It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
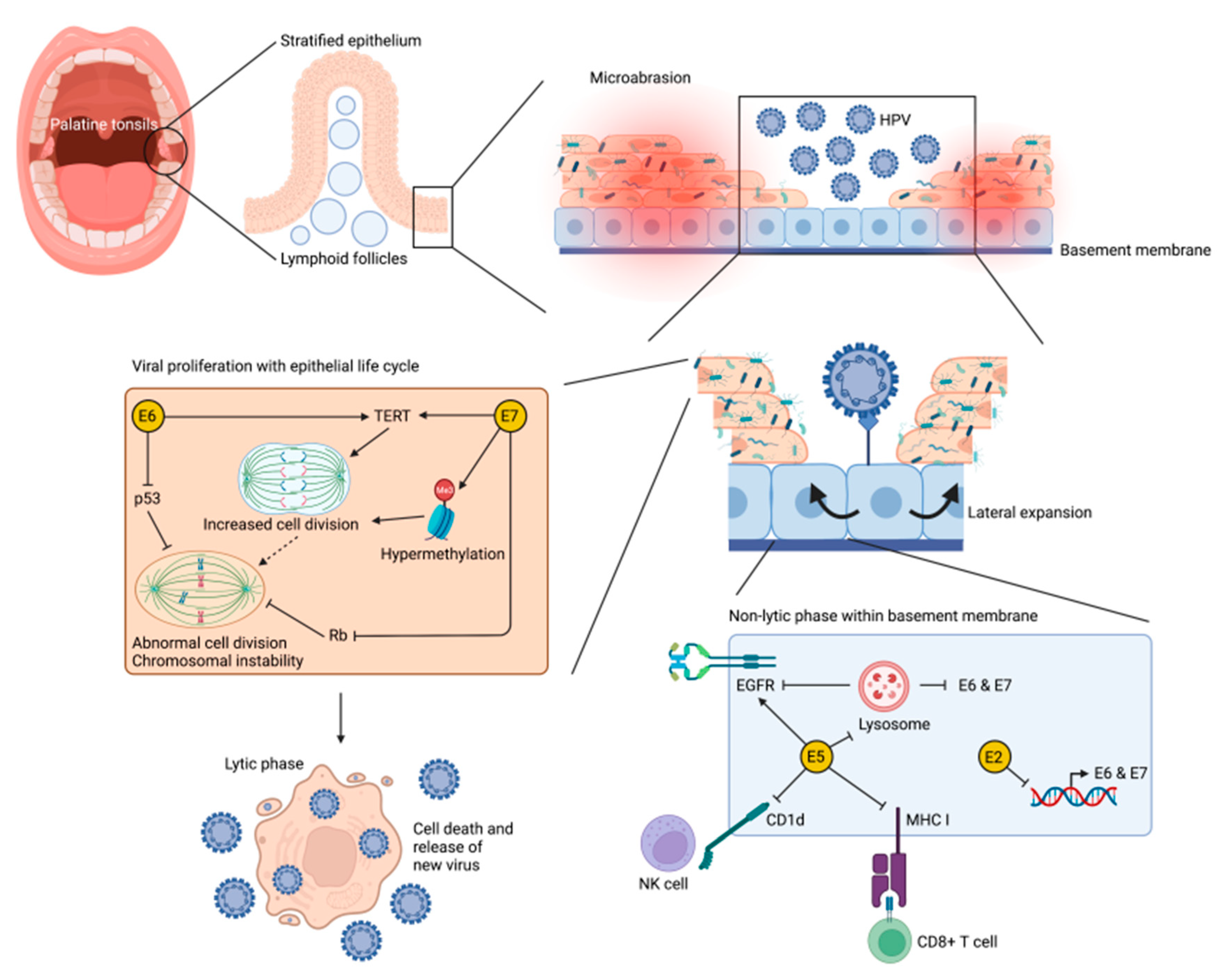
3.1. Human Papillomavirus and Oropharyngeal Carcinoma
3.2. Cellullar Mechanisims of HPV-Mediated Oropharyngeal Carcinogenesis
3.3. Role of the Immune System in HPV-Mediated Oropharyngeal Carcinogenesis
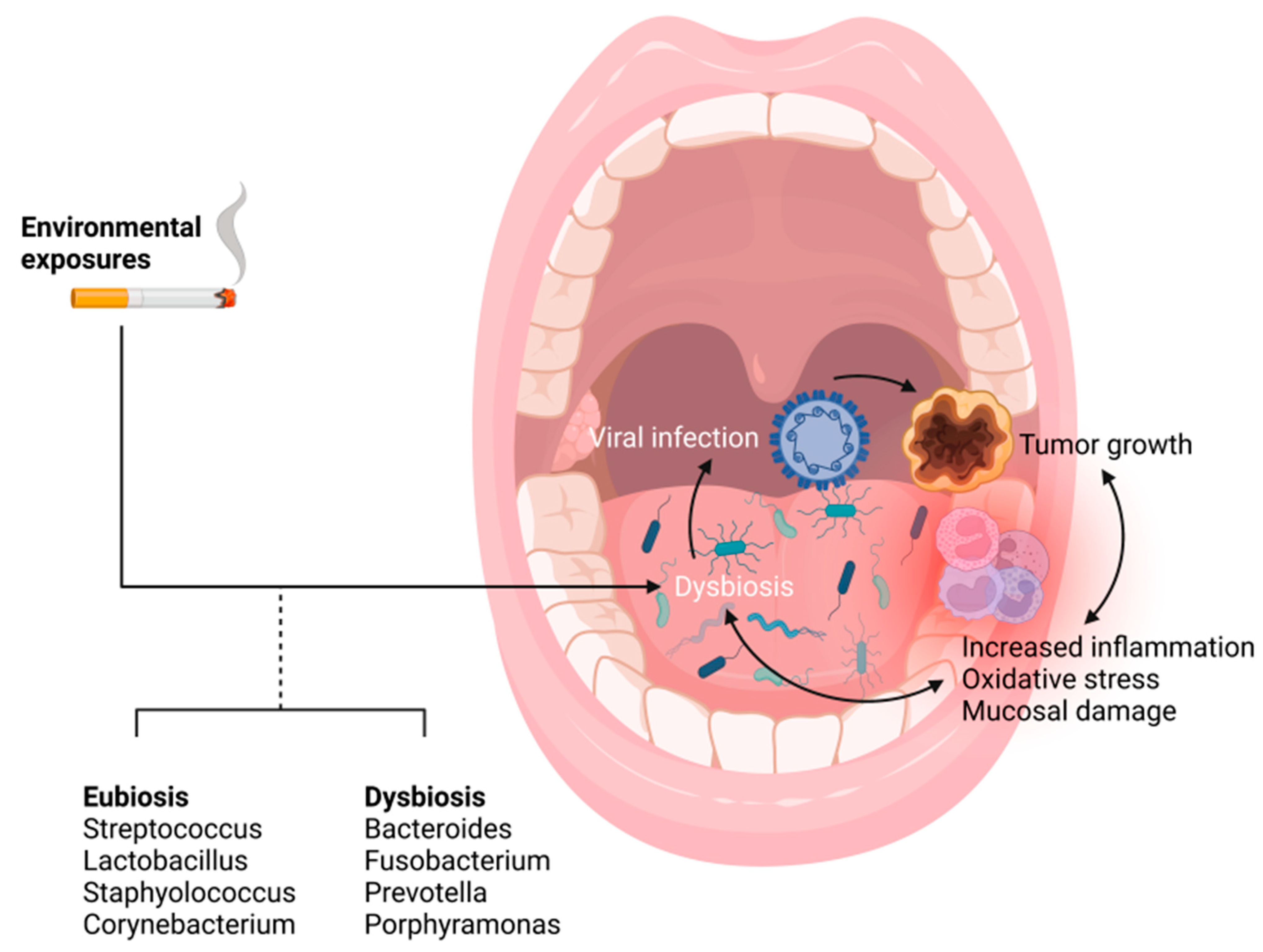
3.4. The Microbiome as a Primer for Inflammation and HPV Mediated Tumor Progression
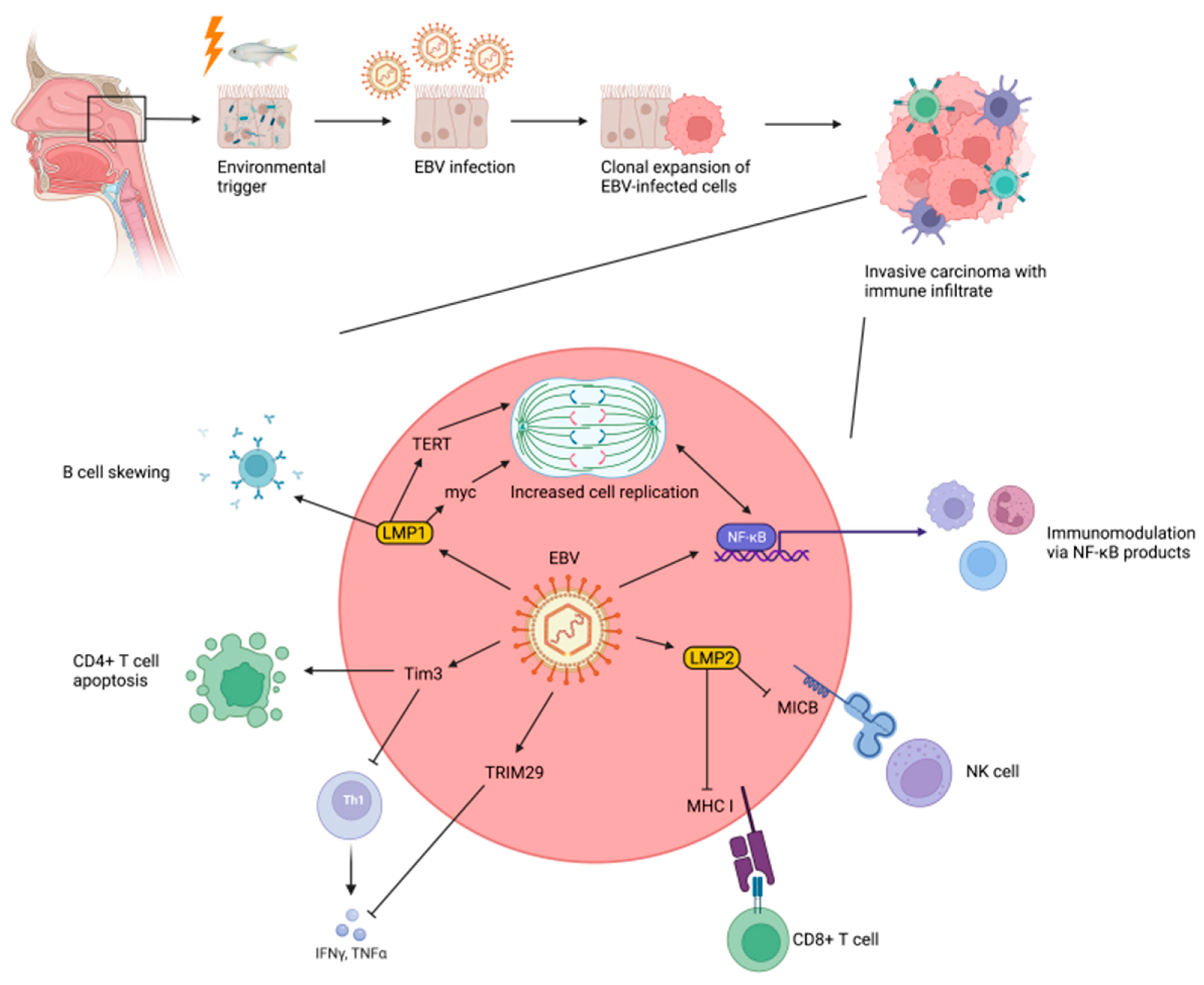
3.5. Epstein–Barr Virus and Nasopharyngeal Carcinogenesis
3.6. Cellular Mechanisms of EBV-Mediated Nasopharyngeal Carcinogenesis
3.7. Role of the Immune System in EBV-Mediated Nasopharyngeal Carcinogenesis
3.8. Does the Microbiome Play a Role in Nasopharyngeal Carcinogenesis?
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamashita, Y.; Takeshita, T. The Oral Microbiome and Human Health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Corbella, S.; Veronesi, P.; Galimberti, V.; Weinstein, R.; Fabbro, M.D.; Francetti, L. Is Periodontitis a Risk Indicator for Cancer? A Meta-Analysis. PLoS ONE 2018, 13, e0195683. [Google Scholar] [CrossRef]
- Welch, J.L.M.; Ramírez-Puebla, S.T.; Borisy, G.G. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe 2020, 28, 160–168. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The Microbiome of the Upper Respiratory Tract in Health and Disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Dinh, S.; Guntinas-Lichius, O.; Wittekindt, C.; Weissenborn, S.; Wieland, U.; Dienes, H.P.; Hoffmann, T.; Smith, E.; Turek, L.; et al. HPV-Associated Tonsillar Cancer. An Update. HNO 2004, 52, 208–218. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Gillison, M.L. Human Papillomavirus-Related Diseases: Oropharynx Cancers and Potential Implications for Adolescent HPV Vaccination. J. Adolesc. Health 2008, 43, S52–S60. [Google Scholar] [CrossRef]
- Goldenberg, D.; Golz, A.; Netzer, A.; Rosenblatt, E.; Rachmiel, A.; Goldenberg, R.F.; Joachims, H.Z. Epstein-Barr Virus and Cancers of the Head and Neck. Am. J. Otolaryngol. 2001, 22, 197–205. [Google Scholar] [CrossRef]
- Tsang, C.M.; Deng, W.; Yip, Y.L.; Zeng, M.-S.; Lo, K.W.; Tsao, S.W. Epstein-Barr Virus Infection and Persistence in Nasopharyngeal Epithelial Cells. Chin. J. Cancer 2014, 33, 549–555. [Google Scholar] [CrossRef]
- Huang, W.B.; Chan, J.Y.W.; Liu, D.L. Human Papillomavirus and World Health Organization Type III Nasopharyngeal Carcinoma: Multicenter Study from an Endemic Area in Southern China. Cancer 2018, 124, 530–536. [Google Scholar] [CrossRef]
- Shukla, S.; Bharti, A.C.; Mahata, S.; Hussain, S.; Kumar, R.; Hedau, S.; Das, B.C. Infection of Human Papillomaviruses in Cancers of Different Human Organ Sites. Indian J. Med. Res. 2009, 130, 222–233. [Google Scholar]
- Hausen, H. zur Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Han, J.J.; Beltran, T.H.; Song, J.W.; Klaric, J.; Choi, Y.S. Prevalence of Genital Human Papillomavirus Infection and Human Papillomavirus Vaccination Rates Among US Adult Men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol. 2017, 3, 810. [Google Scholar] [CrossRef]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H. Primary Epstein-Barr Virus Infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef]
- Fernandes, J.V.; de Fernandes, T.A.A.M.; de Azevdeo, J.C.V.; Cobucci, R.N.O.; de Carvalho, M.G.F.; Andrade, V.S.; de Araujo, J.M.G. Link between Chronic Inflammation and Human Papillomavirus-Induced Carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Feller, L.; Altini, M.; Lemmer, J. Inflammation in the Context of Oral Cancer. Oral Oncol. 2013, 49, 887–892. [Google Scholar] [CrossRef]
- Heusinkveld, M.; Goedemans, R.; Briet, R.J.P.; Gelderblom, H.; Nortier, J.W.R.; Gorter, A.; Smit, V.T.H.B.M.; Langeveld, A.P.M.; Jansen, J.C.; Burg, S.H. van der Systemic and Local Human Papillomavirus 16-specific T-cell Immunity in Patients with Head and Neck Cancer. Int. J. Cancer 2012, 131, E74–E85. [Google Scholar] [CrossRef]
- Bruce, J.P.; To, K.-F.; Lui, V.W.Y.; Chung, G.T.Y.; Chan, Y.-Y.; Tsang, C.M.; Yip, K.Y.; Ma, B.B.Y.; Woo, J.K.S.; Hui, E.P.; et al. Whole-Genome Profiling of Nasopharyngeal Carcinoma Reveals Viral-Host Co-Operation in Inflammatory NF-ΚB Activation and Immune Escape. Nat. Commun. 2021, 12, 4193. [Google Scholar] [CrossRef]
- Lun, S.W.-M.; Cheung, S.-T.; Lo, K.-W. Cancer Stem-like Cells in Epstein-Barr Virus-Associated Nasopharyngeal Carcinoma. Chin. J. Cancer 2014, 33, 529–538. [Google Scholar] [CrossRef]
