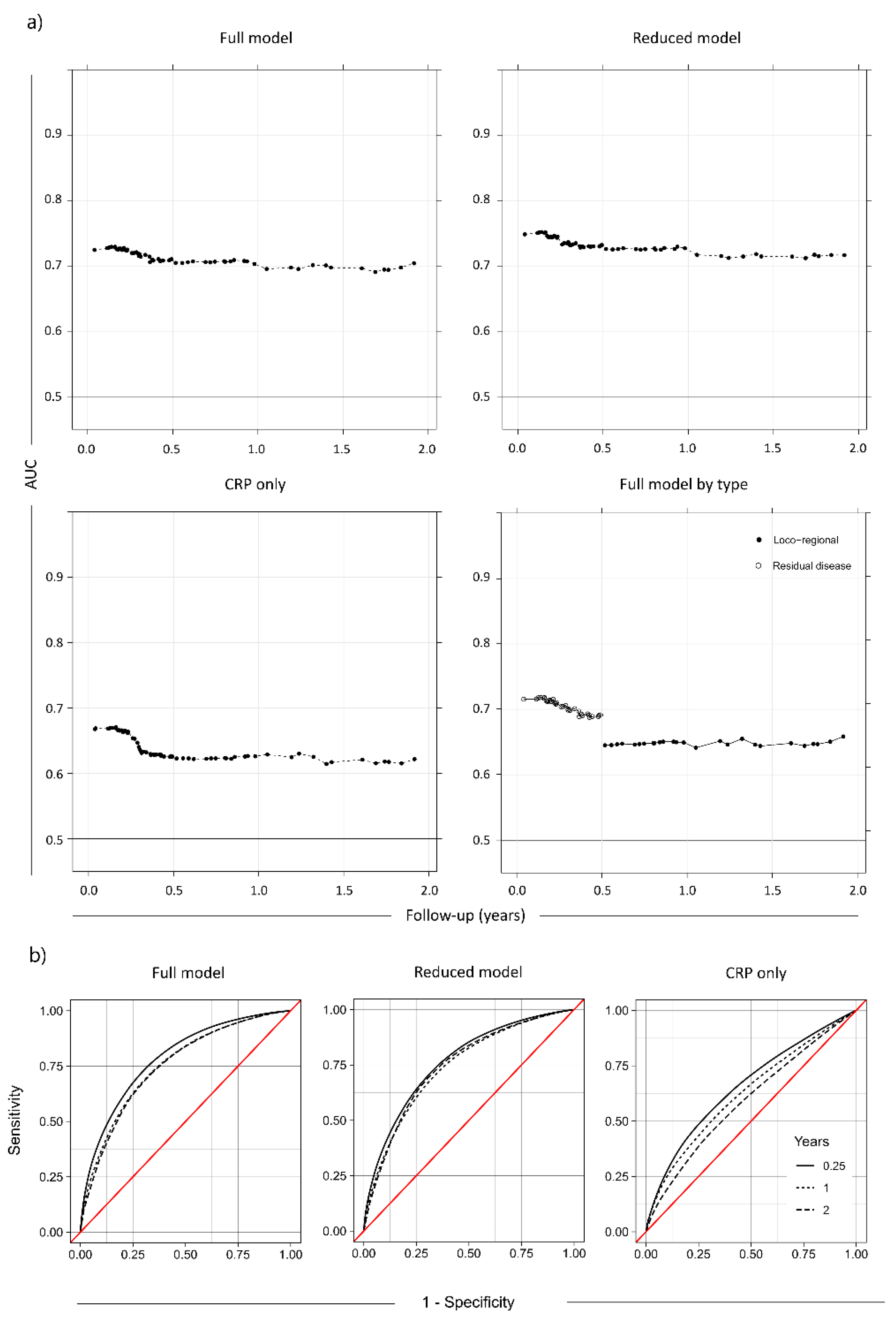
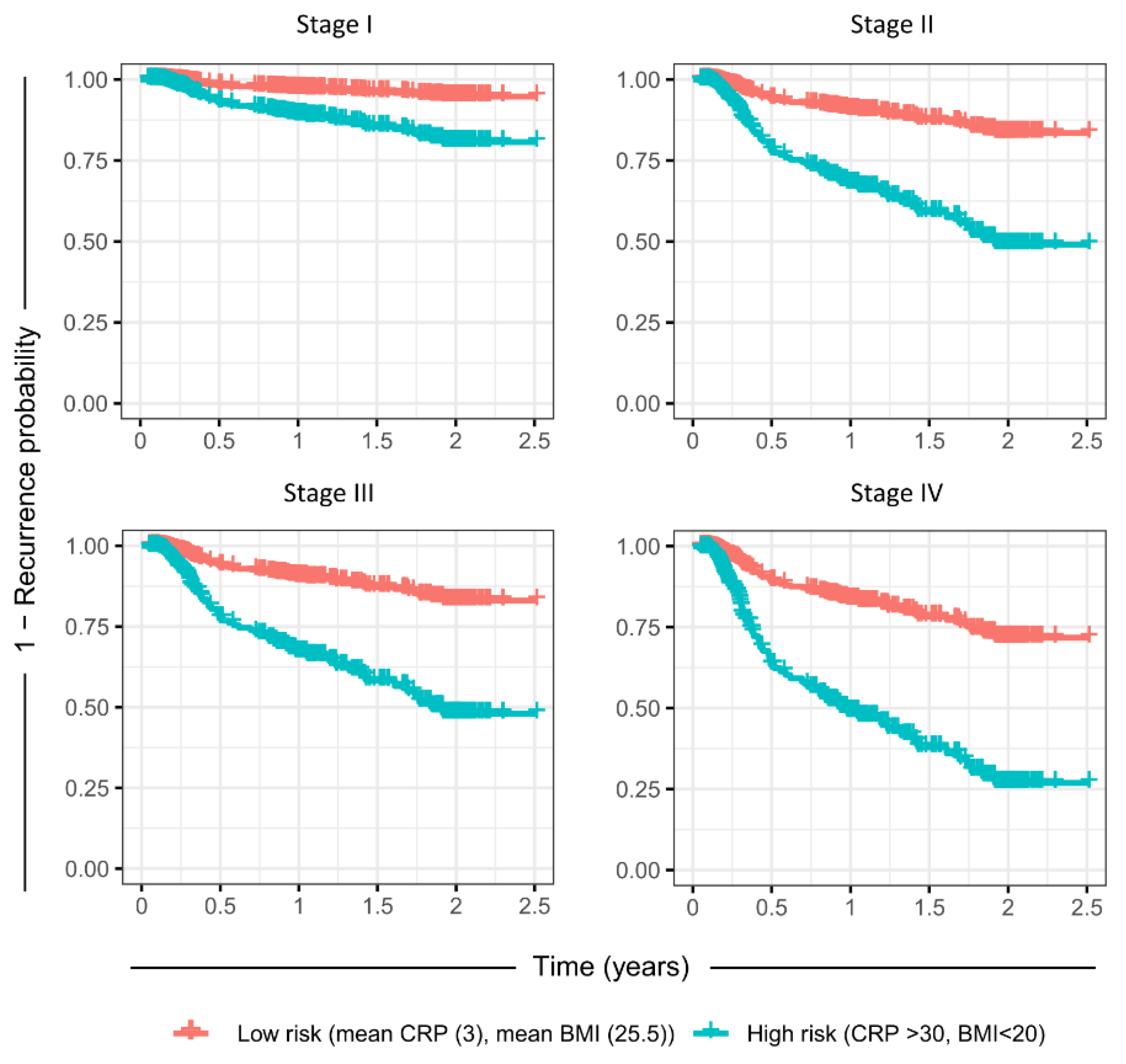
3.4. Cox Proportional Hazards Model of Outcome Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel Rebecca, L.; Miller Kimberly, D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Syrjänen, S.; Lodi, G.; von Bültzingslöwen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.M.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17, 58–72. [Google Scholar] [CrossRef]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef] [PubMed]
- De Stavola, B.L.; Pizzi, C.; Meade, T.W.; dos Santos Silva, I. Circulating levels of coagulation and inflammation markers and cancer risks: Individual participant analysis of data from three long-term cohorts. Int. J. Epidemiol. 2010, 39, 699–709. [Google Scholar]
- Allin, K.H.; Bojesen, S.E.; Nordestgaard, B.G. Baseline C-Reactive Protein Is Associated With Incident Cancer and Survival in Patients With Cancer. J. Clin. Oncol. 2009, 27, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Kasymjanova, G.; MacDonald, N.; Agulnik, J.S.; Cohen, V.; Pepe, C.; Kreisman, H.; Sharma, R.; Small, D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr. Oncol. 2010, 17, 52–58. [Google Scholar]
- Saito, K.; Kihara, K. Role of C-reactive protein in urological cancers: A useful biomarker for predicting outcomes. Int. J. Urol. 2013, 20, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Geissbühler, P.; Mermillod, B.; Rapin, C.-H. Elevated Serum Vitamin B12 Levels Associated With CRP as a Predictive Factor of Mortality in Palliative Care Cancer Patients: A Prospective Study Over Five Years. J. Pain Symptom Manag. 2000, 20, 93–103. