Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
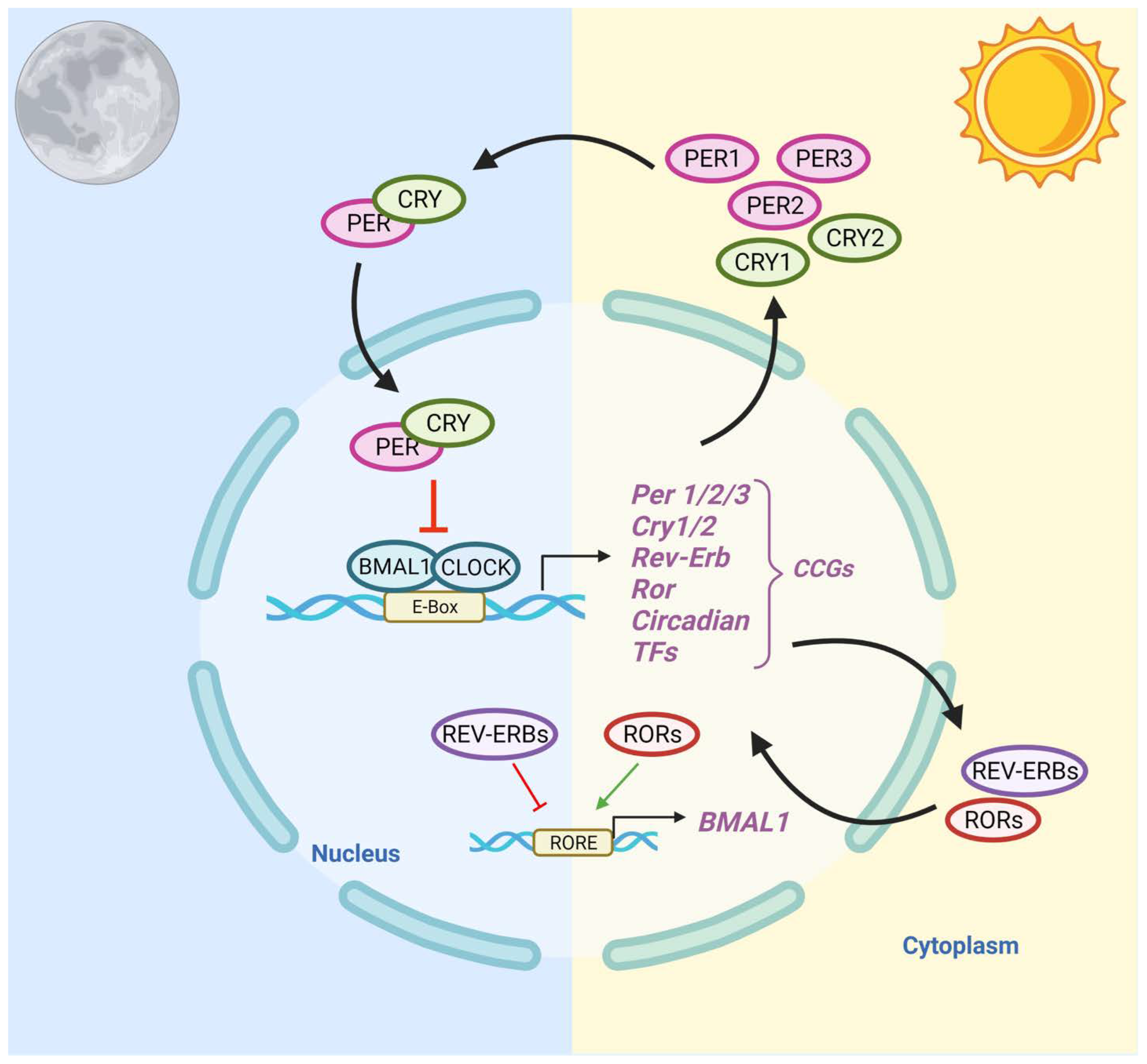
1.1. Regulation of the Circadian Clock System
1.2. Epidemiological Evidence—The Link between CRDs and Prostate Cancer Risk
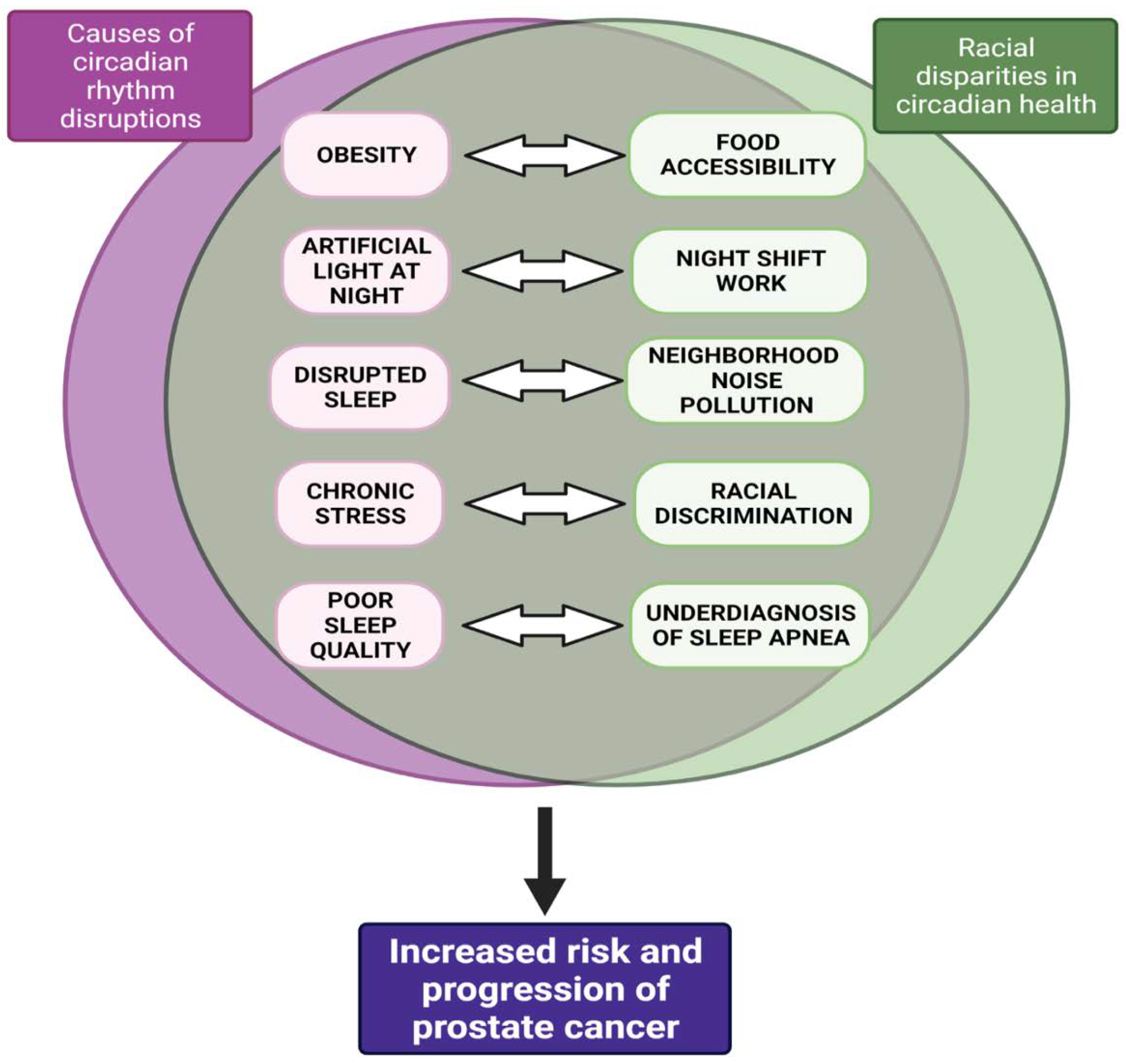
1.2.1. Causes of CRD
1.2.2. Consequences of CRD
1.3. Racial Disparities in Circadian Health—Implications for Prostate Cancer
1.4. The Consequences of CRD for Prostate Cancer Risk and Progression
2. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | African American |
| ALAN | artificial light at night |
| AMPK | AMP-activated protein kinase |
| AR | androgen receptor |
| ARE | androgen response element |
| BMAL1 | brain and Muscle ARNT-Like |
| c-Myc | cellular Myc |
| cAMP | cyclic adenosine monophosphate |
| CCGs | clock-controlled genes |
| CLOCK | circadian Locomotor Output Cycles Kaput |
| CLU | Clusterin |
| CNS | central nervous system |
| CRD | circadian rhythm disruptions |
| CRY | Cryptochrome |
| CSNK1E | casein kinase 1 isoform epsilon |
| GLUT/SLC2A | facilitative glucose transporters |
| GR | glucocorticoid receptor |
| HFD | high fat diet |
| HPA | hypothalamic-pituitary-adrenal |