- Andersen, A.S.; Sølling, A.S.K.; Ovesen, T.; Rusan, M. The Interplay between HPV and Host Immunity in Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2014, 134, 2755–2763. [Google Scholar] [CrossRef]
- Colman, G.; Beighton, D.; Chalk, A.J.; Wake, S. Cigarette Smoking and the Microbial Flora of the Mouth. Aust. Dent. J. 1976, 21, 111–118. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the Respiratory Microbiome in Healthy Nonsmokers and Smokers. Am. J. Respir. Crit. Care 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The Subgingival Microbiome of Clinically Healthy Current and Never Smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. Chapter Four The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef]
- Tada, A.; Senpuku, H. The Impact of Oral Health on Respiratory Viral Infection. Dent. J. 2021, 9, 43. [Google Scholar] [CrossRef]
- Shetty, S.S.; Padam, K.S.R.; Hunter, K.D.; Kudva, A.; Radhakrishnan, R. Biological Implications of the Immune Factors in the Tumour Microenvironment of Oral Cancer. Arch. Oral Biol. 2021, 133, 105294. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, Y.; Liao, B.; Liao, M.; Shi, Y.; Wei, Y.; Huang, Y.; Zhou, X.; Cheng, L.; Ren, B. The Interaction Between the Microbiome and Tumors. Front. Cell Infect. Microbiol. 2021, 11, 673724. [Google Scholar] [CrossRef]
- Kurago, Z.; Loveless, J. Microbial Colonization and Inflammation as Potential Contributors to the Lack of Therapeutic Success in Oral Squamous Cell Carcinoma. Front. Oral Health 2021, 2, 739499. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-Sexual HPV Transmission and Role of Vaccination for a Better Future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-Associated Oropharyngeal Cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef]
- Bohr, A.; Grønhøj, C.; Lajer, C.; Gerstoft, J.; von Buchwald, C. Transmission of and Infection with Human Papillomavirus in the Oropharynx. Ugeskr. Laeg. 2017, 179, V10160700. [Google Scholar]
- Szymonowicz, K.A.; Chen, J. Biological and Clinical Aspects of HPV-Related Cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- El-Mofty, S.K. Human Papillomavirus (HPV) Related Carcinomas of the Upper Aerodigestive Tract. Head Neck Pathol. 2007, 1, 181–185. [Google Scholar] [CrossRef]
- Santos, J.M.O.; da Silva, S.P.; Costa, N.R.; da Costa, R.M.G.; Medeiros, R. The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers. Cancers 2018, 10, 493. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular Pathways and Targets. Curr. Prob. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef]
- Ilmarinen, T.; Munne, P.; Hagström, J.; Haglund, C.; Auvinen, E.; Virtanen, E.I.; Haesevoets, A.; Speel, E.J.M.; Aaltonen, L.-M. Prevalence of High-Risk Human Papillomavirus Infection and Cancer Gene Mutations in Nonmalignant Tonsils. Oral Oncol. 2017, 73, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Herberhold, S.; Hellmich, M.; Panning, M.; Bartok, E.; Silling, S.; Akgül, B.; Wieland, U. Human Polyomavirus and Human Papillomavirus Prevalence and Viral Load in Non-Malignant Tonsillar Tissue and Tonsillar Carcinoma. Med. Microbiol. Immun. 2016, 206, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The Smallest Oncoprotein with Many Functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Crusius, K.; Auvinen, E.; Steuer, B.; Gaissert, H.; Alonso, A. The Human Papillomavirus Type 16 E5-Protein Modulates Ligand-Dependent Activation of the EGF Receptor Family in the Human Epithelial Cell Line HaCaT. Exp. Cell Res. 1998, 241, 76–83. [Google Scholar] [CrossRef]
- Miura, S.; Kawana, K.; Schust, D.J.; Fujii, T.; Yokoyama, T.; Iwasawa, Y.; Nagamatsu, T.; Adachi, K.; Tomio, A.; Tomio, K.; et al. CD1d, a Sentinel Molecule Bridging Innate and Adaptive Immunity, Is Downregulated by the Human Papillomavirus (HPV) E5 Protein: A Possible Mechanism for Immune Evasion by HPV. J. Virol. 2010, 84, 11614–11623. [Google Scholar] [CrossRef]
- Marchetti, B.; Ashrafi, G.H.; Tsirimonaki, E.; O’Brien, P.M.; Campo, M.S. The Bovine Papillomavirus Oncoprotein E5 Retains MHC Class I Molecules in the Golgi Apparatus and Prevents Their Transport to the Cell Surface. Oncogene 2002, 21, 7808–7816. [Google Scholar] [CrossRef][Green Version]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Briones-Herrera, A.; Pedraza-Chaverri, J. Regulation of Autophagy by High- and Low-risk Human Papillomaviruses. Rev. Med. Virol. 2021, 31, e2169. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef]
- Doorslaer, K.V.; Burk, R.D. Association between HTERT Activation by HPV E6 Proteins and Oncogenic Risk. Virology 2012, 433, 216–219. [Google Scholar] [CrossRef]
