Longitudinally Heterogeneous Tumor Dose Optimizes Proton Broadbeam, Interlaced Minibeam, and FLASH Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
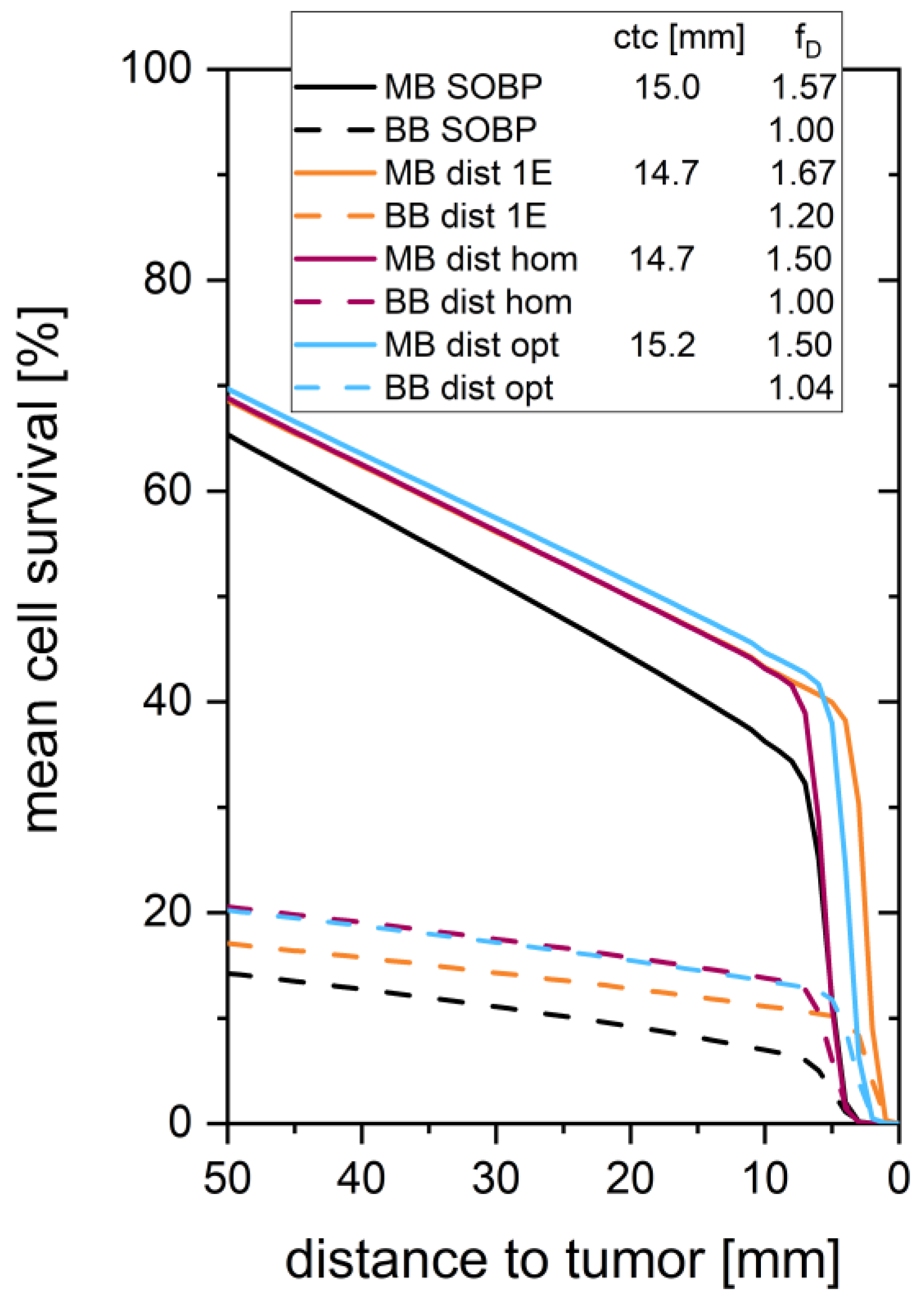
3. Results
4. Discussion
4.1. Longitudinally Heterogeneous Broadbeam Irradiation Modes
4.2. Proton Minibeam Irradiation Modes
4.3. 1E and Proton Minibeam Irradiation Modes in Conjunction with FLASH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Competing Interests
References
- Borras, J.M.; Lievens, Y.; Barton, M.; Corral, J.; Ferlay, J.; Bray, F.; Grau, C. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother. Oncol. 2016, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.P.; Barton, M.B. Evidence-based estimates of the demand for radiotherapy. Clin. Oncol. 2015, 27, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, M.; Tucker, S.L. Quantifying the position and steepness of radiation dose-response curves. Int. J. Radiat. Biol. 1997, 71, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Koonce, N.A.; Dings, R.P.M.; Siegel, E.; Moros, E.G.; Bräuer-Krisch, E.; Corry, P.M. Microbeam radiation therapy alters vascular architecture and tumor oxygenation and is enhanced by a galectin-1 targeted anti-angiogenic peptide. Radiat. Res. 2012, 177, 804–812. [Google Scholar] [CrossRef]
- Morgan-Fletcher, S.L. Prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50). Br. J. Radiol. 2001, 74, 294. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements. ICRU Report 50: Prescribing, Recording, and Reporting Photon Beam Therapy; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1993. [Google Scholar]