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Ukoumunne, O.C.; Shephard, E.A.; Hamilton, W. Clinical relevance of thrombocytosis in primary care: A prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2017, 67, e405–e413. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, P.P.; Strzelec, B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: A review. Folia Morphol. 2018, 77, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tiblom Ehrsson, Y.; Hellström, P.M.; Brismar, K.; Sharp, L.; Langius-Eklöf, A.; Laurell, G. Explorative study on the predictive value of systematic inflammatory and metabolic markers on weight loss in head and neck cancer patients undergoing radiotherapy. Support. Care Cancer 2010, 18, 1385–1391. [Google Scholar] [CrossRef]
- Andersson, B.-Å.; Lewin, F.; Lundgren, J.; Nilsson, M.; Rutqvist, L.-E.; Löfgren, S.; Laytragoon-Lewin, N. Plasma tumor necrosis factor-α and C-reactive protein as biomarker for survival in head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, S.; Söderström, K.; Kjellén, E.; Nilsson, P.; Zackrisson, B.; Laurell, G. Weight and body mass index in relation to irradiated volume and to overall survival in patients with oropharyngeal cancer: A retrospective cohort study. Radiat. Oncol. 2014, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Strulov Shachar, S.; Williams, G.R. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology 2017, 26, 13–16. [Google Scholar]
- Gorphe, P.; Moya-Plana, A.; Guerlain, J.; Tao, Y.; Nguyen, F.; Breuskin, I.; Blanchard, P.; Temam, S. Disease-free time stratification in locally recurrent head and neck carcinoma after definitive radiotherapy or chemoradiotherapy. Eur. Arch. Otorhinolaryngol. 2022, 279, 3063–3069. [Google Scholar] [CrossRef]