- Burgers, W.A.; Blanchon, L.; Pradhan, S.; de Launoit, Y.; Kouzarides, T.; Fuks, F. Viral Oncoproteins Target the DNA Methyltransferases. Oncogene 2007, 26, 1650–1655. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral Cavity and Oropharyngeal Squamous Cell Carcinoma—An Update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco Smoking and Cancer: A Meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- NCCN. NCCN Guidelines Version 1. 2022 Head and Neck Cancers; NCCN: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Cohan, D.M.; Popat, S.; Kaplan, S.E.; Rigual, N.; Loree, T.; Hicks, W.L. Oropharyngeal Cancer: Current Understanding and Management. Curr. Opin. Otolaryngol. 2009, 17, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ernster, J.A.; Sciotto, C.G.; O’Brien, M.M.; Robinson, L.J.; Willson, T. Prevalence of Oncogenic Human Papillomavirus 16 and 18 in the Palatine Tonsils of the General Adult Population. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 554–557. [Google Scholar] [CrossRef][Green Version]
- Tanaka, T.I.; Alawi, F. Human Papillomavirus and Oropharyngeal Cancer. Dent. Clin. N. Am. 2018, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Bhatia, R.K.; Messeguer, A.L.; González, P.; Herrero, R.; Giuliano, A.R. Oral Human Papillomavirus in Healthy Individuals; A Systematic Review of the Literature. Sex. Transm. Dis. 2010, 37, 386–391. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.; Cho, K.-J.; Kim, S.B.; Roh, J.-L.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y.; Song, J.S. Association Between Fibroblast Growth Factor Receptor 1 Gene Amplification and Human Papillomavirus Prevalence in Tonsillar Squamous Cell Carcinoma With Clinicopathologic Analysis. J. Histochem. Cytochem. 2018, 66, 511–522. [Google Scholar] [CrossRef]
- Ilahi, N.E.; Bhatti, A. Impact of HPV E5 on Viral Life Cycle via EGFR Signaling. Microb. Pathog. 2019, 139, 103923. [Google Scholar] [CrossRef]
- Gutierrez-Xicotencatl, L.; Pedroza-Saavedra, A.; Chihu-Amparan, L.; Salazar-Piña, A.; Maldonado-Gama, M.; Esquivel-Guadarrama, F. Cellular Functions of HPV16 E5 Oncoprotein during Oncogenic Transformation. Mol. Cancer Res. 2021, 19, 167–179. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Annunziata, C.; Tornesello, A.L.; Buonaguro, L.; Buonaguro, F.M. Human Oncoviruses and P53 Tumor Suppressor Pathway Deregulation at the Origin of Human Cancers. Cancers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.-E.A.; Foulkes, W.D.; Benlimame, N.; Wong, A.; Yen, L.; Bergeron, J.; Batist, G.; Alpert, L.; Alaoui-Jamali, M.A. E6/E7 Proteins of HPV Type 16 and ErbB-2 Cooperate to Induce Neoplastic Transformation of Primary Normal Oral Epithelial Cells. Oncogene 2004, 23, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lichtig, H.; Gilboa, D.A.; Jackman, A.; Gonen, P.; Levav-Cohen, Y.; Haupt, Y.; Sherman, L. HPV16 E6 Augments Wnt Signaling in an E6AP-Dependent Manner. Virology 2010, 396, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shimane, T.; Kawano, S.; Alshaikh, A.; Kim, S.Y.; Chung, S.H.; Kim, R.H.; Shin, K.H.; Walentin, K.; Park, N.H.; et al. Human Papillomavirus 16 E6 Induces FoxM1B in Oral Keratinocytes through GRHL2. J. Dent. Res. 2018, 97, 795–802. [Google Scholar] [CrossRef]
- Spanos, W.C.; Geiger, J.; Anderson, M.E.; Harris, G.F.; Bossler, A.D.; Smith, R.B.; Klingelhutz, A.J.; Lee, J.H. Deletion of the PDZ Motif of HPV16 E6 Preventing Immortalization and Anchorage-Independent Growth in Human Tonsil Epithelial Cells. Head Neck 2008, 30, 139–147. [Google Scholar] [CrossRef]
- Songock, W.K.; Kim, S.; Bodily, J.M. The Human Papillomavirus E7 Oncoprotein as a Regulator of Transcription. Virus Res. 2017, 231, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Munger, K. The Papillomavirus E7 Proteins. Virology 2013, 445, 138–168. [Google Scholar] [CrossRef]
- Camuzi, D.; de Simão, T.A.; Dias, F.; Pinto, L.F.R.; Soares-Lima, S.C. Head and Neck Cancers Are Not Alike When Tarred with the Same Brush: An Epigenetic Perspective from the Cancerization Field to Prognosis. Cancers 2021, 13, 5630. [Google Scholar] [CrossRef]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef]
- Hoover, A.C.; Spanos, W.C.; Harris, G.F.; Anderson, M.E.; Klingelhutz, A.J.; Lee, J.H. The Role of Human Papillomavirus 16 E6 in Anchorage-Independent and Invasive Growth of Mouse Tonsil Epithelium. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 495–502. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Schiller, J.T.; Viscidi, R.P.; Strickler, H.D.; Coursaget, P.; Tan, T.; Halsey, N.; Jenkins, D. Clinician’s Guide to Human Papillomavirus Immunology: Knowns and Unknowns. Lancet Infect. Dis. 2009, 9, 347–356. [Google Scholar] [CrossRef]