| IGF | insulin growth factor |
| LEDGF/p75 | lens epithelium-derived growth factor p75 |
| mCRPC | metastatic castration resistant prostate cancer |
| Mdm2 | mouse double minute 2 homolog |
| MT | Melatonin |
| MT1/2 | melatonin receptor ½ |
| NPAS2 | neuronal PAS domain protein 2 |
| PCa | prostate cancer |
| PER | Period |
| PI3K | phosphatidylinositol 3-kinase |
| PPAR | peroxisome proliferator-activated receptors |
| PSA | prostate specific antigen |
| REV-ERBα (NR1D1) | nuclear receptor subfamily 1 group D member 1 |
| RMR | resting metabolic rate |
| RORE | retinoid-related orphan receptors response elements |
| RORα | retinoid-related orphan receptor alpha |
| SCN | suprachiasmatic nucleus |
| SGK1 | serum and glucocorticoid-regulated kinase 1 |
| SIRT1 | Sirtuin 1 |
| Trp53 | tumor protein p53 |
| TTFLs | transcriptional-translational feedback loops |
| 6-STM | 6-sulfaxoymelatonin |
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Estimated New Cases and Deaths. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html#:~:text=Prostate%20cancer%20is%20the%20second,do%20not%20die%20from%20it (accessed on 15 October 2021).