- Miller, J.A.; Moradi, F.; Sundaram, V.; Liang, R.; Zhang, C.; Nguyen, N.K.; Akhtar, F.; Liu, Y.; Ren, Y.; Harandi, N.; et al. Posttreatment FDG-PET/CT Hopkins criteria predict locoregional recurrence after definitive radiotherapy for oropharyngeal squamous cell carcinoma. Head Neck 2022, 44, 2491–2504. [Google Scholar] [CrossRef]
- McSpadden, R.; Zender, C.; Eskander, A. AHNS series: Do you know your guidelines? Guideline recommendations for recurrent and persistent head and neck cancer after primary treatment. Head Neck 2019, 41, 7–15. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Hemant, I.; Udaya, B.K.; Eugene, H.B.; Michael, S.L. Random survival forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar]
- Efron, B.; Gong, G. A Leisurely Look at the Bootstrap, the Jackknife, and Cross-Validation. Am. Stat. 1983, 37, 36–48. [Google Scholar]
- Harrell, F. Regression Modelling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis; Springer: Manhattan, NY, USA, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2018. Available online: https://www.R-project.org/ (accessed on 1 August 2022).
- Therneau, T.; Grambsch, P.; Fleming, T. A Package for Survival Analysis in S. 2015. Available online: https://CRANR-project.org/package=survival (accessed on 1 August 2022).
- Bendix Carstensen, M.P.; Laara, E.; Hills, M.; Epi: A Package for Statistical Analysis in Epidemiology. R Package Version 2.38. 2019. Available online: https://CRANR-project.org/package=Epi (accessed on 1 August 2022).
- Saha-Chaudhuri. PJ. risksetROC: Riskset ROC Curve Estimation from Censored Survival Data. R Package Version 1.0.4. 2012. Available online: https://CRANR-project.org/package=risksetROC (accessed on 1 August 2022).