- Rosenberger, S.; Arce, J.D.-C.; Langbein, L.; Steenbergen, R.D.M.; Rösl, F. Alternative Splicing of Human Papillomavirus Type-16 E6/E6* Early MRNA Is Coupled to EGF Signaling via Erk1/2 Activation. Proc. Natl. Acad. Sci. USA 2010, 107, 7006–7011. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S. Papillomavirus Transcripts and Posttranscriptional Regulation. Virology 2013, 445, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, N. Human Papillomavirus-16 E5 Protein: Oncogenic Role and Therapeutic Value. Cell Oncol. 2012, 35, 67–76. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Human Papillomaviruses: Diversity, Infection and Host Interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Egawa, N.; Shiraz, A.; Katakuse, M.; Okamura, M.; Griffin, H.M.; Doorbar, J. The Reservoir of Persistent Human Papillomavirus Infection; Strategies for Elimination Using Anti-Viral Therapies. Viruses 2022, 14, 214. [Google Scholar] [CrossRef]
- Doorbar, J.; Zheng, K.; Aiyenuro, A.; Yin, W.; Walker, C.M.; Chen, Y.; Egawa, N.; Griffin, H.M. Principles of Epithelial Homeostasis Control during Persistent Human Papillomavirus Infection and Its Deregulation at the Cervical Transformation Zone. Curr. Opin. Virol. 2021, 51, 96–105. [Google Scholar] [CrossRef]
- Barros, M.R.; de Oliveira, T.H.A.; de Melo, C.M.L.; Venuti, A.; de Freitas, A.C. Viral Modulation of TLRs and Cytokines and the Related Immunotherapies for HPV-Associated Cancers. J. Immunol. Res. 2018, 2018, 2912671. [Google Scholar] [CrossRef]
- Jouhi, L.; Datta, N.; Renkonen, S.; Atula, T.; Mäkitie, A.; Haglund, C.; Ahmed, A.; Syrjänen, S.; Grénman, R.; Auvinen, E.; et al. Expression of Toll-like Receptors in HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma—An in Vivo and in Vitro Study. Tumour Biol. 2015, 36, 7755–7764. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like Receptor Control of the Adaptive Immune Responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Thorstad, W.L.; Chernock, R.D.; Haughey, B.H.; Yip, J.H.; Zhang, Q.; El-Mofty, S.K. P16 Positive Oropharyngeal Squamous Cell Carcinoma: An Entity with a Favorable Prognosis Regardless of Tumor HPV Status. Am. J. Surg. Pathol. 2010, 34, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.K.; Divine, G.; Chen, K.M.; Chitale, D.; Havard, S.; Worsham, M.J. Significance of P16 in Site-Specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin. Oncol. 2013, 2, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Luginbuhl, A.; Sanders, M.; Spiro, J.D. Prevalence, Morphology, and Prognosis of Human Papillomavirus in Tonsillar Cancer. Ann. Otol. Rhinol. Laryngol. 2009, 118, 742–749. [Google Scholar] [CrossRef]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M.L. Stromal Infiltration of CD8 T Cells Is Associated with Improved Clinical Outcome in HPV-Positive Oropharyngeal Squamous Carcinoma. Brit. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef]
- Wilczynski, S.P.; Lin, B.T.; Xie, Y.; Paz, I.B. Detection of Human Papillomavirus DNA and Oncoprotein Overexpression Are Associated with Distinct Morphological Patterns of Tonsillar Squamous Cell Carcinoma. Am. J. Pathol. 1998, 152, 145–156. [Google Scholar]
- Begum, S.; Cao, D.; Gillison, M.; Zahurak, M.; Westra, W.H. Tissue Distribution of Human Papillomavirus 16 DNA Integration in Patients with Tonsillar Carcinoma. Clin. Cancer Res. 2005, 11, 5694–5699. [Google Scholar] [CrossRef]
- Hibbert, J.; Halec, G.; Baaken, D.; Waterboer, T.; Brenner, N. Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 3010. [Google Scholar] [CrossRef]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M.; Zábrodský, M.; Vošmik, M.; Rozkošová, K.; Vošmiková, H.; et al. Tumor-Infiltrating B Cells Affect the Progression of Oropharyngeal Squamous Cell Carcinoma via Cell-to-Cell Interactions with CD8+ T Cells. J. Immunother. Cancer 2019, 7, 261. [Google Scholar] [CrossRef]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors Associated with Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Hanasoge, S.; Magliocca, K.R.; Switchenko, J.M.; Saba, N.F.; Wadsworth, J.T.; El-Deiry, M.W.; Shin, D.M.; Khuri, F.; Beitler, J.J.; Higgins, K.A. Clinical Outcomes in Elderly Patients with Human Papillomavirus–Positive Squamous Cell Carcinoma of the Oropharynx Treated with Definitive Chemoradiation Therapy. Head Neck 2016, 38, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical Cancer Prevention and Control in Women Living with Human Immunodeficiency Virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.J.; Palefsky, J.M. HIV/AIDS-Associated Viral Oncogenesis. Cancer Treat. 2018, 177, 183–209. [Google Scholar] [CrossRef]