- Jiang, S.; Narayan, V.; Warlick, C. Racial disparities and considerations for active surveillance of prostate cancer. Transl. Androl. Urol. 2018, 7, 214–220. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Jackson, L.A.; Crawford, T.V. Prostate cancer and metabolic syndrome: Is there a link? Asian Pac. J. Cancer Prev. 2012, 13, 1–13. [Google Scholar] [CrossRef]
- Zuniga, K.B.; Chan, J.M.; Ryan, C.J.; Kenfield, S.A. Diet and lifestyle considerations for patients with prostate cancer. Urol. Oncol. 2020, 38, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Davidson, A.J. Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. Transl. Sci. 2013, 119, 283–323. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef]
- Cooksey-Stowers, K.; Schwartz, M.B.; Brownell, K.D. Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. Int. J. Environ. Res. Public Health 2017, 14, 1366. [Google Scholar] [CrossRef]
- Seltenrich, N. Inequality of Noise Exposures: A Portrait of the United States. Environ. Health Perspect. 2017, 125, 094003. [Google Scholar] [CrossRef]
- Bower, K.M.; Thorpe, R.J., Jr.; Rohde, C.; Gaskin, D.J. The intersection of neighborhood racial segregation, poverty, and urbanicity and its impact on food store availability in the United States. Prev. Med. 2014, 58, 33–39. [Google Scholar] [CrossRef]
- Diez Roux, A.V. Neighborhoods and Health: What Do We Know? What Should We Do? Am. J. Public Health 2016, 106, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.A.; Morello-Frosch, R.; Mennitt, D.J.; Fristrup, K.; Ogburn, E.L.; James, P. Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environ. Health Perspect. 2017, 125, 077017. [Google Scholar] [CrossRef] [PubMed]
- Von Allmen, D.C.; Francey, L.J.; Rogers, G.M.; Ruben, M.D.; Cohen, A.P.; Wu, G.; Schmidt, R.E.; Ishman, S.L.; Amin, R.S.; Hogenesch, J.B.; et al. Circadian Dysregulation: The Next Frontier in Obstructive Sleep Apnea Research. Otolaryngol. Head Neck Surg. 2018, 159, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef] [PubMed]
- Koritala, B.S.C.; Conroy, Z.; Smith, D.F. Circadian Biology in Obstructive Sleep Apnea. Diagnostics 2021, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Turkiewicz, S.; Karuga, F.F.; Sochal, M.; Strzelecki, D.; Białasiewicz, P. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients—Possible Mechanisms Involved and Clinical Implication. Int. J. Mol. Sci. 2022, 23, 709. [Google Scholar] [CrossRef] [PubMed]
- Raslau, D.; Summerfield, D.T.; Abu Dabrh, A.M.; Steinkraus, L.W.; Murad, M.H. The risk of prostate cancer in pilots: A meta-analysis. Aerosp. Med. Hum. Perform. 2015, 86, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, E.M.; Hrafnkelsson, J.; Rafnsson, V. Incidence of cancer among licenced commercial pilots flying North Atlantic routes. Environ. Health 2017, 16, 86. [Google Scholar] [CrossRef]
- Wang, F.; Yeung, K.L.; Chan, W.C.; Kwok, C.C.; Leung, S.L.; Wu, C.; Chan, E.Y.; Yu, I.T.; Yang, X.R.; Tse, L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732. [Google Scholar] [CrossRef]
- Rao, D.; Yu, H.; Bai, Y.; Zheng, X.; Xie, L. Does night-shift work increase the risk of prostate cancer? a systematic review and meta-analysis. Oncotargets Ther. 2015, 8, 2817–2826. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Mucci, L.A.; Fall, K.; Rider, J.R.; Schernhammer, E.; Czeisler, C.A.; Launer, L.; Harris, T.; Stampfer, M.J.; et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Markt, S.C.; Grotta, A.; Nyren, O.; Adami, H.O.; Mucci, L.A.; Valdimarsdottir, U.A.; Stattin, P.; Bellocco, R.; Lagerros, Y.T. Insufficient Sleep and Risk of Prostate Cancer in a Large Swedish Cohort. Sleep 2015, 38, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Wendeu-Foyet, M.G.; Cénée, S.; Koudou, Y.; Trétarre, B.; Rébillard, X.; Cancel-Tassin, G.; Cussenot, O.; Boland, A.; Olaso, R.; Deleuze, J.F.; et al. Circadian genes polymorphisms, night work and prostate cancer risk: Findings from the EPICAP study. Int. J. Cancer 2020, 147, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Aronson, W.J. Examining the relationship between obesity and prostate cancer. Rev. Urol. 2004, 6, 73–81. [Google Scholar]
- Lavalette, C.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Cénée, S.; Menegaux, F. Abdominal obesity and prostate cancer risk: Epidemiological evidence from the EPICAP study. Oncotarget 2018, 9, 34485–34494. [Google Scholar] [CrossRef]
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R., Jr.; Danial, N.; et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 2013, 123, 874–886. [Google Scholar] [CrossRef]
- Jayadevappa, R.; Malkowicz, S.B.; Chhatre, S.; Johnson, J.C.; Gallo, J.J. The burden of depression in prostate cancer. Psychooncology 2012, 21, 1338–1345. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, R.G.; Hoffman, A.E.; Fitzgerald, L.M.; Kwon, E.M.; Ostrander, E.A.; Davis, S.; Zheng, T.; Stanford, J.L. Testing the circadian gene hypothesis in prostate cancer: A population-based case-control study. Cancer Res. 2009, 69, 9315–9322. [Google Scholar] [CrossRef]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401. [Google Scholar] [CrossRef]
- Li, Q.; Xia, D.; Wang, Z.; Liu, B.; Zhang, J.; Peng, P.; Tang, Q.; Dong, J.; Guo, J.; Kuang, D.; et al. Circadian Rhythm Gene PER3 Negatively Regulates Stemness of Prostate Cancer Stem Cells via WNT/β-Catenin Signaling in Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 656981. [Google Scholar] [CrossRef]