- Federal Office of Justice. German Radiation Protection Act. In Strahlenschutzgesetz vom 27. Juni 2017 (BGBl. I S. 1966), das Zuletzt Durch die Bekanntmachung vom 3. Januar 2022 (BGBl. I S. 15) Geändert Worden ist; Federal Office of Justice: Bonn, Germany, 2017. [Google Scholar]
- Brahme, A. Dosimetric precision requirements and quantities for characterizing the response of tumors and normal tissues. In IAEA-TECDOC-896, Radiation Dose in Radiotherapy from Prescription to Delivery; IAEA: Vienna, Austria, 1996. [Google Scholar]
- Sammer, M.; Greubel, C.; Girst, S.; Dollinger, G. Optimization of beam arrangements in proton minibeam radiotherapy by cell survival simulations. Med. Phys. 2017, 44, 6096–6104. [Google Scholar] [CrossRef]
- Sammer, M.; Girst, S.; Dollinger, G. Optimizing proton minibeam radiotherapy by interlacing and heterogeneous tumor dose on the basis of calculated clonogenic cell survival. Sci. Rep. 2021, 11, 3533. [Google Scholar] [CrossRef]
- MATLAB. The Mathworks. Available online: http://www.mathworks.com/ (accessed on 8 September 2022).
- Deasy, J.O.; Blanco, A.I.; Clark, V.H. CERR: A computational environment for radiotherapy research. Med. Phys. 2003, 30, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Schell, S.; Wilkens, J.J. Advanced treatment planning methods for efficient radiation therapy with laser accelerated proton and ion beams. Med. Phys. 2010, 37, 5330–5340. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Datzmann, G.; Degiovanni, A.; Dimov, V.; Dollinger, G. Magnetically focused 70 MeV proton minibeams for preclinical experiments combining a tandem accelerator and a 3 GHz linear post-accelerator. Med. Phys. 2022, 48, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Sammer, M.; Teiluf, K.; Girst, S.; Greubel, C.; Reindl, J.; Ilicic, K.; Walsh, D.W.M.; Aichler, M.; Walch, A.; Combs, S.E. Beam size limit for pencil minibeam radiotherapy determined from side effects in an in-vivo mouse ear model. PLoS ONE 2019, 14, e0221454. [Google Scholar] [CrossRef] [PubMed]
- Sammer, M.; Zahnbrecher, E.; Dobiasch, S.; Girst, S.; Greubel, C.; Ilicic, K.; Reindl, J.; Schwarz, B.; Siebenwirth, C.; Walsh, D.W.M.; et al. Proton pencil minibeam irradiation of an in-vivo mouse ear model spares healthy tissue dependent on beam size. PLoS ONE 2019, 14, 221454. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef] [PubMed]
- Sammer, M.; Dombrowsky, A.C.; Schauer, J.; Oleksenko, K.; Bicher, S.; Schwarz, B.; Rudigkeit, S.; Matejka, N.; Reindl, J.; Bartzsch, S.; et al. Normal Tissue Response of Combined Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 76–83. [Google Scholar] [CrossRef]
- Friedrich, T.; Scholz, U.; Elsässer, T.; Durante, M.; Scholz, M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J. Radiat. Res. 2012, 54, 494–514. [Google Scholar] [CrossRef] [PubMed]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Dilmanian, F.A.; Zhong, Z.; Bacarian, T.; Benveniste, H.; Romanelli, P.; Wang, R.; Welwart, J.; Yuasa, T.; Rosen, E.M.; Anschel, D.J. Interlaced x-ray microplanar beams: A radiosurgery approach with clinical potential. Proc. Natl. Acad. Sci. USA 2006, 103, 9709–9714. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Jouvion, G.; Hardy, D.; Patriarca, A.; Nauraye, C.; Bergs, J.; González, W.; Guardiola, C.; Juchaux, M.; Labiod, D. Proton minibeam radiation therapy spares normal rat brain: Long-Term Clinical, Radiological and Histopathological Analysis. Sci. Rep. 2017, 7, 14403. [Google Scholar] [CrossRef] [PubMed]
- Deasy, J.O.; Mackie, T.R.; DeLuca, P.M. Conformal proton tomotherapy using distal-edge tracking. Radiother. Oncol. 1995, 37, S43. [Google Scholar]
- Liu, W.; Li, Y.; Li, X.; Cao, W.; Zhang, X. Influence of robust optimization in intensity-modulated proton therapy with different dose delivery techniques. Med. Phys. 2022, 39, 3089–3101. [Google Scholar] [CrossRef]
- Parodi, K.; Polf, J.C. In vivo range verification in particle therapy. Med. Phys. 2018, 45, e1036–e1050. [Google Scholar] [CrossRef] [PubMed]
- Smeets, J.; Roellinghoff, F.; Prieels, D.; Stichelbaut, F.; Benilov, A.; Busca, P.; Fiorini, C.; Peloso, R.; Basilavecchia, M.; Frizzi, T.; et al. Prompt gamma imaging with a slit camera for real-time range control in proton therapy. Phys. Med. Biol. 2012, 57, 3371. [Google Scholar] [CrossRef]
- Verburg, J.M.; Seco, J. Proton range verification through prompt gamma-ray spectroscopy. Phys. Med. Biol. 2014, 59, 7089. [Google Scholar] [CrossRef] [PubMed]