- Beachler, D.C.; D’Souza, G. Oral Human Papillomavirus Infection and Head and Neck Cancers in HIV-Infected Individuals. Curr. Opin. Oncol. 2013, 25, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Massa, S.; Mazul, A.L.; Kallogjeri, D.; Yaeger, L.; Jackson, R.S.; Zevallos, J.; Pipkorn, P. The Association of Smoking and Outcomes in HPV-Positive Oropharyngeal Cancer: A Systematic Review. Am. J. Otolaryngol. 2020, 41, 102592. [Google Scholar] [CrossRef]
- Villepelet, A.; Hugonin, S.; Atallah, S.; Job, B.; Baujat, B.; Guily, J.L.S.; Lacave, R. Effects of Tobacco Abuse on Major Chromosomal Instability in Human Papilloma Virus 16-Positive Oropharyngeal Squamous Cell Carcinoma. Int. J. Oncol. 2019, 55, 527–535. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Elinav, E.; Garrett, W.S.; Trinchieri, G.; Wargo, J. The Cancer Microbiome. Nat. Rev. Cancer 2019, 19, 371–376. [Google Scholar] [CrossRef]
- Cullin, N.; Antunes, C.A.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and Cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the Microbiome and TP53 in Human Lung Cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, P.; Murugesan, S.; Vetizou, M.; McCulloch, J.; Badger, J.H.; Trinchieri, G.; Khodor, S.A. Microbiome as an Immunological Modifier. Methods Mol. Biol. Clifton N. J. 2020, 2055, 595–638. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, C.; Jobin, C.; Trinchieri, G.; Howcroft, T.K.; Seifried, H.; Espey, M.G.; Flores, R.; Kim, Y.S.; Daschner, P.J. Workshop Report: Modulation of Antitumor Immune Responses by Dietary and Microbial Metabolites. J. Natl. Cancer Inst. 2017, 109, djx040. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Trinchieri, G. The Interplay between Neutrophils and Microbiota in Cancer. J. Leukoc. Biol. 2018, 104, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2016, 35, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Fusobacterium Nucleatum—Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Healy, C.M.; Moran, G.P. The Microbiome and Oral Cancer: More Questions than Answers. Oral Oncol. 2019, 89, 30–33. [Google Scholar] [CrossRef]
- Shillitoe, E.J. The Microbiome of Oral Cancer. Crit. Rev. Oncog. 2018, 23, 153–160. [Google Scholar] [CrossRef]
- Vanhoecke, B.; Stringer, A. Host–Microbe Cross Talk in Cancer Therapy. Curr. Opin. Support. Palliat. Care 2015, 9, 174–181. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kitamura, N.; Yoshida, K.; Yamamoto, T.; Ozaki, K.; Kudo, Y. Involvement of Fusobacterium Species in Oral Cancer Progression: A Literature Review Including Other Types of Cancer. Int. J. Mol. Sci. 2020, 21, 6207. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Hilario, A.R.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-Resolution Microbiome Profiling Uncovers Fusobacterium Nucleatum, Lactobacillus Gasseri/Johnsonii, and Lactobacillus Vaginalis Associated to Oral and Oropharyngeal Cancer in Saliva from HPV Positive and HPV Negative Patients Trea. Oncotarget 2014, 5, 110931–110948. [Google Scholar] [CrossRef]
- Shin, J.M.; Luo, T.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Microbial Communities Associated with Primary and Metastatic Head and Neck Squamous Cell Carcinoma–A High Fusobacterial and Low Streptococcal Signature. Sci. Rep. 2017, 7, 9934. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Yost, S.; Choi, Y.; Danciu, T.; Chen, T.; Yoganathan, S.; Kressirer, C.; Ruiz-Tourrella, M.; Das, B.; Kokaras, A.; et al. The Oral Mouse Microbiome Promotes Tumorigenesis in Oral Squamous Cell Carcinoma. Msystems 2019, 4, e00323-19. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.-R.; Chang, C.-C.; Lee, W.-T.; Huang, C.-C.; Ou, C.-Y.; Tsai, S.-T.; Chen, K.-C.; Huang, J.-S.; Wong, T.-Y.; Lai, Y.-H.; et al. The Interplay between Oral Microbiome, Lifestyle Factors and Genetic Polymorphisms in the Risk of Oral Squamous Cell Carcinoma. Carcinogenesis 2018, 39, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, G.; Guo, Y.; Zhao, Y.; Zhao, M.; Lai, Y.; Sui, P.; Shi, T.; Guo, W.; Huang, Z. Variations in Oral Microbiota Composition Are Associated with a Risk of Throat Cancer. Front. Cell Infect. Microbiol. 2019, 9, 205. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; D’Souza, G.; Fakhry, C.; Bigelow, E.O.; Usyk, M.; Burk, R.D.; Zhao, N. Oral HPV Associated with Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358. [Google Scholar] [CrossRef]