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Woods-Burnham, L.; Cajigas-Du Ross, C.K.; Love, A.; Basu, A.; Sanchez-Hernandez, E.S.; Martinez, S.R.; Ortiz-Hernández, G.L.; Stiel, L.; Durán, A.M.; Wilson, C.; et al. Glucocorticoids Induce Stress Oncoproteins Associated with Therapy-Resistance in African American and European American Prostate Cancer Cells. Sci. Rep. 2018, 8, 15063. [Google Scholar] [CrossRef] [PubMed]
- Frankenberry, K.A.; Somasundar, P.; McFadden, D.W.; Vona-Davis, L.C. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am. J. Surg. 2004, 188, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Calastretti, A.; Gatti, G.; Lucini, V.; Dugnani, S.; Canti, G.; Scaglione, F.; Bevilacqua, A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1505. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef]
- Rivkees, S.A. The Development of Circadian Rhythms: From Animals To Humans. Sleep Med. Clin. 2007, 2, 331–341. [Google Scholar] [CrossRef]
- Shi, S.Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef]
- Astiz, M.; Heyde, I.; Oster, H. Mechanisms of Communication in the Mammalian Circadian Timing System. Int. J. Mol. Sci. 2019, 20, 343. [Google Scholar] [CrossRef]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef]
- Carmona-Alcocer, V.; Rohr, K.E.; Joye, D.A.M.; Evans, J.A. Circuit development in the master clock network of mammals. Eur. J. Neurosci. 2020, 51, 82–108. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Sassone-Corsi, P. Mammalian circadian clock and metabolism—The epigenetic link. J. Cell Sci. 2010, 123, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Bernal, I.; Becerril-Pérez, F.; Aguilar-Arnal, L. Circadian rhythms in the three-dimensional genome: Implications of chromatin interactions for cyclic transcription. Clin. Epigenetics 2019, 11, 79. [Google Scholar] [CrossRef]
- Milev, N.B.; Reddy, A.B. Circadian redox oscillations and metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Shostak, A. Circadian Clock, Cell Division, and Cancer: From Molecules to Organism. Int. J. Mol. Sci. 2017, 18, 873. [Google Scholar] [CrossRef]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Potter, G.D.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212. [Google Scholar] [CrossRef]
- Daiber, A.; Frenis, K.; Kuntic, M.; Li, H.; Wolf, E.; Kilgallen, A.B.; Lecour, S.; Van Laake, L.W.; Schulz, R.; Hahad, O.; et al. Redox Regulatory Changes of Circadian Rhythm by the Environmental Risk Factors Traffic Noise and Air Pollution. Antioxid. Redox Signal. 2022, 37, 679–703. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.C.; Imboden, M.; Foraster, M.; Schaffner, E.; Kumar, A.; Vienneau, D.; Héritier, H.; Rudzik, F.; Thiesse, L.; Pieren, R.; et al. Exposure to Night-Time Traffic Noise, Melatonin-Regulating Gene Variants and Change in Glycemia in Adults. Int. J. Environ. Res. Public Health 2017, 14, 1492. [Google Scholar] [CrossRef] [PubMed]
- Comperatore, C.A.; Krueger, G.P. Circadian rhythm desynchronosis, jet lag, shift lag, and coping strategies. Occup. Med. 1990, 5, 323–341. [Google Scholar]
- Gander, P.; Mulrine, H.M.; van den Berg, M.J.; Wu, L.; Smith, A.; Signal, L.; Mangie, J. Does the circadian clock drift when pilots fly multiple transpacific flights with 1- to 2-day layovers? Chronobiol. Int. 2016, 33, 982–994. [Google Scholar] [CrossRef]
- Arendt, J. Shift work: Coping with the biological clock. Occup. Med. 2010, 60, 10–20. [Google Scholar] [CrossRef]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607–608, 1073–1084. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Fall, K.; Rider, J.R.; Lockley, S.W.; Schernhammer, E.; Mucci, L.A. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1002–1011. [Google Scholar] [CrossRef]
- Halperin, D. Environmental noise and sleep disturbances: A threat to health? Sleep Sci. 2014, 7, 209–212. [Google Scholar] [CrossRef]
- Muzet, A. Environmental noise, sleep and health. Sleep Med. Rev. 2007, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Harrison, D.G. Nocturnal noise knocks NOS by Nox: Mechanisms underlying cardiovascular dysfunction in response to noise pollution. Eur. Heart J. 2018, 39, 3540–3542. [Google Scholar] [CrossRef] [PubMed]
- Raslau, D.; Abu Dabrh, A.M.; Summerfield, D.T.; Wang, Z.; Steinkraus, L.W.; Murad, M.H. Prostate Cancer in Pilots. Aerosp. Med. Hum. Perform. 2016, 87, 565–570. [Google Scholar] [CrossRef]
- Krstev, S.; Baris, D.; Stewart, P.A.; Hayes, R.B.; Blair, A.; Dosemeci, M. Risk for prostate cancer by occupation and industry: A 24-state death certificate study. Am. J. Ind. Med. 1998, 34, 413–420. [Google Scholar] [CrossRef]
- Reynolds, R.; Little, M.P.; Day, S.; Charvat, J.; Blattnig, S.; Huff, J.; Patel, Z.S. Cancer incidence and mortality in the USA Astronaut Corps, 1959-2017. Occup. Environ. Med. 2021, 78, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Lange, J.H.; Perissinotto, E.; Rausa, G.; Grigoletto, F.; Canova, C.; Mastrangelo, G. Cancer incidence among male military and civil pilots and flight attendants: An analysis on published data. Toxicol. Ind. Health 2005, 21, 273–282. [Google Scholar] [CrossRef]
- Webber, B.J.; Tacke, C.D.; Wolff, G.G.; Rutherford, A.E.; Erwin, W.J.; Escobar, J.D.; Simon, A.A.; Reed, B.H.; Whitaker, J.G.; Gambino-Shirley, K.J.; et al. Cancer Incidence and Mortality Among Fighter Aviators in the United States Air Force. J. Occup. Environ. Med. 2021, 64, 71. [Google Scholar] [CrossRef]
- Erren, T.C.; Falaturi, P.; Morfeld, P.; Knauth, P.; Reiter, R.J.; Piekarski, C. Shift work and cancer: The evidence and the challenge. Dtsch. Arztebl. Int. 2010, 107, 657–662. [Google Scholar] [CrossRef]