- Assmann, W.; Kellnberger, S.; Reinhardt, S.; Lehrack, S.; Edlich, A.; Thirolf, P.G.; Moser, M.; Dollinger, G.; Omar, M.; Ntziachristos, V.; et al. Ionoacoustic characterization of the proton Bragg peak with submillimeter accuracy. Med. Phys. 2015, 42, 567–574. [Google Scholar] [CrossRef]
- Wieser, H.P.; Huang, Y.; Schauer, J.; Lascaud, J.; Würl, M.; Lehrack, S.; Radonic, D.; Vidal, M.; Hérault, J.; Chmyrov, A.; et al. Experimental demonstration of accurate Bragg peak localization with ionoacoustic tandem phase detection (iTPD). Phys. Med. Biol. 2021, 66, 245020. [Google Scholar] [CrossRef] [PubMed]
- Zlobinskaya, O.; Girst, S.; Greubel, C.; Hable, V.; Siebenwirth, C.; Walsh, D.W.M.; Multhoff, G.; Wilkens, J.J.; Schmid, T.E.; Dollinger, G. Reduced side effects by proton microchannel radiotherapy: Study in a human skin model. Radiat. Env. Biophys. 2013, 52, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Girst, S.; Greubel, C.; Reindl, J.; Siebenwirth, C.; Zlobinskaya, O.; Walsh, D.W.M.; Ilicic, K.; Aichler, M.; Walch, A.; Wilkens, J.J. Proton minibeam radiation therapy reduces side effects in an in vivo mouse ear model. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 234–241. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Eley, J.G.; Rusek, A.; Krishnan, S. Charged Particle Therapy with Mini-Segmented Beams. Front. Oncol. 2015, 5, 269. [Google Scholar] [CrossRef]
- Eley, J.; Chadha, A.; Quini, C.; Vichaya, E.; Zhang, C.; Davis, J.; Sahoo, N.; Waddell, J.; Leiser, D.; Dilmanian, A.; et al. Pilot study of neurologic toxicity in mice after proton minibeam therapy. Sci. Rep. 2020, 10, 11368. [Google Scholar] [CrossRef]