- Shigeishi, H.; Sugiyama, M.; Ohta, K. Relationship between the Prevalence of Oral Human Papillomavirus DNA and Periodontal Disease (Review). Biomed. Rep. 2021, 14, 40. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Gannon, O.M.; Panizza, B.J.; Saunders, N.A.; Antonsson, A. Human Papillomavirus Infection and Tumor Microenvironment Are Associated with the Microbiota in Patients with Oropharyngeal Cancers—Pilot Study. Head Neck 2021, 43, 3324–3330. [Google Scholar] [CrossRef]
- Astl, J.; Holy, R.; Maute, E.; Rotnágl, J.; Kalfeřt, D.; Drnková, B.; Younus, T.; Pavlík, E. Genome of Helicobacter Pylori and Serotype of HPV Detected in Oropharyngeal and Laryngeal Cancer and Chronic Inflammation Patients. Int. J. Environ. Res. Public Health 2021, 18, 9545. [Google Scholar] [CrossRef]
- Bouland, C.; Dequanter, D.; Lechien, J.R.; Hanssens, C.; Aubain, N.D.S.; Digonnet, A.; Javadian, R.; Yanni, A.; Rodriguez, A.; Loeb, I.; et al. Prognostic Significance of a Scoring System Combining P16, Smoking, and Drinking Status in a Series of 131 Patients with Oropharyngeal Cancers. Int. J. Otolaryngol. 2021, 2021, 8020826. [Google Scholar] [CrossRef] [PubMed]
- Ference, R.; Liao, D.; Gao, Q.; Mehta, V. Impact of Smoking on Survival Outcomes in HPV-Related Oropharyngeal Carcinoma: A Meta-Analysis. Otolaryngol. Head Neck Surg. 2020, 163, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Börnigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in Oral Bacterial Communities Are Associated with Risk Factors for Oral and Oropharyngeal Cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Al-hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Bauer, M.; Jasinski-Bergner, S.; Mandelboim, O.; Wickenhauser, C.; Seliger, B. Epstein–Barr Virus—Associated Malignancies and Immune Escape: The Role of the Tumor Microenvironment and Tumor Cell Evasion Strategies. Cancers 2021, 13, 5189. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet 1964, 283, 702–703. [Google Scholar] [CrossRef]
- Hsu, J.L.; Glaser, S.L. Epstein–Barr Virus-Associated Malignancies: Epidemiologic Patterns and Etiologic Implications. Crit. Rev. Oncol. 2000, 34, 27–53. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal Carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Stan, D.J.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Vesa, D.; Bezman, L.; Bujoreanu, F.C.; Sarbu, M.I.; Mihailov, R.; et al. Nasopharyngeal Carcinoma: A New Synthesis of Literature Data (Review). Exp. Ther. Med. 2022, 23, 136. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Ng, W.T.; Chan, L.L.K.; Hung, W.M.; Chan, C.C.C.; Sze, H.C.K.; Chan, O.S.H.; Chang, A.T.Y.; Yeung, R.M.W. Evolution of Treatment for Nasopharyngeal Cancer–Success and Setback in the Intensity-Modulated Radiotherapy Era. Radiother. Oncol. 2014, 110, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Vasef, M.A.; Ferlito, A.; Weiss, L.M. Nasopharyngeal Carcinoma, with Emphasis on Its Relationship to Epstein-Barr Virus. Ann. Otol. Rhinol. Laryngol. 1997, 106, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.W.; Hui, E.P.; Lo, K.-W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.-F.; King, A.D.; et al. Nasopharyngeal Carcinoma: An Evolving Paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Pathmanathan, R.; Prasad, U.; Sadler, R.; Flynn, K.; Raab-Traub, N. Clonal Proliferations of Cells Infected with Epstein-Barr Virus in Preinvasive Lesions Related to Nasopharyngeal Carcinoma. N. Engl. J. Med. 1995, 333, 693–698. [Google Scholar] [CrossRef]
- Biggi, A.F.B.; de Oliveira, D.E. The Epstein-Barr Virus Hacks Immune Checkpoints: Evidence and Consequences for Lymphoproliferative Disorders and Cancers. Biomolecules 2022, 12, 397. [Google Scholar] [CrossRef]
- Van Senten, J.R.; Fan, T.S.; Siderius, M.; Smit, M.J. Viral G Protein-Coupled Receptors as Modulators of Cancer Hallmarks. Pharmacol. Res. 2020, 156, 104804. [Google Scholar] [CrossRef]
- Wang, L.; Ning, S. New Look of EBV LMP1 Signaling Landscape. Cancers 2021, 13, 5451. [Google Scholar] [CrossRef]
- Cen, O.; Longnecker, R. Epstein Barr Virus Volume 2, One Herpes Virus: Many Diseases. Curr. Top Microbiol. 2015, 391, 151–180. [Google Scholar] [CrossRef]