- Pepłońska, B.; Burdelak, W.; Bukowska, A.; Krysicka, J.; Konieczko, K. Night shift work characteristics and occupational co-exposures in industrial plants in Łódź, Poland. Int. J. Occup. Med. Environ. Health 2013, 26, 522–534. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef]
- Conlon, M.; Lightfoot, N.; Kreiger, N. Rotating shift work and risk of prostate cancer. Epidemiology 2007, 18, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Oyama, I.; Nakamura, T.; Kunimoto, M.; Kadowaki, K.; Otomo, H.; Fujino, Y.; Fujimoto, N.; Matsumoto, T.; Matsuda, S. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int. J. Urol. 2011, 18, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; El-Zein, M.; Rousseau, M.C.; Pintos, J.; Siemiatycki, J. Night work and the risk of cancer among men. Am. J. Epidemiol. 2012, 176, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragonés, N.; Pérez-Gómez, B.; Burgos, J.; Gómez-Acebo, I.; Llorca, J.; Peiró, R.; Jimenez-Moleón, J.J.; et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer 2015, 137, 1147–1157. [Google Scholar] [CrossRef]
- Mancio, J.; Leal, C.; Ferreira, M.; Norton, P.; Lunet, N. Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 337–344. [Google Scholar] [CrossRef]
- Davis, S.; Mirick, D.K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control 2006, 17, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Blettner, M.; Emrich, K.; Nasterlack, M.; Oberlinner, C.; Hammer, G.P. A retrospective cohort study of shift work and risk of incident cancer among German male chemical workers. Scand. J. Work Environ. Health 2014, 40, 502–510. [Google Scholar] [CrossRef]
- Barul, C.; Richard, H.; Parent, M.E. Night-Shift Work and Risk of Prostate Cancer: Results From a Canadian Case-Control Study, the Prostate Cancer and Environment Study. Am. J. Epidemiol. 2019, 188, 1801–1811. [Google Scholar] [CrossRef]
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch. Environ. Occup. Health 2017, 72, 111–122. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y.J.; Kim, J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobiol. Int. 2017, 34, 203–211. [Google Scholar] [CrossRef]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.E.; Wagner, S.E.; Burch, J.; Bayakly, R.; Vena, J.E. A case-referent study: Light at night and breast cancer risk in Georgia. Int. J. Health Geogr. 2013, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Nelson, D.; Hertz, A.; Horn-Ross, P.L.; Bernstein, L.; Reynolds, P. Light at night and breast cancer risk among California teachers. Epidemiology 2014, 25, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Haim, A.; Portnov, B.A. Light-at-Night (LAN) as a General Stressor. In Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers, Haim, A., Portnov, B.A., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 67–70. [Google Scholar]
- Kloog, I.; Haim, A.; Stevens, R.G.; Portnov, B.A. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol. Int. 2009, 26, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Al-Naggar, R.A.; Anil, S. Artificial Light at Night and Cancer: Global Study. Asian Pac. J. Cancer Prev. 2016, 17, 4661–4664. [Google Scholar] [CrossRef]
- Samanta, S. The Potential Oncostatic Effects of Melatonin against Prostate Cancer. Crit. Rev. Oncog. 2021, 26, 53–67. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Schulmeister, K. Melatonin and cancer risk: Does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br. J. Cancer 2004, 90, 941–943. [Google Scholar] [CrossRef]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef]
- Ullrich, P.M.; Carson, M.R.; Lutgendorf, S.K.; Williams, R.D. Cancer fear and mood disturbance after radical prostatectomy: Consequences of biochemical evidence of recurrence. J. Urol. 2003, 169, 1449–1452. [Google Scholar] [CrossRef]
- Kotwal, A.A.; Schumm, P.; Mohile, S.G.; Dale, W. The influence of stress, depression, and anxiety on PSA screening rates in a nationally representative sample. Med. Care 2012, 50, 1037–1044. [Google Scholar] [CrossRef]
- Fabre, B.; Grosman, H.; Gonzalez, D.; Machulsky, N.F.; Repetto, E.M.; Mesch, V.; Lopez, M.A.; Mazza, O.; Berg, G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac. J. Cancer Prev. 2016, 17, 3167–3171. [Google Scholar] [PubMed]
- Gidron, Y.; Fabre, B.; Grosman, H.; Nolazco, C.; Mesch, V.; Mazza, O.; Berg, G. Life events, cortisol and levels of prostate specific antigen: A story of synergism. Psychoneuroendocrinology 2011, 36, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Kuan, P.; Kotov, R.; Mukherjee, S.; Thompson-Carino, P.; Bromet, E.J.; Luft, B.J. Risk factors for incident prostate cancer in a cohort of world trade center responders. BMC Psychiatry 2019, 19, 389. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Bonn, S.E.; Sjölander, A.; Wiklund, F.; Stattin, P.; Holmberg, E.; Grönberg, H.; Bälter, K. The roles of stress and social support in prostate cancer mortality. Scand. J. Urol. 2016, 50, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.L.; Sanderson, M.; Ellison, G.L.; Fadden, M.K. Stress, coping, social support, and prostate cancer risk among older African American and Caucasian men. Ethn. Dis. 2006, 16, 978–987. [Google Scholar]
- Isikbay, M.; Otto, K.; Kregel, S.; Kach, J.; Cai, Y.; Vander Griend, D.J.; Conzen, S.D.; Szmulewitz, R.Z. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm. Cancer 2014, 5, 72–89. [Google Scholar] [CrossRef]
- Møller, H.; Roswall, N.; Van Hemelrijck, M.; Larsen, S.B.; Cuzick, J.; Holmberg, L.; Overvad, K.; Tjønneland, A. Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. Int. J. Cancer 2015, 136, 1940–1947. [Google Scholar] [CrossRef]