- Eley, J.G.; Haga, C.W.; Keller, A.; Lazenby, E.M.; Raver, C.; Rusek, A.; Dilmanian, F.A.; Krishnan, S.; Waddell, J. Heavy Ion Minibeam Therapy: Side Effects in Normal Brain. Cancers 2021, 13, 6207. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, T.; Mairani, A.; Brons, S.; Haberer, T.; Debus, J.; Parodi, K. Experimental dosimetric comparison of 1H, 4He, 12C and 16O scanned ion beams. Phys. Med. Biol. 2017, 62, 3958. [Google Scholar] [CrossRef]
- Schneider, T.; Patriarca, A.; Prezado, Y. Improving the dose distributions in minibeam radiation therapy: Helium ions vs protons. Med. Phys. 2019, 46, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.-C.; de Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Greubel, C. Einfluss der Zeitlichen und Räumlichen Fokussierung auf die Strahlenbiologische Wirksamkeit von Protonen. Ph.D. Thesis, Universität der Bundeswehr München: Neubiberg, Germany, 2013. [Google Scholar]
- Degiovanni, A.; Stabile, P.; Ungaro, D. Application of Detectors and Accelerators to Medicine. LIGHT: A linear accelerator for proton therapy. In Proceedings of the NAPAC2016, Chicago, IL, USA, 14 October 2016. [Google Scholar]
- Ronsivalle, C.; Picardi, L.; Ampollini, A.; Bazzano, G.; Marracino, F.; Nenzi, P.; Snels, C.; Surrenti, V.; Vadrucci, M.; Ambrosini, F. First acceleration of a proton beam in a Side Coupled Drift tube Linac. EPL (Europhys. Lett.) 2015, 111, 14002. [Google Scholar] [CrossRef][Green Version]