- Chen, J.; Fu, L.; Zhang, L.-Y.; Kwong, D.L.; Yan, L.; Guan, X.-Y. Tumor Suppressor Genes on Frequently Deleted Chromosome 3p in Nasopharyngeal Carcinoma. Chin. J. Cancer 2012, 31, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Ye, W.; Zeng, Y.-X.; Adami, H.-O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Prev. Biomark. 2021, 30, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Yip, Y.L.; Lo, K.W.; Deng, W.; To, K.F.; Hau, P.M.; Lau, V.M.Y.; Takada, K.; Lui, V.W.Y.; Lung, M.L.; et al. Cyclin D1 Overexpression Supports Stable EBV Infection in Nasopharyngeal Epithelial Cells. Proc. Natl. Acad. Sci. USA 2012, 109, E3473–E3482. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, W.; Ye, Q.; Zhou, Y.; Xiong, W.; He, W.; Deng, M.; Zhou, M.; Guo, X.; Chen, P.; et al. Inhibition of Epstein-Barr Virus Infection by Lactoferrin. J. Innate Immun. 2012, 4, 387–398. [Google Scholar] [CrossRef]
- Roberts, M.L.; Cooper, N.R. Activation of a Ras–MAPK-Dependent Pathway by Epstein–Barr Virus Latent Membrane Protein 1 Is Essential for Cellular Transformation. Virology 1998, 240, 93–99. [Google Scholar] [CrossRef]
- Endo, K.; Shackelford, J.; Aga, M.; Yoshizaki, T.; Pagano, J.S. Upregulation of Special AT-Rich-Binding Protein 1 by Epstein–Barr Virus Latent Membrane Protein 1 in Human Nasopharyngeal Cells and Nasopharyngeal Cancer. J. Gen. Virol. 2013, 94, 507–513. [Google Scholar] [CrossRef]
- Lo, A.K.F.; Liu, Y.; Wang, X.H.; Huang, D.P.; Yuen, P.W.; Wong, Y.C.; Tsao, G.S.W. Alterations of Biologic Properties and Gene Expression in Nasopharyngeal Epithelial Cells by the Epstein-Barr Virus–Encoded Latent Membrane Protein 1. Lab. Investig. 2003, 83, 697–709. [Google Scholar] [CrossRef]
- Yang, J.; Deng, X.; Deng, L.; Gu, H.; Fan, W.; Cao, Y. Telomerase Activation by Epstein-Barr Virus Latent Membrane Protein 1 Is Associated with c-Myc Expression in Human Nasopharyngeal Epithelial Cells. J. Exp. Clin. Cancer Res. 2004, 23, 495–506. [Google Scholar]
- Yip, Y.; Tsang, C.; Deng, W.; Cheung, P.; Jin, Y.; Cheung, A.L.; Lung, M.L.; Tsao, S. Expression of Epstein-Barr Virus-encoded LMP1 and HTERT Extends the Life Span and Immortalizes Primary Cultures of Nasopharyngeal Epithelial Cells. J. Med. Virol. 2010, 82, 1711–1723. [Google Scholar] [CrossRef]
- Yang, J.; Deng, X.Y.; Gu, H.H.; Xia, L.Q.; Yi, W.; Cao, Y. Epstein-Barr Virus Latent Membrane Protein 1 Induces the Telomerase Activity in Human Nasopharyngeal Epithelial Cells. Shi Yan Sheng Wu Xue Bao 2001, 34, 207–211. [Google Scholar]
- Mordasini, V.; Ueda, S.; Aslandogmus, R.; Berger, C.; Gysin, C.; Hühn, D.; Sartori, A.A.; Bernasconi, M.; Nadal, D. Activation of ATR-Chk1 Pathway Facilitates EBV-Mediated Transformation of Primary Tonsillar B-Cells. Oncotarget 2016, 8, 6461–6474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo, A.K.; Lo, K.; Ko, C.; Young, L.S.; Dawson, C.W. Inhibition of the LKB1–AMPK Pathway by the Epstein–Barr Virus-encoded LMP1 Promotes Proliferation and Transformation of Human Nasopharyngeal Epithelial Cells. J. Pathol. 2013, 230, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Glatzel-Plucińska, N.; Piotrowska, A.; Dzięgiel, P.; Podhorska-Okołów, M. The Role of SATB1 in Tumour Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 4156. [Google Scholar] [CrossRef]
- Brinkmann, M.M.; Schulz, T.F. Regulation of Intracellular Signalling by the Terminal Membrane Proteins of Members of the Gammaherpesvirinae. J. Gen. Virol. 2006, 87, 1047–1074. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.-F.; Dawson, C.W.; Young, L.S.; Ko, C.-W.; Hau, P.-M.; Lo, K.-W. Activation of the FGFR1 Signalling Pathway by the Epstein-Barr Virus-Encoded LMP1 Promotes Aerobic Glycolysis and Transformation of Human Nasopharyngeal Epithelial Cells. J. Pathol. 2015, 237, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Subramanian, A.; Sheen, T.-S.; Tsai, C.-H.; Golub, T.R.; Thorley-Lawson, D.A. Epstein-Barr-Virus-Encoded LMP2A Induces Primary Epithelial Cell Migration and Invasion: Possible Role in Nasopharyngeal Carcinoma Metastasis. J. Virol. 2005, 79, 15430–15442. [Google Scholar] [CrossRef]
- Port, R.J.; Pinheiro-Maia, S.; Hu, C.; Arrand, J.R.; Wei, W.; Young, L.S.; Dawson, C.W. Epstein–Barr Virus Induction of the Hedgehog Signalling Pathway Imposes a Stem Cell Phenotype on Human Epithelial Cells. J. Pathol. 2013, 231, 367–377. [Google Scholar] [CrossRef]
- Baizig, N.M.; Wided, B.A.; Amine, O.E.; Gritli, S.; ElMay, M. The Clinical Significance of IGF-1R and Relationship with Epstein–Barr Virus Markers: LMP1 and EBERs in Tunisian Patients with Nasopharyngeal Carcinoma. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1011–1019. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Li, H.; Shi, F.; Xie, L.; Zhao, L.; Tang, M.; Luo, X.; Jia, W.; Fan, J.; et al. Targeting Epstein-Barr Virus Oncoprotein LMP1-Mediated High Oxidative Stress Suppresses EBV Lytic Reactivation and Sensitizes Tumors to Radiation Therapy. Theranostics 2020, 10, 11921–11937. [Google Scholar] [CrossRef]