- Fowke, J.H.; Motley, S.S.; Concepcion, R.S.; Penson, D.F.; Barocas, D.A. Obesity, body composition, and prostate cancer. BMC Cancer 2012, 12, 23. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Weikert, S.; Allen, N.; Key, T.; Johnsen, N.F.; Tjønneland, A.; Severinsen, M.T.; Overvad, K.; Rohrmann, S.; et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3252–3261. [Google Scholar] [CrossRef]
- Engeland, A.; Tretli, S.; Bjørge, T. Height, body mass index, and prostate cancer: A follow-up of 950,000 Norwegian men. Br. J. Cancer 2003, 89, 1237–1242. [Google Scholar] [CrossRef]
- Barrington, W.E.; Schenk, J.M.; Etzioni, R.; Arnold, K.B.; Neuhouser, M.L.; Thompson, I.M., Jr.; Lucia, M.S.; Kristal, A.R. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015, 1, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Giovannucci, E.; Platz, E.A. Are findings from studies of obesity and prostate cancer really in conflict? Cancer Causes Control 2006, 17, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Keto, C.J.; Aronson, W.J.; Terris, M.K.; Presti, J.C.; Kane, C.J.; Amling, C.L.; Freedland, S.J. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: Results from the SEARCH database. BJU Int. 2012, 110, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Walker, J.R.; Brown, M.K.; Das, R.; Jones, N.L. A workshop report on the causes and consequences of sleep health disparities. Sleep 2020, 43, zsaa037. [Google Scholar] [CrossRef]
- Kingsbury, J.H.; Buxton, O.M.; Emmons, K.M. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2013, 7, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Lunsford-Avery, J.R.; Engelhard, M.M.; Navar, A.M.; Kollins, S.H. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci. Rep. 2018, 8, 14158. [Google Scholar] [CrossRef]
- Huang, S.J.; Sehgal, N.J. Association of historic redlining and present-day health in Baltimore. PLoS ONE 2022, 17, e0261028. [Google Scholar] [CrossRef]
- Johnson, D.A.; Lisabeth, L.; Hickson, D.; Johnson-Lawrence, V.; Samdarshi, T.; Taylor, H.; Diez Roux, A.V. The Social Patterning of Sleep in African Americans: Associations of Socioeconomic Position and Neighborhood Characteristics with Sleep in the Jackson Heart Study. Sleep 2016, 39, 1749–1759. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Kaplan, G.A.; Roberts, R.E. Short sleep duration across income, education, and race/ethnic groups: Population prevalence and growing disparities during 34 years of follow-up. Ann. Epidemiol. 2007, 17, 948–955. [Google Scholar] [CrossRef]
- Ertel, K.A.; Berkman, L.F.; Buxton, O.M. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep 2011, 34, 509–518. [Google Scholar] [CrossRef]
- Ghosh-Dastidar, B.; Cohen, D.; Hunter, G.; Zenk, S.N.; Huang, C.; Beckman, R.; Dubowitz, T. Distance to store, food prices, and obesity in urban food deserts. Am. J. Prev. Med. 2014, 47, 587–595. [Google Scholar] [CrossRef]
- Chen, D.; Jaenicke, E.C.; Volpe, R.J. Food Environments and Obesity: Household Diet Expenditure Versus Food Deserts. Am. J. Public Health 2016, 106, 881–888. [Google Scholar] [CrossRef]
- Slopen, N.; Lewis, T.T.; Williams, D.R. Discrimination and sleep: A systematic review. Sleep Med. 2016, 18, 88–95. [Google Scholar] [CrossRef]
- Hale, L.; Do, D.P. Racial Differences in Self-Reports of Sleep Duration in a Population-Based Study. Sleep 2007, 30, 1096–1103. [Google Scholar] [CrossRef]
- Egan, K.J.; Knutson, K.L.; Pereira, A.C.; von Schantz, M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med. Rev. 2017, 33, 70–78. [Google Scholar] [CrossRef]
- Eastman, C.I.; Tomaka, V.A.; Crowley, S.J. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci. Rep. 2016, 6, 36716. [Google Scholar] [CrossRef]
- Jackson, C.L. Determinants of racial/ethnic disparities in disordered sleep and obesity. Sleep Health 2017, 3, 401–415. [Google Scholar] [CrossRef]
- Grandner, M.A.; Petrov, M.E.; Rattanaumpawan, P.; Jackson, N.; Platt, A.; Patel, N.P. Sleep symptoms, race/ethnicity, and socioeconomic position. J. Clin. Sleep Med. 2013, 9, 897–905; 905a–905d. [Google Scholar] [CrossRef]
- Friedman, M.; Bliznikas, D.; Klein, M.; Duggal, P.; Somenek, M.; Joseph, N.J. Comparison of the incidences of obstructive sleep apnea-hypopnea syndrome in African-Americans versus Caucasian-Americans. Otolaryngol. Head Neck Surg. 2006, 134, 545–550. [Google Scholar] [CrossRef]
- Kimoff, R.J. Sleep Fragmentation in Obstructive Sleep Apnea. Sleep 1996, 19, S61–S66. [Google Scholar] [CrossRef]
- Zanobetti, A.; Redline, S.; Schwartz, J.; Rosen, D.; Patel, S.; O’Connor, G.T.; Lebowitz, M.; Coull, B.A.; Gold, D.R. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am. J. Respir. Crit. Care Med. 2010, 182, 819–825. [Google Scholar] [CrossRef]
- Kiss, Z.; Ghosh, P.M. Women in cancer thematic review: Circadian rhythmicity and the influence of ‘clock’ genes on prostate cancer. Endocr. Relat. Cancer 2016, 23, T123–T134. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Huang, W.; Reiter, R.J.; Ahmad, N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res. 2010, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Gery, S.; Dashti, A.; Yin, D.; Zhou, Y.; Gu, J.; Koeffler, H.P. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009, 69, 7619–7625. [Google Scholar] [CrossRef]
- Woods-Burnham, L.; Stiel, L.; Martinez, S.R.; Sanchez-Hernandez, E.S.; Ruckle, H.C.; Almaguel, F.G.; Stern, M.C.; Roberts, L.R.; Williams, D.R.; Montgomery, S. Psychosocial stress, glucocorticoid signaling, and prostate cancer health disparities in African American men. Cancer Health Disparities 2020, 4. [Google Scholar]