- Talani, C.; Mäkitie, A.; Beran, M.; Holmberg, E.; Laurell, G.; Farnebo, L. Early mortality after diagnosis of cancer of the head and neck-A population-based nationwide study. PLoS ONE 2019, 14, e0223154. [Google Scholar] [CrossRef] [PubMed]
- Valdes, M.; Villeda, J.; Mithoowani, H.; Pitre, T.; Chasen, M. Inflammatory markers as prognostic factors of recurrence in advanced-stage squamous cell carcinoma of the head and neck. Curr. Oncol. 2020, 27, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chamchod, S.; Fuller, C.D.; Mohamed, A.S.R.; Heukelom, J.; Eichelberger, H.; Kantor, M.E.; Hutcheson, K.A.; Gunn, G.B.; Garden, A.S.; et al. Association of Body Composition with Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016, 2, 782–789. [Google Scholar] [CrossRef]
- Potharaju, M.; Mangaleswaran, B.; Mathavan, A.; John, R.; Thamburaj, V.; Ghosh, S.; Ganesh, S.; Kalvakonda, G.; Loganathan, M.; Bapu, S.; et al. Body Mass Index as a Prognostic Marker in Glioblastoma Multiforme: A Clinical Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 204–209. [Google Scholar] [CrossRef]
- Pahwa, R.G.A.; Jialal, I. Chronic Inflammation. [Updated 2021 Sep 28]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 12 February 2022).
- Hannoodee, S.N.D. Acute Inflammatory Response. [Updated 2021 Nov 21]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://wwwncbinlmnihgov/books/NBK556083/ (accessed on 12 February 2022).
- Sabrkhany, S.; Kuijpers, M.J.E.; oude Egbrink, M.G.A.; Griffioen, A.W. Platelets as messengers of early-stage cancer. Cancer Metastasis Rev. 2021, 40, 563–573. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-Reactive Protein to Abdominal Adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and Haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef]
- Chen, Y.; Cong, R.; Ji, C.; Ruan, W. The prognostic role of C-reactive protein in patients with head and neck squamous cell carcinoma: A meta-analysis. Cancer Med. 2020, 9, 9541–9553. [Google Scholar] [CrossRef]
- Pagh, A.; Grau, C.; Overgaard, J. Failure pattern and salvage treatment after radical treatment of head and neck cancer. Acta Oncol. 2016, 55, 625–632. [Google Scholar] [CrossRef]
- Astradsson, T.; Sellberg, F.; Tiblom Ehrsson, Y.; Sandström, K.; Laurell, G. Serum Proteomics in Patients with Head and Neck Cancer: Peripheral Blood Immune Response to Treatment. Int. J. Mol. Sci. 2022, 23, 6304. [Google Scholar] [CrossRef]
- Mehanna, H.; Kong, A.; Ahmed, S.K. Recurrent head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S181–S190. [Google Scholar] [CrossRef]
- Kawecki, A.; Krajewski, R. Follow-up in patients treated for head and neck cancer. Memo 2014, 7, 87–91. [Google Scholar] [CrossRef][Green Version]



| Anatomic Site | N | Stage I | Stage II | Stage III | Stage IV | Treatment | N |
|---|---|---|---|---|---|---|---|
| Oropharynx | 124 | 68 | 21 | 31 | 4 | Surgery only | 30 |
| Oral cavity | 78 | 18 | 20 | 12 | 28 | Radiotherapy ** | 150 |