- Li, H.M.; Zhuang, Z.H.; Wang, Q.; Pang, J.C.S.; Wang, X.H.; Wong, H.L.; Feng, H.C.; Jin, D.Y.; Ling, M.T.; Wong, Y.C.; et al. Epstein–Barr Virus Latent Membrane Protein 1 (LMP1) Upregulates Id1 Expression in Nasopharyngeal Epithelial Cells. Oncogene 2004, 23, 4488–4494. [Google Scholar] [CrossRef][Green Version]
- Shi, F.; Zhou, M.; Shang, L.; Du, Q.; Li, Y.; Xie, L.; Liu, X.; Tang, M.; Luo, X.; Fan, J.; et al. EBV(LMP1)-Induced Metabolic Reprogramming Inhibits Necroptosis through the Hypermethylation of the RIP3 Promoter. Theranostics 2019, 9, 2424–2438. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.F.; Lo, K.W.; Tsao, S.W.; Wong, H.L.; Hui, J.W.Y.; To, K.F.; Hayward, S.D.; Chui, Y.L.; Lau, Y.L.; Takada, K.; et al. Epstein-Barr Virus Infection Alters Cellular Signal Cascades in Human Nasopharyngeal Epithelial Cells. Neoplasia 2006, 8, 173–180. [Google Scholar] [CrossRef]
- Sun, J.; Hu, C.; Zhu, Y.; Sun, R.; Fang, Y.; Fan, Y.; Xu, F. LMP1 Increases Expression of NADPH Oxidase (NOX) and Its Regulatory Subunit P22 in NP69 Nasopharyngeal Cells and Makes Them Sensitive to a Treatment by a NOX Inhibitor. PLoS ONE 2015, 10, e0134896. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-W.; Chung, G.T.-Y.; To, K.-F. Deciphering the Molecular Genetic Basis of NPC through Molecular, Cytogenetic, and Epigenetic Approaches. Semin. Cancer Biol. 2012, 22, 79–86. [Google Scholar] [CrossRef]
- Iwakiri, D. Multifunctional Non-Coding Epstein–Barr Virus Encoded RNAs (EBERs) Contribute to Viral Pathogenesis. Virus Res. 2016, 212, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Tsai, K.; Allan, L.L.; Zheng, D.J.; Nie, J.C.; Biggs, C.M.; Hasan, M.R.; Kozak, F.K.; van den Elzen, P.; Priatel, J.J.; et al. Innate Immune Control of EBV-Infected B Cells by Invariant Natural Killer T Cells. Blood 2013, 122, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, E.T.; Liu, Q.; Cai, Y.; Zhang, Z.; Chen, G.; Huang, Q.; Xie, S.; Cao, S.; Jia, W.; et al. Occupational Exposures and Risk of Nasopharyngeal Carcinoma in a High-risk Area: A Population-based Case-control Study. Cancer 2021, 127, 2724–2735. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Huang, Q.; Yang, G.; Zhang, M.; Bi, J.; Shan, J.; Li, E.; He, S. Characterization of Tonsil Microbiota and Their Effect on Adenovirus Reactivation in Tonsillectomy Samples. Microbiol. Spectr. 2021, 9, e01246-21. [Google Scholar] [CrossRef]
- Zeidler, R.; Meissner, P.; Eissner, G.; Lazis, S.; Hammerschmidt, W. Rapid Proliferation of B Cells from Adenoids in Response to Epstein-Barr Virus Infection. Cancer Res. 1996, 56, 5610–5614. [Google Scholar]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between Human Papilloma Virus/Epstein-Barr Virus Coinfection and Oral Carcinogenesis. J. Oral Pathol. Med. 2014, 44, 28–36. [Google Scholar] [CrossRef]
- Lewis, J.S. Human Papillomavirus Testing in Head and Neck Squamous Cell Carcinoma in 2020: Where Are We Now and Where Are We Going? Head Neck Pathol. 2020, 14, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Phua, S.K.A.; Soong, Y.L.; Oon, L.L.E.; Chan, K.S.; Lucky, S.S.; Mong, J.; Tan, M.H.; Lim, C.M. Clinical Utility of Epstein-Barr Virus DNA and Other Liquid Biopsy Markers in Nasopharyngeal Carcinoma. Cancer Commun. 2020, 40, 564–585. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Kuhs, K.A.L.; Waterboer, T. Biomarkers for Early Identification of Recurrences in HPV-Driven Oropharyngeal Cancer. Oral Oncol. 2018, 82, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Jud, A.; Kotur, M.; Berger, C.; Gysin, C.; Nadal, D.; Lünemann, A. Tonsillar CD56brightNKG2A+ NK Cells Restrict Primary Epstein-Barr Virus Infection in B Cells via IFN-γ. Oncotarget 2016, 8, 6130–6141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKeon, M.G.; Gallant, J.-N.; Kim, Y.J.; Das, S.R. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers 2022, 14, 3120. https://doi.org/10.3390/cancers14133120
McKeon MG, Gallant J-N, Kim YJ, Das SR. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers. 2022; 14(13):3120. https://doi.org/10.3390/cancers14133120
Chicago/Turabian StyleMcKeon, Mallory G., Jean-Nicolas Gallant, Young J. Kim, and Suman R. Das. 2022. "It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer" Cancers 14, no. 13: 3120. https://doi.org/10.3390/cancers14133120
APA StyleMcKeon, M. G., Gallant, J.-N., Kim, Y. J., & Das, S. R. (2022). It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers, 14(13), 3120. https://doi.org/10.3390/cancers14133120