- Kroon, J.; Puhr, M.; Buijs, J.T.; van der Horst, G.; Hemmer, D.M.; A Marijt, K.; Hwang, M.S.; Masood, M.; Grimm, S.; Storm, G.; et al. Glucocorticoid receptor antagonism reverts docetaxel resistance in human prostate cancer. Endocrine-Related Cancer 2015, 23, 35–45. [Google Scholar] [CrossRef]
- Kumar, R. Emerging role of glucocorticoid receptor in castration resistant prostate cancer: A potential therapeutic target. J. Cancer 2020, 11, 696–701. [Google Scholar] [CrossRef]
- Sherk, A.B.; Frigo, D.E.; Schnackenberg, C.G.; Bray, J.D.; Laping, N.J.; Trizna, W.; Hammond, M.; Patterson, J.R.; Thompson, S.K.; Kazmin, D.; et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008, 68, 7475–7483. [Google Scholar] [CrossRef]
- Kach, J.; Long, T.M.; Selman, P.; Tonsing-Carter, E.Y.; Bacalao, M.A.; Lastra, R.R.; de Wet, L.; Comiskey, S.; Gillard, M.; VanOpstall, C.; et al. Selective Glucocorticoid Receptor Modulators (SGRMs) Delay Castrate-Resistant Prostate Cancer Growth. Mol. Cancer Ther. 2017, 16, 1680–1692. [Google Scholar] [CrossRef]
- Puhr, M.; Hoefer, J.; Eigentler, A.; Ploner, C.; Handle, F.; Schaefer, G.; Kroon, J.; Leo, A.; Heidegger, I.; Eder, I.E.; et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef]
- Haus, E.; Dumitriu, L.; Nicolau, G.Y.; Bologa, S.; Sackett-Lundeen, L. Circadian rhythms of basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), cortisol, and melatonin in women with breast cancer. Chronobiol. Int. 2001, 18, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.; Haneuse, S.; Fall, K.; Schernhammer, E.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary Melatonin Levels, Sleep Disruption, and Risk of Prostate Cancer in Elderly Men. Eur. Urol. 2015, 67, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Shiu, S.Y. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: Potential implications for therapeutic strategies against prostate cancer. J Pineal Res 2011, 51, 297–312. [Google Scholar] [CrossRef]
- Tai, S.Y.; Huang, S.P.; Bao, B.Y.; Wu, M.T. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: A case-control study. Sci. Rep. 2016, 6, 29606. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Gonzalez-Menendez, P.; Fernandez-Fernandez, M.; Cueto, S.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Mayo, J.C.; Sainz, R.M. Melatonin Decreases Glucose Metabolism in Prostate Cancer Cells: A (13)C Stable Isotope-Resolved Metabolomic Study. Int. J. Mol. Sci. 2017, 18, 1620. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lee, C.C. The circadian clock: Pacemaker and tumour suppressor. Nat. Rev. Cancer 2003, 3, 350–361. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, Z.; Li, Z.; Huang, Q. Circadian clock is associated with tumor microenvironment in kidney renal clear cell carcinoma. Aging 2020, 12, 14620–14632. [Google Scholar] [CrossRef]
- Chu, L.W.; Zhu, Y.; Yu, K.; Zheng, T.; Yu, H.; Zhang, Y.; Sesterhenn, I.; Chokkalingam, A.P.; Danforth, K.N.; Shen, M.C.; et al. Variants in circadian genes and prostate cancer risk: A population-based study in China. Prostate Cancer Prostatic Dis. 2008, 11, 342–348. [Google Scholar] [CrossRef]
- Fang, M.Z.; Ohman-Strickland, P.; Kelly-McNeil, K.; Kipen, H.; Crabtree, B.F.; Lew, J.P.; Zarbl, H. Sleep interruption associated with house staff work schedules alters circadian gene expression. Sleep Med. 2015, 16, 1388–1394. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chen, L.C.; Chiou, C.Y.; Chang, Y.J.; Lin, V.C.; Huang, C.Y.; Lin, I.L.; Chang, T.Y.; Lu, T.L.; Lee, C.H.; et al. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Till, C.; Yang, B.; Tangen, C.M.; Goodman, P.J.; Yu, K.; Zhu, Y.; Han, S.; Hoque, A.M.; Ambrosone, C.; et al. Circadian genes and risk of prostate cancer in the prostate cancer prevention trial. Mol. Carcinog. 2018, 57, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Markt, S.C.; Valdimarsdottir, U.A.; Shui, I.M.; Sigurdardottir, L.G.; Rider, J.R.; Tamimi, R.M.; Batista, J.L.; Haneuse, S.; Flynn-Evans, E.; Lockley, S.W.; et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control 2015, 26, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Hoogstraat, M.; Stelloo, S.; Eickhoff, N.; Schuurman, K.; de Barros, H.; Alkemade, M.; Bekers, E.M.; Severson, T.M.; Sanders, J.; et al. Drug-induced epigenomic plasticity reprograms circadian rhythm regulation to drive prostate cancer towards androgen-independence. Cancer Discov. 2022, 19, 2074–2097. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Chrousos, G.P. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. 2), S50–S55. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Sookoian, S.; Gemma, C.; Fernández Gianotti, T.; Burgueño, A.; Alvarez, A.; González, C.D.; Pirola, C.J. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J. Intern. Med. 2007, 261, 285–292. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Cuevas, A.G.; Trudel-Fitzgerald, C.; Cofie, L.; Zaitsu, M.; Allen, J.; Williams, D.R. Placing prostate cancer disparities within a psychosocial context: Challenges and opportunities for future research. Cancer Causes Control 2019, 30, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.H.C.; Strong, L.L.; Nguyen, N.T.; Cho, D.; John, J.; McNeill, L.H. Psychosocial Stressors, Depression, and Physical Activity among African Americans. Am. J. Health Behav. 2019, 43, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Lewis, T.T.; Guo, N.; Jackson, C.L.; Sims, M.; Wilson, J.G.; Diez Roux, A.V.; Williams, D.R.; Redline, S. Associations between everyday discrimination and sleep quality and duration among African-Americans over time in the Jackson Heart Study. Sleep 2021, 44, zsab162. [Google Scholar] [CrossRef]