| Larynx | 31 | 14 | 6 | 6 | 5 | Chemoradiation *** | 75 |
| Hypopharynx | 6 | 0 | 0 | 1 | 5 | Radiotherapy with | |
| Nasopharynx | 8 | 1 | 3 | 1 | 3 | Cetuximab **** | 17 |
| Unknown primary | 9 | 4 | 0 | 4 | 1 | ||
| Salivary gland | 8 | 1 | 2 | 1 | 4 | ||
| Nose and sinus | 4 | 3 | 1 | 0 | 0 | ||
| Other | 4 * | 1 | 0 | 1 | 0 |
| Covariates | Initial Visit (Pre-Treatment Baseline) | Post-Treatment | 3 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|
| Total (N) | 276 | 272 | 259 | 211 | 154 |
| BMI | 26.7 (23.4–29.3) | 25.5 (22.5–28.0) | 25.1 (22.2–27.7) | 25.6 (22.3–28.4) | 26.1 (23.0–28.4) |
| Sex ratio (M/F) | 2.6 | 2.7 | 2.8 | 2.6 | 2.6 |
| CRP | 7.9 (1.1–8.2) | 19.8 (2.6–26.0) | 5.1 (1.0–5.0) | 4.7 (0.8–4.0) | - |
| Trc | 274.4 (228.0–311.2) | 258.7 (199.0–300.0) | 245.1 (194.0–273.5) | 230.9 (193.0–265.0) | - |
| Lkc | 7.5 (5.9–8.8) | 5.9 (4.2–7.3) | 5.6 (4.2–6.6) | 5.6 (4.2–6.5) | - |
| Hb | 139.8 (131.1–149.0) | 130.0 (119.2–139.0) | 136.4 (128.0–146.0) | 141.0 (133.0–149.0) | - |
| BMI change | - | 0 | −0.5 (−1.2–0.3) | −0.3 (−1.2–0.95) | -0.1 (-0.8–1.3) |
| Age | 63.1 (56.0–71.0) | 63 (56.0–71.0) | 63.0 (56.0–71.0) | 62.6 (55.5–70.5) | 63.1 (56.3–71.0) |
| Smoking status: | |||||
| Never | 94 (34.1) | 91 (33.5) | 86 (33.2) | 70 (33.2) | 50 (32.5) |
| Former | 157 (56.9) | 156 (57.4) | 149 (57.5) | 123 (58.3) | 91 (59.1) |
| Current | 25 (9.1) | 25 (9.2) | 24 (9.3) | 18 (8.5) | 13 (8.4) |
| Stage: | |||||
| I | 110 (39.9) | 110 (41.6) | 108 (41.9) | 103 (48.8) | 81 (52.6) |
| II | 53 (19.2) | 53 (18.7) | 53 (20.5) | 43 (20.4) | 27 (17.5) |
| III | 58 (21.0) | 57 (18.2) | 53 (20.5) | 39 (18.5) | 25 (16.2) |
| IV | 53 (19.2) | 50 (17.3) | 44 (17.1) | 26 (12.3) | 21 (13.6) |
| Unknown | 2 (0.7) | 2 (0.7) | 1 (0.4) | 0 (0) | 0 (0) |
| Outcome Variables * | Initial Visit–Post Treatment | Post-Treatment–3 Months | 3 Months–12 Months | 12 Months–24 Months | >24 Months |
| Recurrence, total (N) | -NA | 12 | 42 | 15 | 2 |
| Residual disease within 6 months (N) | - | 12 | 28 | 0 | 0 |
| Recurrence of disease after 6 months (N) | - | 0 | 14 | 15 | 2 |
| Loco-regional failure (N) | - | 10 | 28 | 9 | 2 |
| General failure (N) | - | 2 | 14 | 6 | 0 |
| Variable | Pre-Treatment | Post-Treatment | 3 Months | 12 Months | 24 Months | ||||
|---|---|---|---|---|---|---|---|---|---|
| N = 276 | Non-recurrence group, N = 259 | Recurrence group, N = 12 | Non-recurrence group, N = 210 | Recurrence group, N = 42 | Non-recurrence group, N = 195 | Recurrence group, N = 15 | Non-recurrence group, N = 151 | Recurrence group, N = 2 | |
| CRP | 7.9 (1.1–8.2) | 18.7 (2.4–24.0) | 36.2 (8.9–53.3) | 4.2 (1.0–4.6) | 7.7 (2.0–7.0) | 3.9 (0.8–4.0) | 4.0 (1.0–3.0) | - | - |
| Trc | 274 (228–311) | 258 (197–296) | 218 (211–218) | 238 (191–268) | 238 (201–325) | 229 (193–264) | 260 (221–295) | - | - |
| Lkc | 7.5 (5.9–8.8) | 5.9 (4.2–7.3) | 5.4 (4.7–6.6) | 5.2 (4.2–6.5) | 6.1 (4.4–7.0) | 5.5 (4.3–6.4) | 7.2 (3.8–8.7) | - | - |