- Schneider, T.; de Marzi, L.; Patriarca, A.; Prezado, Y. Advancing proton minibeam radiation therapy: Magnetically focussed proton minibeams at a clinical centre. Sci. Rep. 2020, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Reindl, J.; Girst, S. pMB FLASH-Status and Perspectives of Combining Proton Minibeam with FLASH Radiotherapy. J. Cancer Immunol. 2019, 1, 14–23. [Google Scholar] [CrossRef]
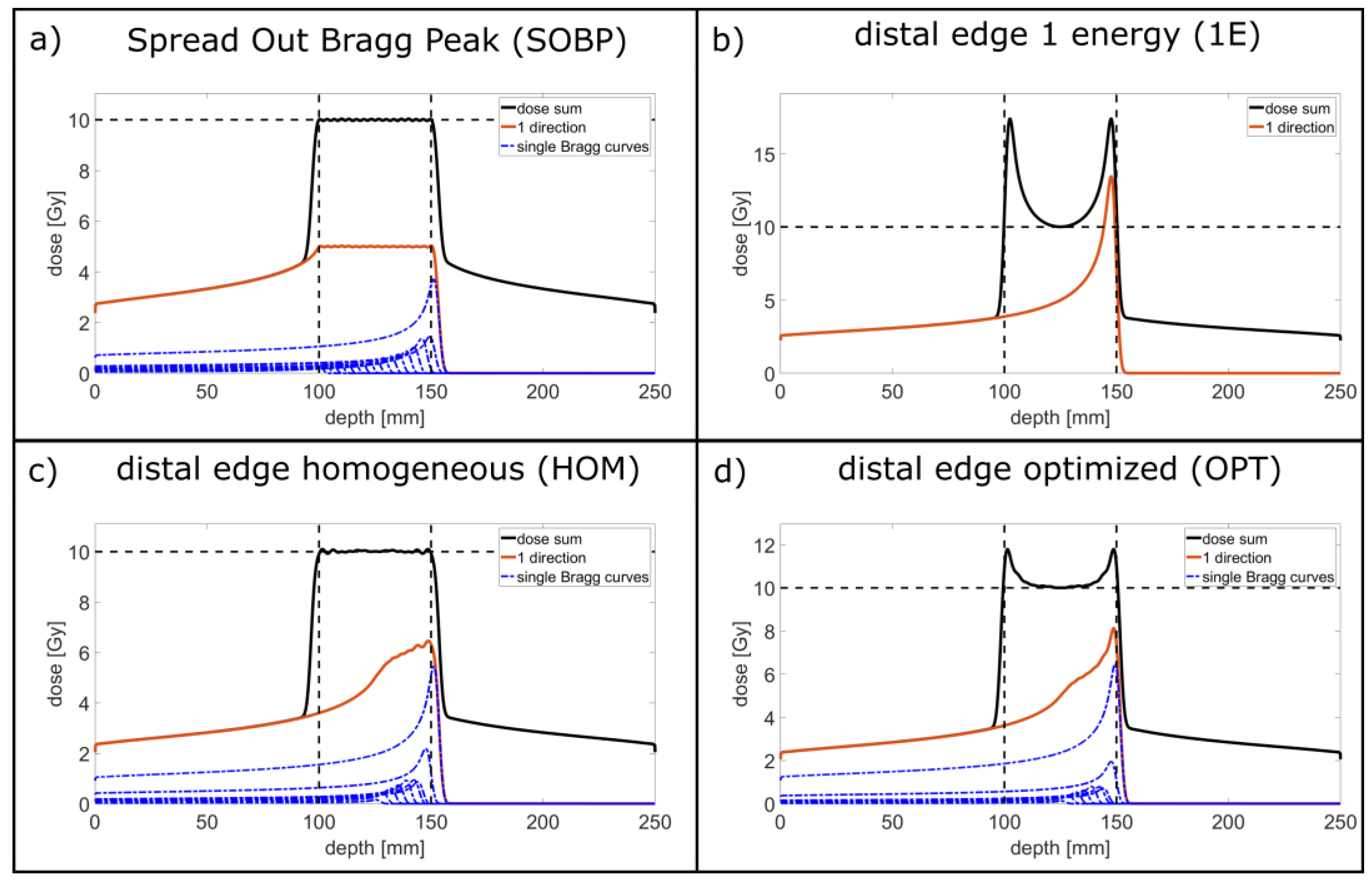
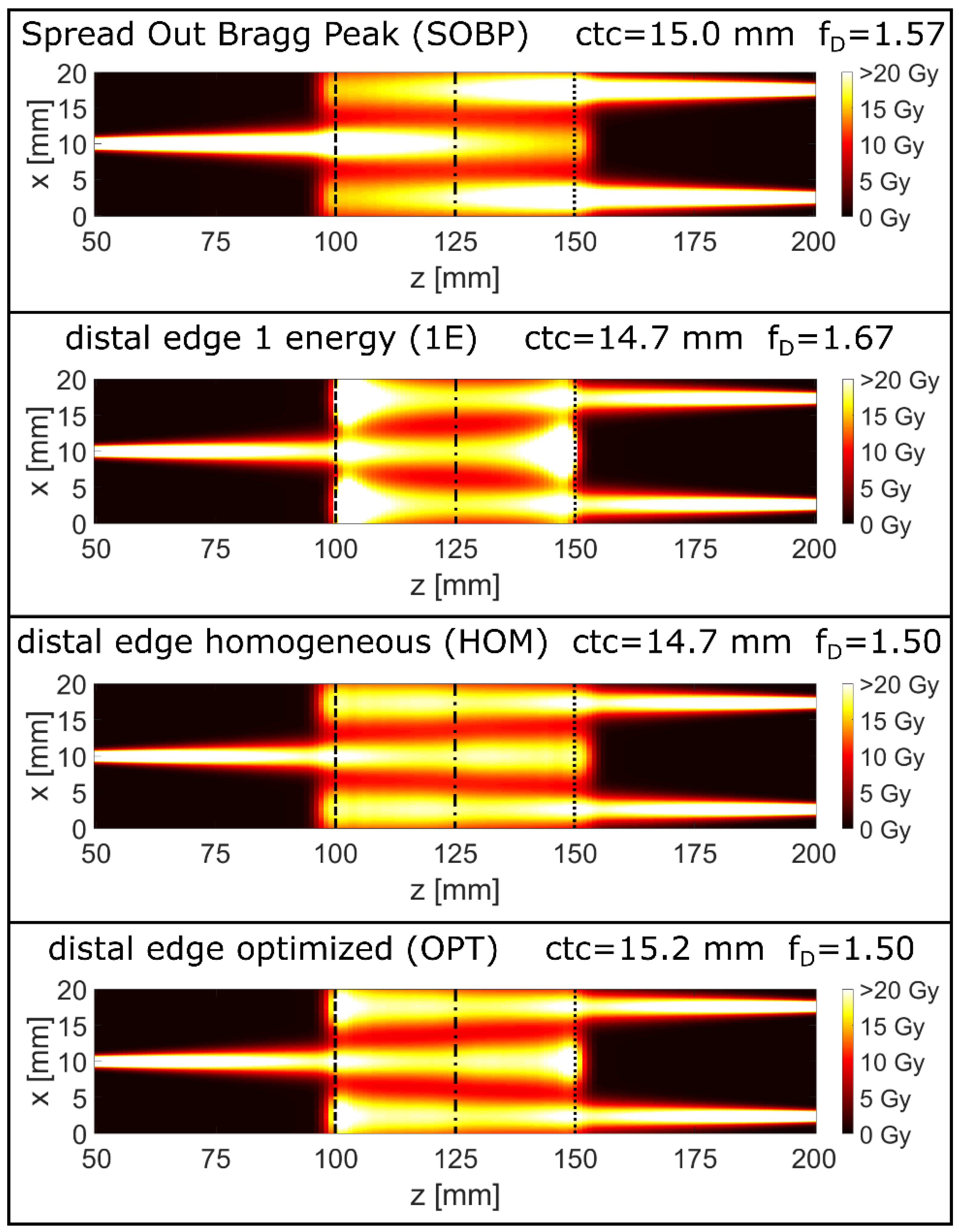
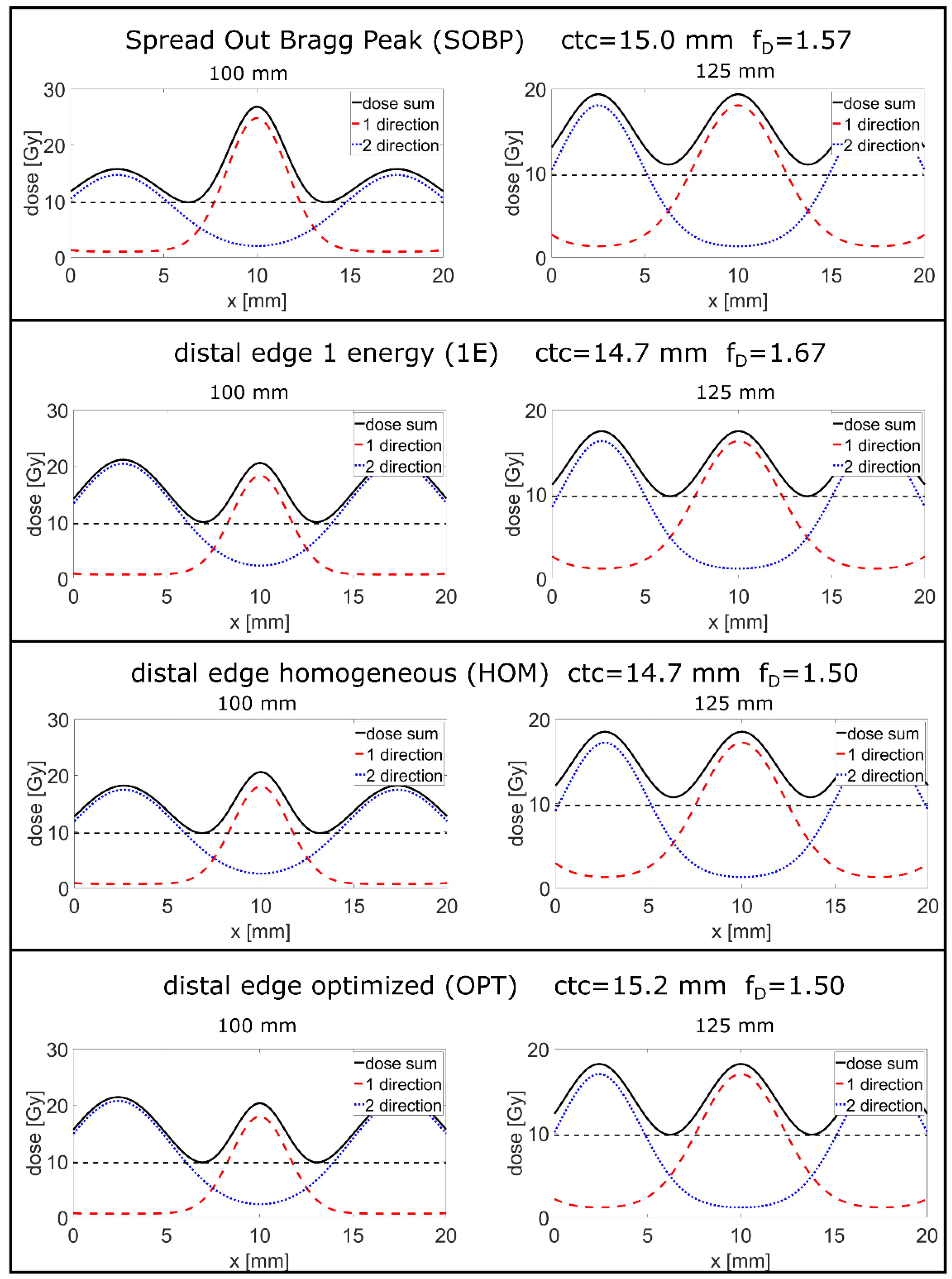
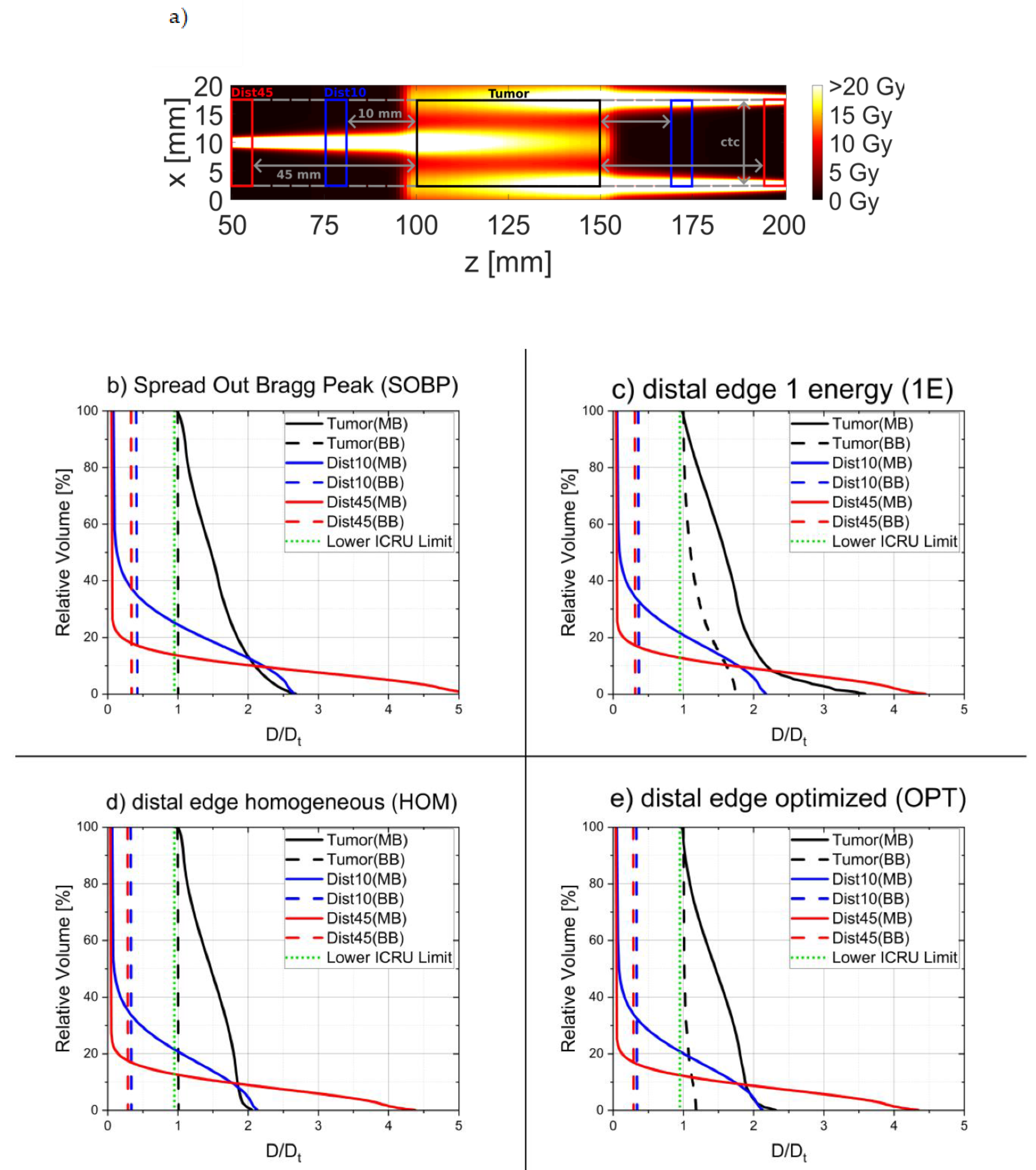
| SOBP | Energy [MeV] | 117 | 119 | 121 | 123 | 125 | 127 | 129 | 131 | 133 |
| Weighting factor | 0.077 | 0.081 | 0.085 | 0.093 | 0.101 | 0.105 | 0.113 | 0.125 | 0.137 | |
| Energy [MeV] | 135 | 137 | 139 | 141 | 143 | 145 | 147 | 148 | ||
| Weighting factor | 0.149 | 0.169 | 0.190 | 0.226 | 0.266 | 0.363 | 0.387 | 1 | ||
| 1E | Energy [MeV] | 146 | ||||||||
| Weighting factor | 1 | |||||||||
| HOM | Energy [MeV] | 133 | 135 | 137 | 139 | 141 | 143 | 144 | 146 | 148 |
| Weighting factor | 0.026 | 0.066 | 0.105 | 0.128 | 0.162 | 0.167 | 0.155 | 0.397 | 1 | |
| OPT | Energy [MeV] | 133 | 135 | 137 | 139 | 141 | 143 | 144 | 146 | 147 |
| Weighting factor | 0.018 | 0.051 | 0.077 | 0.090 | 0.118 | 0.118 | 0.109 | 0.300 | 1 | |
| Irradiation Mode | ctc [mm] | fD | Depth = 90 mm and 160 mm, thus, 10 mm from Tumor Edges Dt = 10 Gy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dmean [Gy] | S [%] | EUD [Gy] | DRFSOBP | deff,10% [mm] | deff,50% [mm] | Δ [mm] | |||
| Broad SOBP | - | 1.00 | 4.23 | 7.0 | 4.23 | 1 | - | - | 4.6 |