- Williams, D.R. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann. N. Y. Acad. Sci. 1999, 896, 173–188. [Google Scholar] [CrossRef]
- Berger, M.; Sarnyai, Z. “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puhr, M.; Eigentler, A.; Handle, F.; Hackl, H.; Ploner, C.; Heidegger, I.; Schaefer, G.; Brandt, M.P.; Hoefer, J.; Van der Pluijm, G.; et al. Targeting the glucocorticoid receptor signature gene Mono Amine Oxidase-A enhances the efficacy of chemo- and anti-androgen therapy in advanced prostate cancer. Oncogene 2021, 40, 3087–3100. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Q. The role of glucocorticoid receptor in prostate cancer progression: From bench to bedside. Int. Urol. Nephrol. 2017, 49, 369–380. [Google Scholar] [CrossRef]
- Narayanan, S.; Srinivas, S.; Feldman, D. Androgen–glucocorticoid interactions in the era of novel prostate cancer therapy. Nat. Rev. Urol. 2016, 13, 47–60. [Google Scholar] [CrossRef]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra143. [Google Scholar] [CrossRef]
- Kuhn, E.; Brodan, V.; Brodanová, M.; Rysánek, K. Metabolic reflection of sleep deprivation. Act. Nerv. Super 1969, 11, 165–174. [Google Scholar]
- Laposky, A.D.; Bass, J.; Kohsaka, A.; Turek, F.W. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008, 582, 142–151. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N. Y. Acad. Sci. 2008, 1129, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Leproult, R.; Van Cauter, E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocrinol. 2009, 5, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rudic, R.D.; McNamara, P.; Curtis, A.M.; Boston, R.C.; Panda, S.; Hogenesch, J.B.; Fitzgerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef]
- Burton, A.J.; Tilling, K.M.; Holly, J.M.; Hamdy, F.C.; Rowlands, M.A.; Donovan, J.L.; Martin, R.M. Metabolic imbalance and prostate cancer progression. Int. J. Mol. Epidemiol. Genet. 2010, 1, 248–271. [Google Scholar]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Ayers, R.T.; Mantzoros, C.S. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef]
- Nguyen, J.; Wright, K.P., Jr. Influence of weeks of circadian misalignment on leptin levels. Nat. Sci. Sleep 2010, 2, 9–18. [Google Scholar] [CrossRef][Green Version]
- Dibner, C.; Gachon, F. Circadian Dysfunction and Obesity: Is Leptin the Missing Link? Cell Metab. 2015, 22, 359–360. [Google Scholar] [CrossRef]
- Tähkämö, L.; Partonen, T.; Pesonen, A.-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Rodriguez-Garcia, A.; Gonzalez-Menendez, P.; Cepas, V.; Gonzalez-Pola, I.; Sainz, R.M. IGFBP3 and MAPK/ERK signaling mediates melatonin-induced antitumor activity in prostate cancer. J. Pineal Res. 2017, 62, e12373. [Google Scholar] [CrossRef]
| CRD-related Pathways | Effect on Prostate Cancer | Therapeutic Targets | ||
|---|---|---|---|---|
| Circadian Gene Variants | Per 1, Per 2, and Clock ↓ and Bmal1 ↑ [124] | Increased risk of Pca [124] | Melatonin ↑ Per 2 and Clock and ↓ Bmal1 levels [125] | |
| Per 1-3, CSNK1E, Cry 1-2, BMAL1, CLOCK and NPAS2 SNPs [29] | Greater risk of aggressive Pca [29] | Overexpression of Per 1 and Per 2 induces growth inhibition [124] | ||
| Per 3 pathway [31] | Regulation of PCSCs [31] | CRY1 levels promote DNA repair and cancer survival [30] | ||
| Per1 decreased AR-related genes in the presence of DHT [126] | ||||
| Stress | Glucocorticoids ↑ CLU and LEDG/p75 [127] | Pca therapy resistance [127] | RU-486 and cyproterone acetate revert docetaxel resistance [128] | |
| ↑ GR transcript expression following anti- androgen therapy [129] | Tumor progression in mCRPC [129] | SGK1 antagonist blocks AR-mediated growth [130] | ||
| SGRMs ↓ GR transcriptional activity and ↓ GR-mediated tumor cell viability post-AR blockade [131] | ||||
| GR upregulation [132] | Bypass AR blockade [132] | |||
| Obesity | ↑ Leptin [34] | Migration [34] | MAPK and PI3K inhibits migration of Pca cells in the presence of leptin [34] | |
| NPAS2, Per 1, Per 3, Cry 2, and CSNK1E [133] | Altered IGF-1 and androgen [133] | |||
| Melatonin Inhibition | ↓ 6-STM serum levels [134] | Increase risk for advanced Pca [134] | Melatonin inhibits Pca cell proliferation, ↓ AR signaling and ↓ p27 pathway [135] | |
| ↓ melatonin: cortisol ratio and PSA levels [136] | Increased risk for primary and advanced Pca [136] | Melatonin inhibits glycolysis and the pentose phosphate pathway [137] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasari, S.S.; Archer, M.; Mohamed, N.E.; Tewari, A.K.; Figueiro, M.G.; Kyprianou, N. Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer. Cancers 2022, 14, 5116. https://doi.org/10.3390/cancers14205116
Dasari SS, Archer M, Mohamed NE, Tewari AK, Figueiro MG, Kyprianou N. Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer. Cancers. 2022; 14(20):5116. https://doi.org/10.3390/cancers14205116
Chicago/Turabian StyleDasari, Sonali S., Maddison Archer, Nihal E. Mohamed, Ashutosh K. Tewari, Mariana G. Figueiro, and Natasha Kyprianou. 2022. "Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer" Cancers 14, no. 20: 5116. https://doi.org/10.3390/cancers14205116
APA StyleDasari, S. S., Archer, M., Mohamed, N. E., Tewari, A. K., Figueiro, M. G., & Kyprianou, N. (2022). Circadian Rhythm Disruption as a Contributor to Racial Disparities in Prostate Cancer. Cancers, 14(20), 5116. https://doi.org/10.3390/cancers14205116