| Hb | 139.8 (131–149) | 131 (120–139) | 130 (122–141) | 138 (128–146) | 132 (125–141) | 141 (134–150) | 141 (124–149) | - | - |
| BMI loss | - | 0 | 0 | −0.5 (−1.2–0.3) | −0.3 (−1.2–0.3) | −0.3 (−1.2–0.9) | 0.3 (−0.4–1.2) | 0.1 (−0.75–1.35) | −1.25 (−2.1-0.42) |
| Variable | Hazard Ratio | |||
|---|---|---|---|---|
| Recurrence, Total (f0) | Residual Disease (f2) | Loco-Regional Recurrence (f) | General Recurrence (f1) | |
| CRP 1 | 1.74 (1.12–2.71), p = 0.018 * | 2.19 (1.29–3.73), p = 0.004 * | 0.87 (0.35–2.15), p = 0.763 | 0.62 (0.12–3.11), p = 0.559 |
| Trc 1 | 1.34 (0.90–1.99), p = 0.138 | 1.38 (0.88–2.18), p = 0.163 | 1.38 (0.68–2.78), p = 0.372 | 1.99 (0.62–6.40), p = 0.250 |
| Lkc 1 | 1.10 (0.74–1.65), p = 0.627 | 1.04 (0.65–1.67), p = 0.872 | 1.49 (0.76–2.91), p = 0.247 | 1.13 (0.39–3.26), p = 0.823 |
| Hb 1 | 0.83 (0.52–1.31), p = 0.423 | 0.85 (0.48–1.53), p = 0.594 | 0.76 (0.36–1.63), p = 0.485 | 0.99 (0.29–3.34), p = 0.983 |
| BMI change 2 | 1.00 (0.86–1.18), p = 0.970 | 1.00 (0.79–1.28), p = 0.909 | 1.00 (0.76–1.33), p = 0.887 | 1.24 (0.84–1.84), p = 0.967 |
| BMI, 7 weeks 2 | 0.54 (0.35–0.83), p = 0.005 * | 0.57 (0.32–1.02), p = 0.058 | 0.44 (0.22–0.91), p = 0.027 * | 0.48 (0.14–1.74), p = 0.267 |
| Age 2 | 0.82 (0.57–1.18), p = 0.284 | 0.87 (0.56–1.36), p = 0.548 | 0.76 (0.39–1.47), p = 0.417 | 0.75 (0.26–2.14), p = 0.589 |
| Sex (m:f) | 0.44 (0.22–0.84), p = 0.015 * | 0.51 (0.23–1.17), p = 0.115 | 0.28 (0.08–0.93), p = 0.038 * | 0.79 (0.15–4.12), p = 0.778 |
| Smoker (y:n) | 1.30 (0.72–2.33), p = 0.383 | 1.35 (0.64–2.83), p = 0.429 | 1.34 (0.51–3.53), p = 0.551 | 0.91 (0.19–4.36), p = 0.916 |
| Stage (I:IV) | 6.00 (2.72–13.3), p < 0.001 * | 5.86 (2.13–16.1), p < 0.001 * | 8.09 (2.15–30.3), p = 0.002 * | 9.88 (1.44–67.6), p = 0.020 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegelberg, D.; Malmberg, C.; Tiblom Ehrsson, Y.; Laurell, G. Higher Risk of Recurrence in Patients Treated for Head and Neck Cancer with Low BMI and Elevated Levels of C-Reactive Protein. Cancers 2022, 14, 5161. https://doi.org/10.3390/cancers14205161
Spiegelberg D, Malmberg C, Tiblom Ehrsson Y, Laurell G. Higher Risk of Recurrence in Patients Treated for Head and Neck Cancer with Low BMI and Elevated Levels of C-Reactive Protein. Cancers. 2022; 14(20):5161. https://doi.org/10.3390/cancers14205161
Chicago/Turabian StyleSpiegelberg, Diana, Christer Malmberg, Ylva Tiblom Ehrsson, and Göran Laurell. 2022. "Higher Risk of Recurrence in Patients Treated for Head and Neck Cancer with Low BMI and Elevated Levels of C-Reactive Protein" Cancers 14, no. 20: 5161. https://doi.org/10.3390/cancers14205161
APA StyleSpiegelberg, D., Malmberg, C., Tiblom Ehrsson, Y., & Laurell, G. (2022). Higher Risk of Recurrence in Patients Treated for Head and Neck Cancer with Low BMI and Elevated Levels of C-Reactive Protein. Cancers, 14(20), 5161. https://doi.org/10.3390/cancers14205161