| Broad 1E | - | 1.20 | 3.66 | 11.1 | 3.66 | 0.87 | - | - | 2.1 |
| Broad HOM | - | 1.00 | 3.37 | 13.8 | 3.37 | 0.80 | - | - | 5.0 |
| Broad OPT | - | 1.04 | 3.40 | 13.5 | 3.40 | 0.80 | - | - | 3.2 |
| MB SOBP | 15.0 | 1.57 | 4.23 | 36.2 | 1.96 | 0.46 | 5.7 | 7.7 | 4.6 |
| MB 1E | 14.7 | 1.67 | 3.66 | 43.2 | 1.66 | 0.39 | 4.9 | 6.6 | 2.1 |
| MB HOM | 14.7 | 1.50 | 3.37 | 43.1 | 1.67 | 0.39 | 5.0 | 6.6 | 5.0 |
| MB OPT | 15.2 | 1.50 | 3.40 | 44.7 | 1.60 | 0.38 | 5.0 | 6.6 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammer, M.; Rousseti, A.; Girst, S.; Reindl, J.; Dollinger, G. Longitudinally Heterogeneous Tumor Dose Optimizes Proton Broadbeam, Interlaced Minibeam, and FLASH Therapy. Cancers 2022, 14, 5162. https://doi.org/10.3390/cancers14205162
Sammer M, Rousseti A, Girst S, Reindl J, Dollinger G. Longitudinally Heterogeneous Tumor Dose Optimizes Proton Broadbeam, Interlaced Minibeam, and FLASH Therapy. Cancers. 2022; 14(20):5162. https://doi.org/10.3390/cancers14205162
Chicago/Turabian StyleSammer, Matthias, Aikaterini Rousseti, Stefanie Girst, Judith Reindl, and Günther Dollinger. 2022. "Longitudinally Heterogeneous Tumor Dose Optimizes Proton Broadbeam, Interlaced Minibeam, and FLASH Therapy" Cancers 14, no. 20: 5162. https://doi.org/10.3390/cancers14205162
APA StyleSammer, M., Rousseti, A., Girst, S., Reindl, J., & Dollinger, G. (2022). Longitudinally Heterogeneous Tumor Dose Optimizes Proton Broadbeam, Interlaced Minibeam, and FLASH Therapy. Cancers, 14(20), 5162. https://doi.org/10.3390/cancers14205162






